Seasonal Antimicrobial Activity of the Airway: Post-Hoc Analysis of a Randomized Placebo-Controlled Double-Blind Trial
Abstract
1. Introduction
2. Materials and Methods
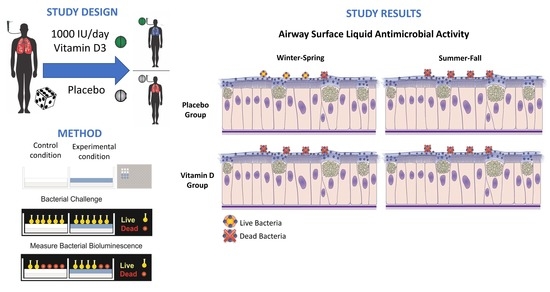
2.1. Study Design
2.2. Participants, Interventions and Randomization
2.3. Outcomes
2.4. Adherence
2.5. Vitamin D Measurement
2.6. Human ASL Samples
2.7. ASL Antimicrobial Activity
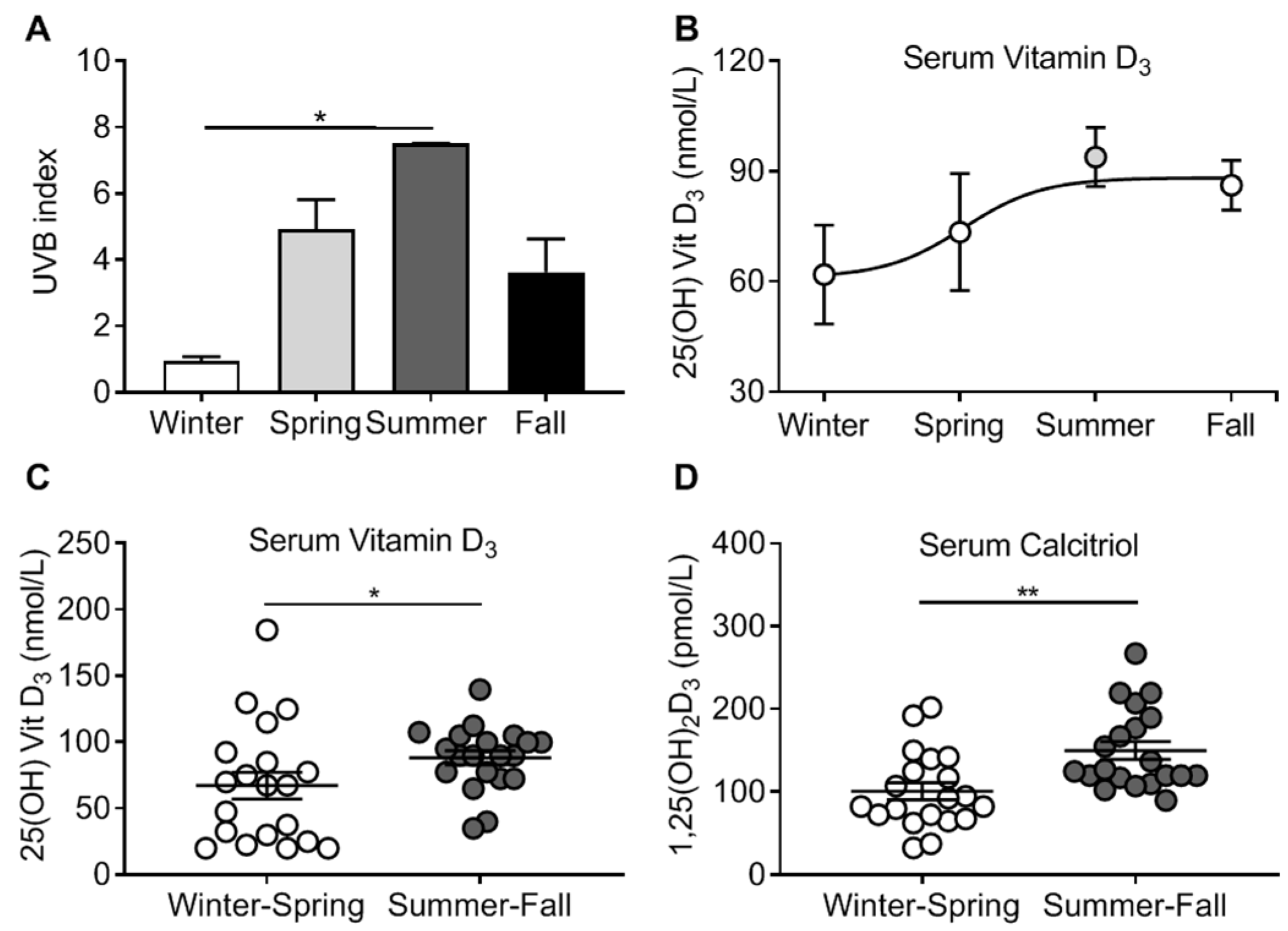
2.8. Seasons and UV Intensity
2.9. LL-37 Antibody Assay
2.10. Statistical Analysis
3. Results
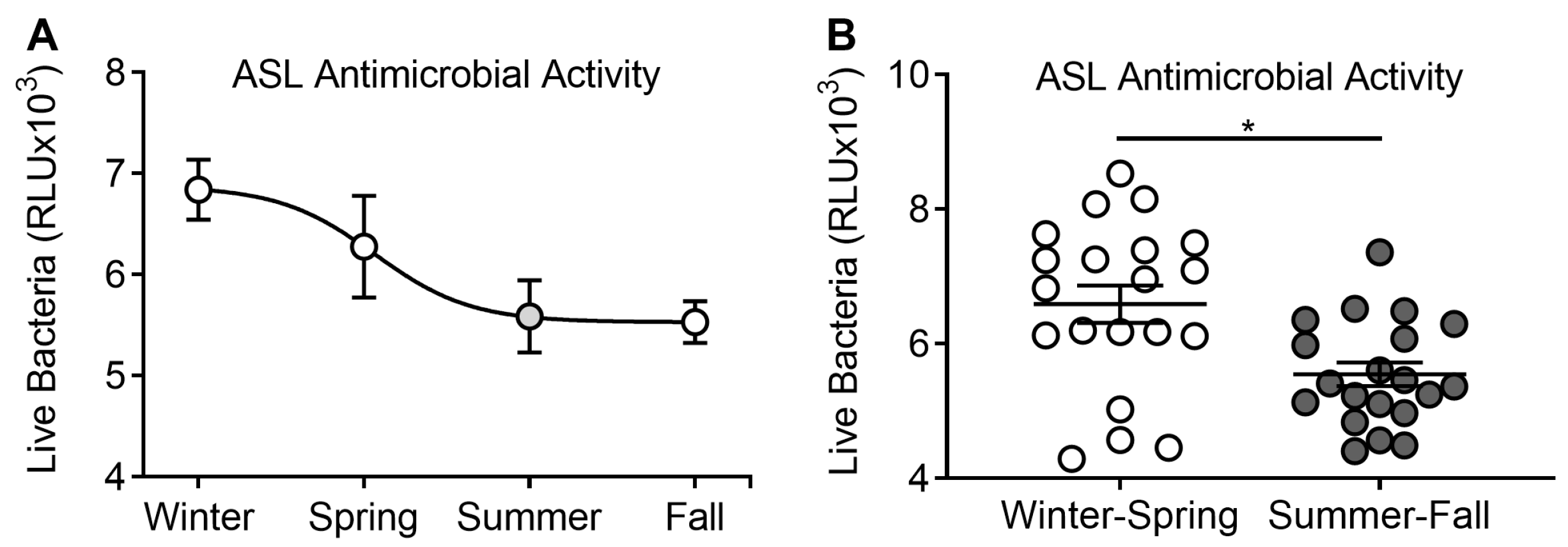
3.1. ASL Antimicrobial Activity Analyzed by Season
3.2. Vitamin D3 Levels and Seasonal Pattern
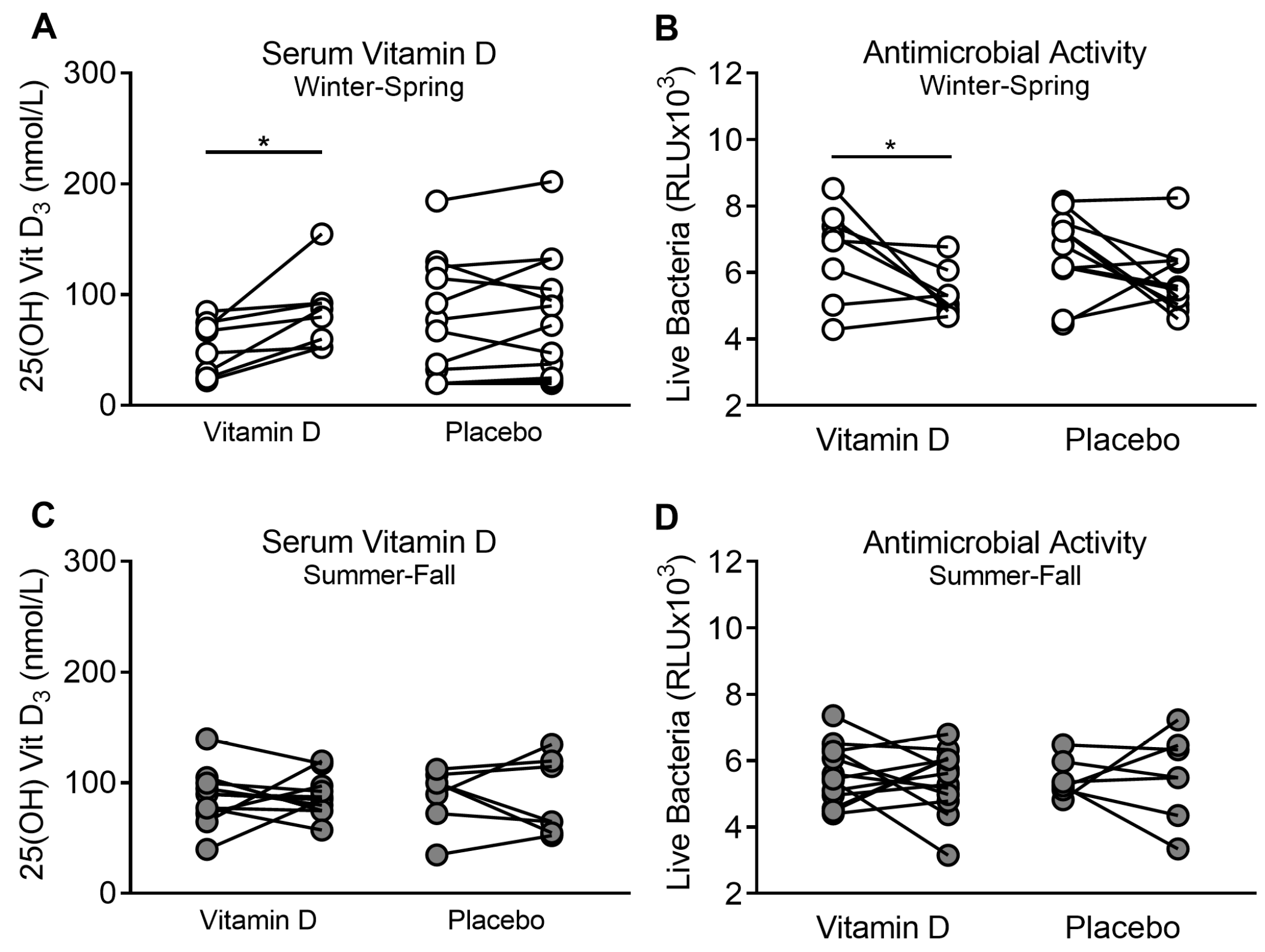
3.3. Supplementation of Vitamin D3 during Winter–Spring, but Not during Summer–Fall, Improves ASL Antimicrobial Activity
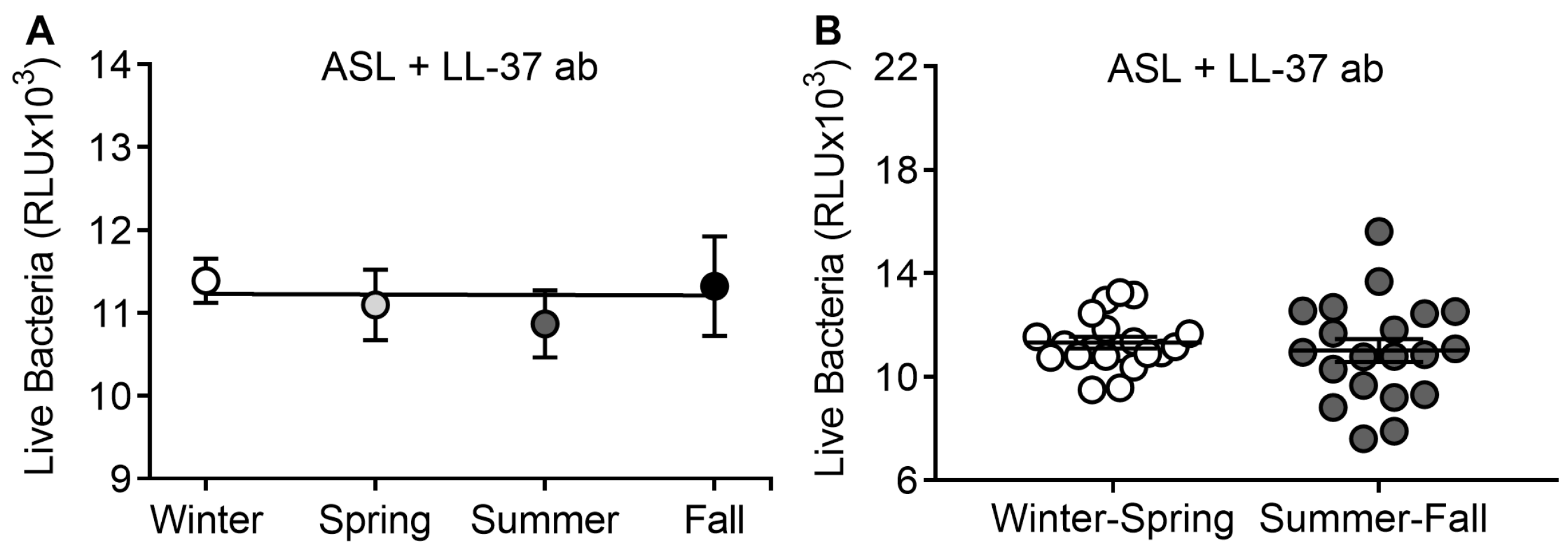
3.4. LL-37 Antibody Abrogates Seasonal Differences in ASL Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hope-Simpson, R.E. The role of season in the epidemiology of influenza. J. Hyg. 1981, 86, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun. Dis. Public Health 2000, 3, 32–38. [Google Scholar] [PubMed]
- Elliot, A.J.; Cross, K.W.; Fleming, D.M. Acute respiratory infections and winter pressures on hospital admissions in England and Wales 1990–2005. J. Public Health 2008, 30, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Eccles, R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Oto-Laryngol. 2002, 122, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Ma, L.W.; Kwitniewski, M.; Polev, G.A.; Lagunova, Z.; Dahlback, A.; Moan, J. The seasonality of pandemic and non-pandemic influenzas: The roles of solar radiation and vitamin D. Int. J. Infect. Dis. 2010, 14, e1099–e1105. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, E.; Fefferman, N.H.; Naumov, Y.N.; Gorski, J.; Naumova, E.N. Influenza seasonality: Underlying causes and modeling theories. J. Virol. 2007, 81, 5429–5436. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, H.; Pournaghi, S.J.; Hashemi, J.; Mohammad-Zadeh, M.; Akaberi, A. Do sufficient vitamin D levels at the end of summer in children and adolescents provide an assurance of vitamin D sufficiency at the end of winter? A cohort study. J. Pediatr. Endocrinol. Metab. 2017, 30, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Vaes, A.M.M.; Van der Zwaluw, N.L.; Van Wijngaarden, J.P.; Swart, K.M.A.; Ham, A.C.; Van Dijk, S.C.; Enneman, A.W.; Sohl, E.; Van Schoor, N.M.; et al. Relative importance of summer sun exposure, vitamin D intake, and genes to vitamin D status in Dutch older adults: The B-PROOF study. J. Steroid Biochem. Mol. Biol. 2016, 164, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Tjonneland, A.; Koster, B.; Brot, C.; Andersen, R.; Lundqvist, M.; Christensen, J.; Olsen, A. Sun Exposure Guidelines and Serum Vitamin D Status in Denmark: The StatusD Study. Nutrients 2016, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Bittner, E.A.; Christopher, K.B.; Camargo, C.A., Jr. Vitamin D status and community-acquired pneumonia: Results from the third National Health and Nutrition Examination Survey. PLoS ONE 2013, 8, e81120. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.J.; Gray, M.A.; Garnett, J.P.; Ward, C.; Brodlie, M. Airway surface liquid homeostasis in cystic fibrosis: Pathophysiology and therapeutic targets. Thorax 2016, 71, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. Off. Publ. Federat. Am. Soc. Exp. Biol. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Gerke, A.K.; Pezzulo, A.A.; Tang, F.; Cavanaugh, J.E.; Bair, T.B.; Phillips, E.; Powers, L.S.; Monick, M.M. Effects of vitamin D supplementation on alveolar macrophage gene expression: Preliminary results of a randomized, controlled trial. Multidiscip. Respir. Med. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Vargas Buonfiglio, L.G.; Cano, M.; Pezzulo, A.A.; Vanegas Calderon, O.G.; Zabner, J.; Gerke, A.K.; Comellas, A.P. Effect of vitamin D3 on the antimicrobial activity of human airway surface liquid: Preliminary results of a randomised placebo-controlled double-blind trial. BMJ Open Respir. Res. 2017, 4, e000211. [Google Scholar] [CrossRef] [PubMed]
- Vargas Buonfiglio, L.G.; Mudunkotuwa, I.A.; Abou Alaiwa, M.H.; Vanegas Calderon, O.G.; Borcherding, J.A.; Gerke, A.K.; Zabner, J.; Grassian, V.H.; Comellas, A.P. Effects of Coal Fly Ash Particulate Matter on the Antimicrobial Activity of Airway Surface Liquid. Environ. Health Perspect. 2017, 125, 077003. [Google Scholar] [CrossRef] [PubMed]
- Jari Hovila, A.A.; Johanna, T. OMI/Aura Surface UVB Irradiance and Erythemal Dose Daily L3 Global Gridded 1.0 Degree × 1.0 Degree V3. DISC; NASA Goddard Earth Sciences Data and Information Services Center (GES DISC); NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2010. Available online: https://mirador.gsfc.nasa.gov/collections/OMUVBd__003.shtml (accessed on 22 February 2018). [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
| Subject Characteristics | Summer–Fall | Winter–Spring | p Value |
|---|---|---|---|
| Number of participants | 20 | 20 | … |
| Age (years) | 28.9 (19–51) | 26.15 (19–60) | 0.41 |
| Male/female sex (%) | 55/45 | 75/25 | 0.32 |
| Ethnicity (% white) | 100 | 80 | 0.11 |
| Smokers (%) | 30 | 30 | 0.21 |
| Pack/years | 24.67 (10–25) | 16.75 (10–45) | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas Buonfiglio, L.G.; Vanegas Calderon, O.G.; Cano, M.; Simmering, J.E.; Polgreen, P.M.; Zabner, J.; Gerke, A.K.; Comellas, A.P. Seasonal Antimicrobial Activity of the Airway: Post-Hoc Analysis of a Randomized Placebo-Controlled Double-Blind Trial. Nutrients 2020, 12, 2602. https://doi.org/10.3390/nu12092602
Vargas Buonfiglio LG, Vanegas Calderon OG, Cano M, Simmering JE, Polgreen PM, Zabner J, Gerke AK, Comellas AP. Seasonal Antimicrobial Activity of the Airway: Post-Hoc Analysis of a Randomized Placebo-Controlled Double-Blind Trial. Nutrients. 2020; 12(9):2602. https://doi.org/10.3390/nu12092602
Chicago/Turabian StyleVargas Buonfiglio, Luis G., Oriana G. Vanegas Calderon, Marlene Cano, Jacob E. Simmering, Philip M. Polgreen, Joseph Zabner, Alicia K. Gerke, and Alejandro P. Comellas. 2020. "Seasonal Antimicrobial Activity of the Airway: Post-Hoc Analysis of a Randomized Placebo-Controlled Double-Blind Trial" Nutrients 12, no. 9: 2602. https://doi.org/10.3390/nu12092602
APA StyleVargas Buonfiglio, L. G., Vanegas Calderon, O. G., Cano, M., Simmering, J. E., Polgreen, P. M., Zabner, J., Gerke, A. K., & Comellas, A. P. (2020). Seasonal Antimicrobial Activity of the Airway: Post-Hoc Analysis of a Randomized Placebo-Controlled Double-Blind Trial. Nutrients, 12(9), 2602. https://doi.org/10.3390/nu12092602