Inputs to Thirst and Drinking during Water Restriction and Rehydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
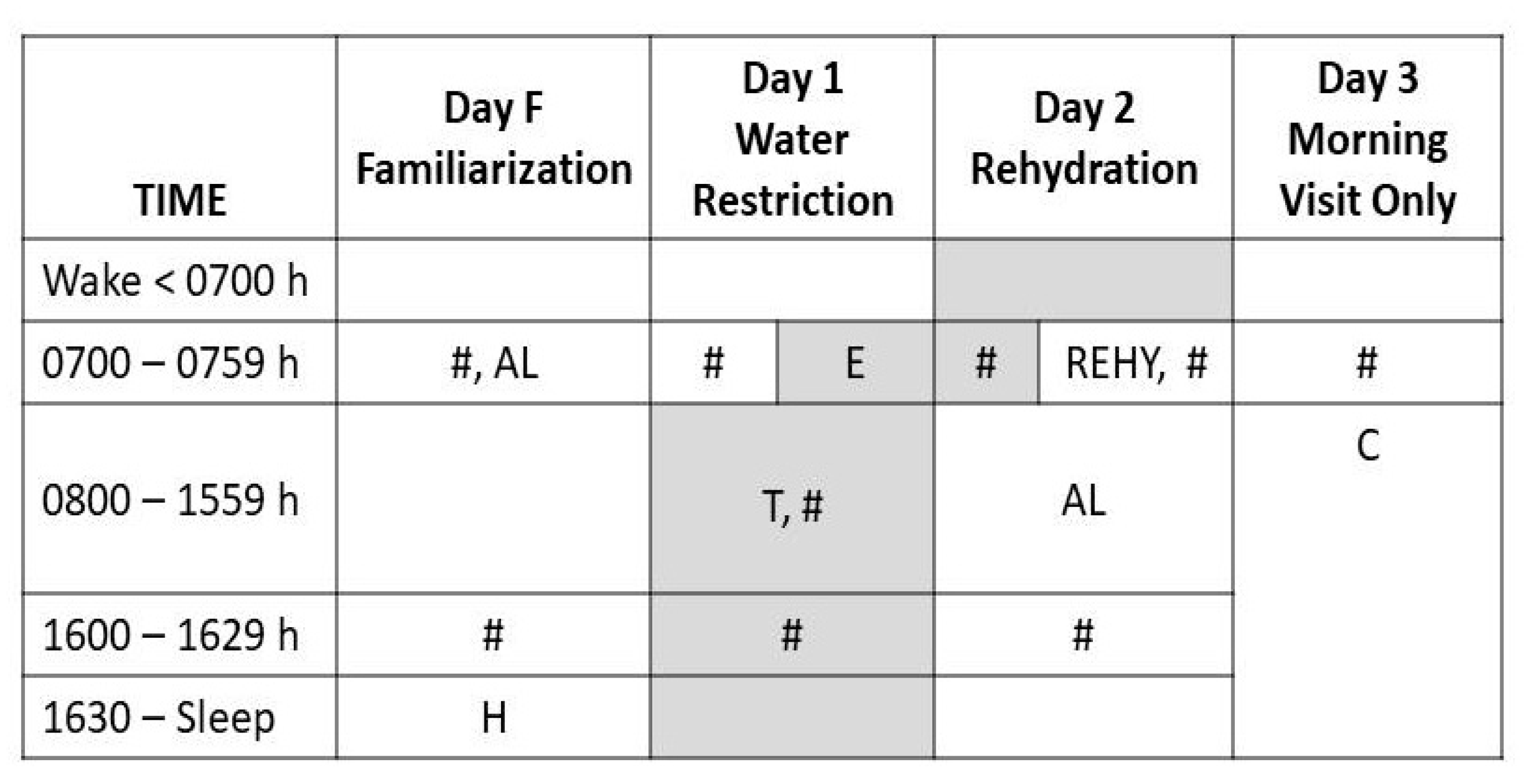
2.2. Experimental Intervention
2.3. Laboratory Measurements and Analyses
2.4. Visual Analog Scales (VAS)
2.5. Statistical Analyses and Figure Construction
2.6. Calculation of Sample Size
3. Results
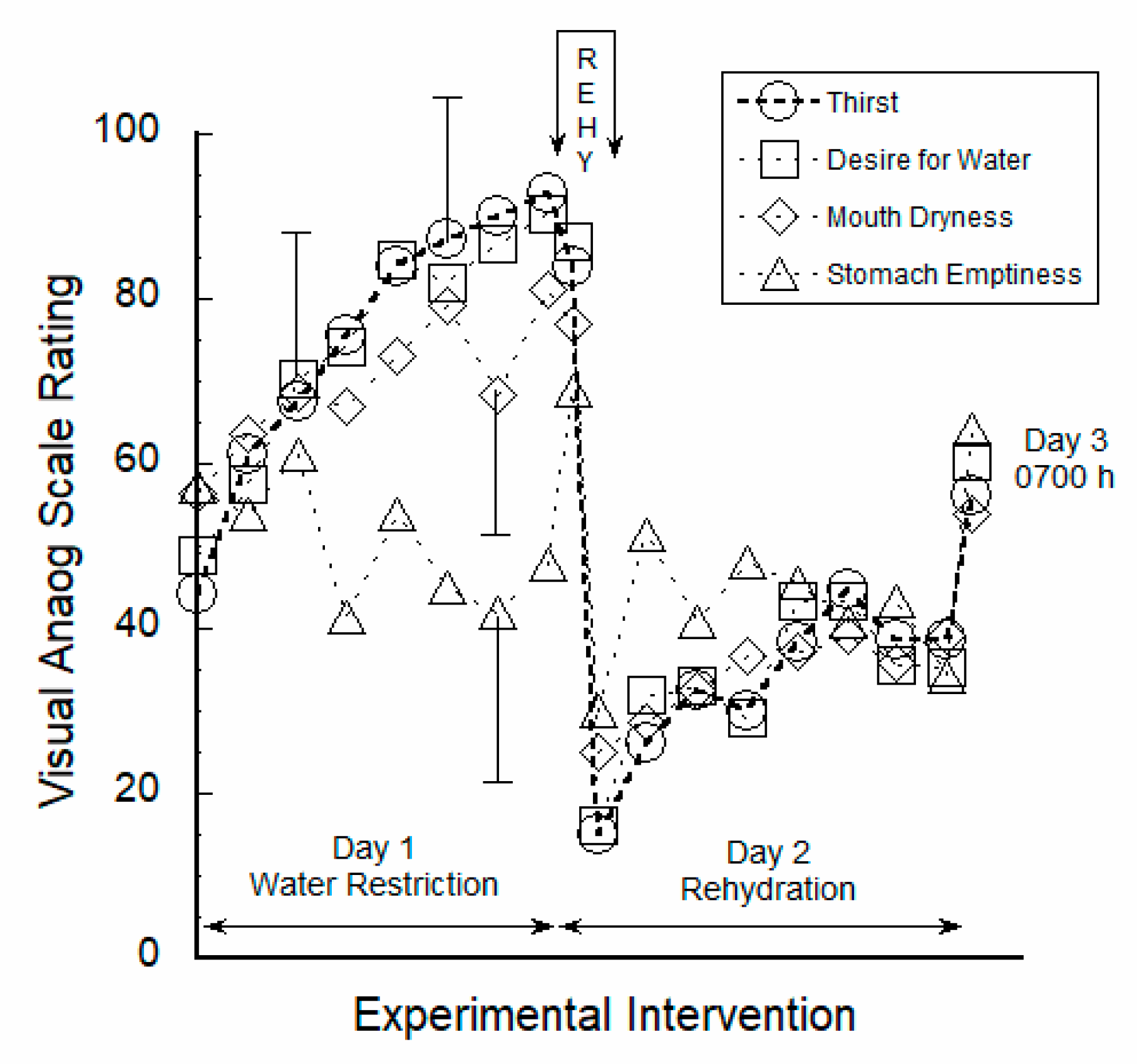
3.1. Perceptual Ratings Were Strongly Correlated to Thirst
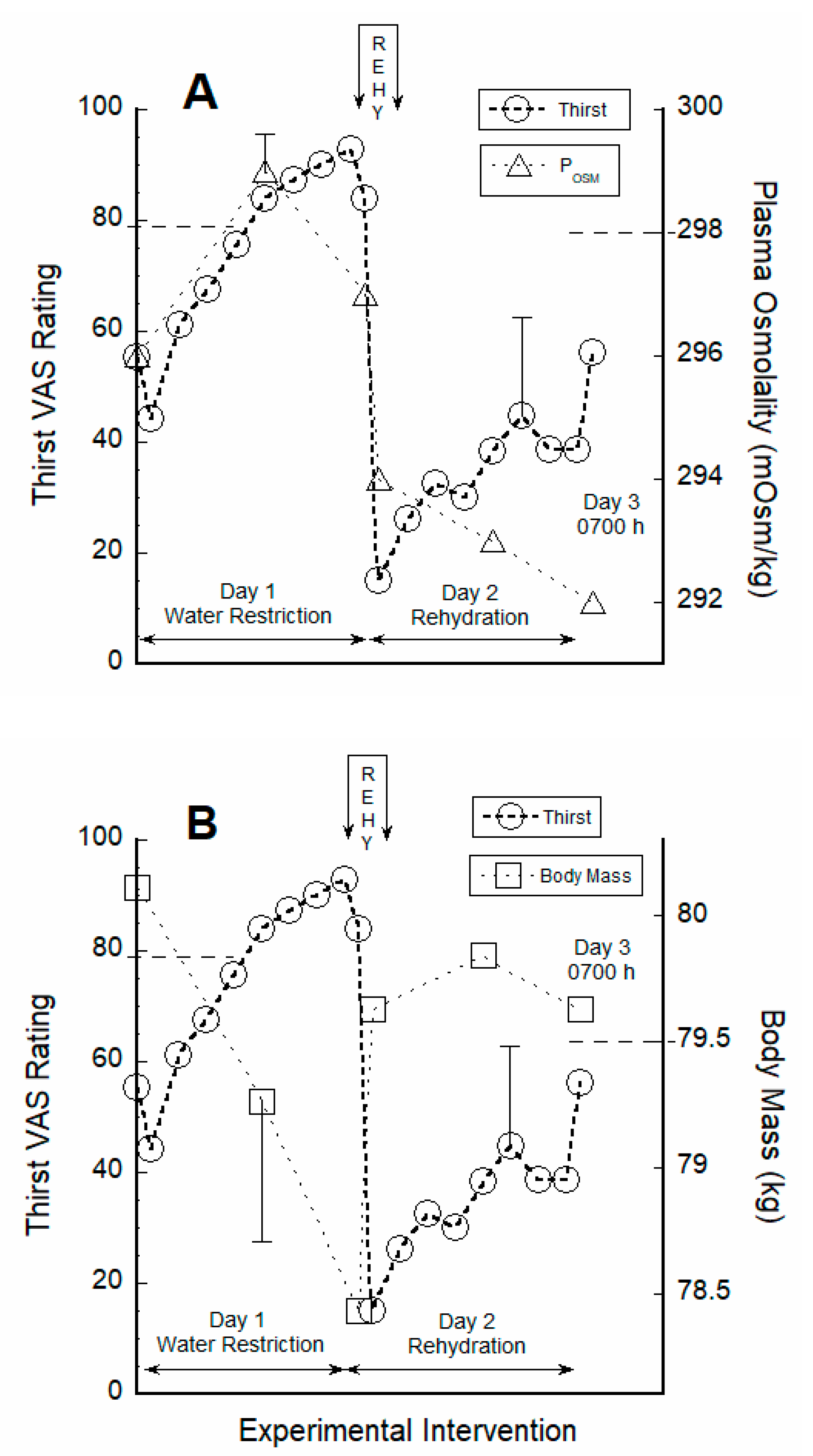
3.2. Thirst Correlated with Hydration Indices during Water Restriction
3.3. VAS Ratings Responded to 30-Min Fluid Intake
3.4. Prior Hydration Status and VAS Ratings Did Not Predict REHY Volume
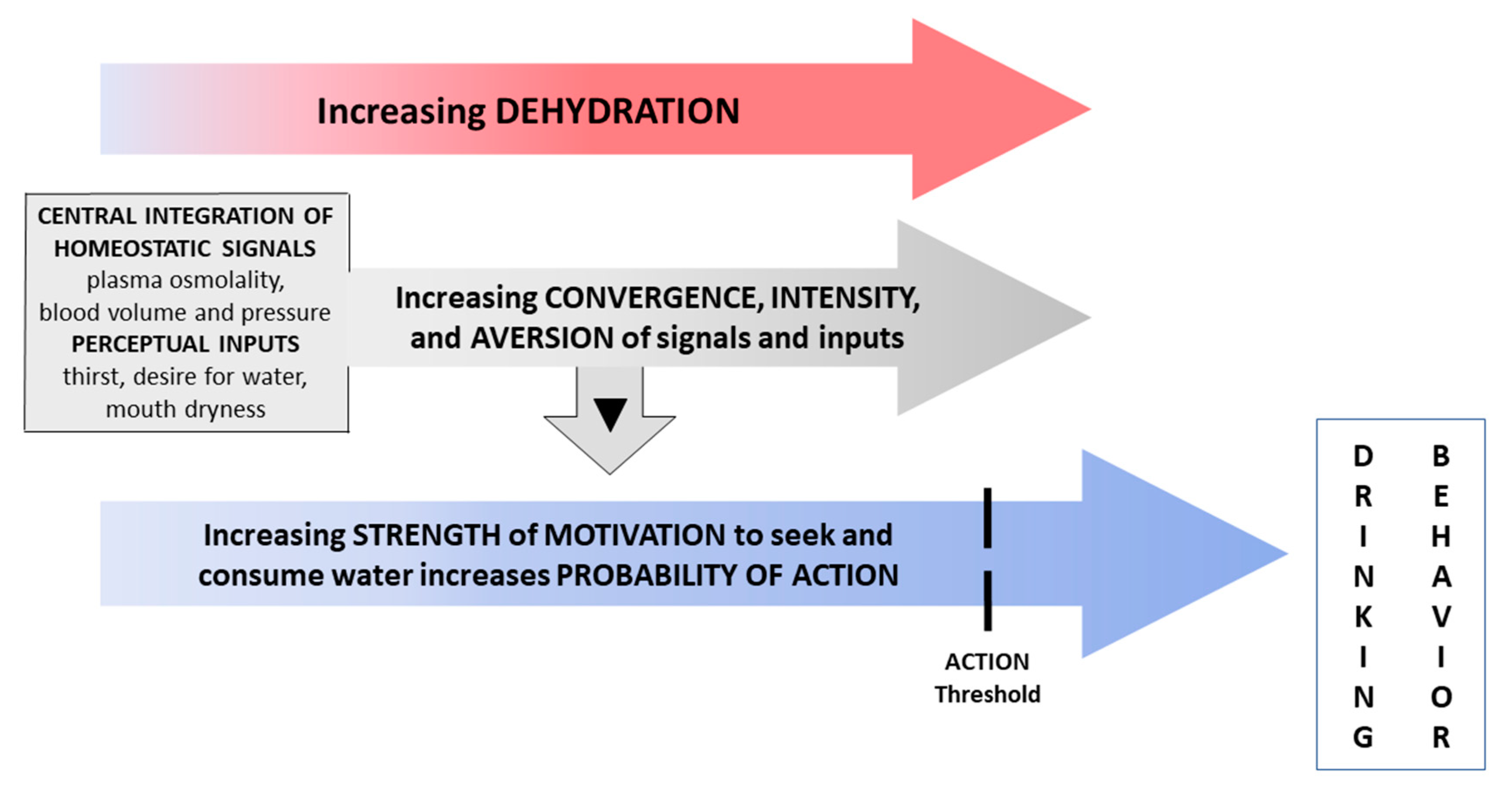
4. Discussion
4.1. Responses to Water Restriction
4.2. Acute 30-Min Rehydration (REHY)
4.3. Re-Emergence of Body Water Homeostasis and Mild Thirst
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Augustine, V.; Gokce, S.K.; Lee, S.; Wang, B.; Davidson, T.J.; Reimann, F.; Gribble, F.M.; Deisseroth, K.; Lois, C.; Oka, Y. Hierarchical neural architecture underlying thirst regulation. Nature 2018, 555, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.N. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 2010, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.A.; Huey, E.L.; Ahn, J.S.; Beutler, L.R.; Tan, C.L.; Kosar, S.; Bai, L.; Chen, Y.; Corpuz, T.V.; Madisen, L.; et al. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 2019, 568, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.W. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Geelen, G.; Keil, L.C.; Kravik, S.E.; Wade, C.E.; Thrasher, T.N.; Barnes, P.R.; Pyka, G.; Nesvig, C.; Greenleaf, J.E. Inhibition of plasma vasopressin after drinking in dehydrated humans. Am. J. Physiol. Integr. Comp. Physiol. 1984, 247, R968–R971. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Bowala, T.K.; Gavrilescu, M.; Phillips, P.A.; McKinley, M.J.; McAllen, R.; Denton, D.A.; Egan, G.F. Cortical activation and lamina terminalis functional connectivity during thirst and drinking in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R623–R631. [Google Scholar] [CrossRef]
- Hollis, J.H.; McKinley, M.J.; D’Souza, M.; Kampe, J.; Oldfield, B.J. The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: A neuroanatomical framework for the generation of thirst. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1390–R1401. [Google Scholar] [CrossRef]
- Zimmerman, C.A.; Lin, Y.-C.; Leib, D.E.; Guo, L.; Huey, E.L.; Daly, G.E.; Chen, Y.; Knight, Z.A. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 2016, 537, 680–684. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Johnson, E.C. Water Intake, Water Balance, and the Elusive Daily Water Requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. Angiotensin, thirst, and sodium appetite. Physiol. Rev. 1998, 78, 583–686. [Google Scholar] [CrossRef]
- Saker, P.; Farrell, M.J.; Egan, G.F.; McKinley, M.J.; Denton, D.A. Overdrinking, swallowing inhibition, and regional brain responses prior to swallowing. Proc. Natl. Acad. Sci. USA 2016, 113, 12274–12279. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Kavouras, S.A. Thirst and Drinking Paradigms: Evolution from Single Factor Effects to Brainwide Dynamic Networks. Nutrients 2019, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Figaro, M.K.; Mack, G.W. Regulation of fluid intake in dehydrated humans: Role of oropharyngeal stimulation. Am. J. Physiol. Content 1997, 272, R1740–R1746. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Rolls, E.T. The control of normal drinking. In Thirst; Cambridge University Press: Cambridge, UK, 1982; pp. 152–166. [Google Scholar]
- Rolls, B.J.; Wood, R.J.; Rolls, E.T.; Lind, H.; Lind, W.; Ledingham, J.G. Thirst following water deprivation in humans. Am. J. Physiol. Integr. Comp. Physiol. 1980, 239, R476–R482. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.A.; Rolls, B.J.; Ledingham, J.G.; Morton, J.J. Body fluid changes, thirst and drinking in man during free access to water. Physiol. Behav. 1984, 33, 357–363. [Google Scholar] [CrossRef]
- Stricker, E.M.; Sved, A.F. Thirst. Nutrition 2000, 16, 821–826. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for water. EFSA J. 2010, 8, 1459–1507. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; Institute of Medicine: Washington, DC, USA, 2004. [Google Scholar]
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, U.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef]
- Denton, D.A.; McKinley, M.J.; Weisinger, R.S. Hypothalamic integration of body fluid regulation. Proc. Natl. Acad. Sci. USA 1996, 93, 7397–7404. [Google Scholar] [CrossRef]
- Spruce, B.A.; Baylis, P.H.; Burd, J.; Watson, M.J. Variation in Osmoregulation of Arginine Vasopressin during the Human Menstrual Cycle. Clin. Endocrinol. 1985, 22, 37–42. [Google Scholar] [CrossRef]
- Zerbe, R.L.; Robertson, G.L. Osmoregulation of thirst and vasopressin secretion in human subjects: Effect of various solutes. Am. J. Physiol. Metab. 1983, 244, E607–E614. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.G.; Blass, E.M. Orogastric determinants of drinking in rats: Interaction between absorptive and peripheral controls. J. Comp. Physiol. Psychol. 1977, 91, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Thrasher, T.N.; Nistal-Herrera, J.F.; Keil, L.C.; Ramsay, D.J. Satiety and inhibition of vasopressin secretion after drinking in dehydrated dogs. Am. J. Physiol. Metab. 1981, 240, E394–E401. [Google Scholar] [CrossRef]
- Wood, R.J.; Maddison, S.; Rolls, E.T.; Rolls, B.J.; Gibbs, J.; Al, E. Drinking in rhesus monkeys: Roles of presystemic and systemic factors in control of drinking. J. Comp. Physiol. Psychol. 1980, 94, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.E.; Chen, M.Z.; Pichamoorthy, N.; Tien, R.H.; Pachitariu, M.; Luo, L.; Deisseroth, K. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 2019, 364, 253. [Google Scholar] [CrossRef]
- Robinson, B.W.; Mishkin, M. Alimentary responses to forebrain stimulation in monkeys. Exp. Brain Res. 1968, 4, 330–366. [Google Scholar] [CrossRef]
- Watson, P.E.; Watson, I.D.; Batt, R.D. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am. J. Clin. Nutr. 1980, 33, 27–39. [Google Scholar] [CrossRef]
| Dietary Components | 24-h Water Restriction (Day 1) | 24-h Ad libitum Rehydration (Day 2) |
|---|---|---|
| Food energy content (Kcal) | 2,219 ± 897 | 2377 ± 989 |
| Sodium (mg) | 4377 ± 2446 | 3755 ± 2090 |
| Carbohydrate (%) a | 42 ± 12 | 46 ± 10 |
| Fat (%) a | 39 ± 10 | 33 ± 7 |
| Protein (%) a | 20 ± 5 | 21 ± 9 |
| Food moisture + water + beverages (L) b | 0.31 ± 0.24 | 1.13 ± 0.52 c |
| REHY water (L) d | 0 ± 0 | 1.46 ± 0.47 |
| Total daily water intake (L) | 0.31 ± 0.24 | 2.60 ± 0.66 c |
| Measured Variables | 24-h Water Restriction (Day 1 Morning to Day 2 Morning) | 24-h Ad libitum Rehydration (Day 2 Morning to Day 3 Morning) |
|---|---|---|
| Body Mass change (kg/24 h) | −1.67 ± 0.35 | +1.19 ± 0.36 a |
| Body Mass change (%/24 h) | −2.12 ± 0.50 | +1.56 ± 0.60 a |
| Plasma Osmolality (mOsm/kg) b | 299 ± 5 | 294 ± 4 a |
| Urine specific gravity b | 1.030 ± 0.003 | 1.021 ± 0.008 a |
| Urine specific gravity c | 1.025 ± 0.005 | 1.017 ± 0.008 a |
| Urine Osmolality (mOsm/kg) c | 893 ± 178 | 613 ± 279 a |
| Urine Volume (L/24 h) c | 0.810 ± 0.275 | 1.770 ± 1.136 a |
| Measurements | Baseline (Day 1, 0700 h) | Thirst Awareness a (Day 1) |
|---|---|---|
| Body mass (kg) | 80.11 ± 10.58 | 79.47 ± 10.55 |
| Plasma osmolality (mOsm/kg) b | 297 ± 4 | 298 ± 4 |
| Urine specific gravity b | 1.020 ± 0.007 | 1.024 ± 0.004 c |
| Thirst d | 55 ± 14 | 79 ± 12 c |
| Desire for water d | 55 ± 18 | 79 ± 14 c |
| Mouth dryness d | 58 ± 15 | 71 ± 22 c |
| Stomach emptiness d | 57 ± 15 | 48 ± 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armstrong, L.E.; Giersch, G.E.W.; Dunn, L.; Fiol, A.; Muñoz, C.X.; Lee, E.C. Inputs to Thirst and Drinking during Water Restriction and Rehydration. Nutrients 2020, 12, 2554. https://doi.org/10.3390/nu12092554
Armstrong LE, Giersch GEW, Dunn L, Fiol A, Muñoz CX, Lee EC. Inputs to Thirst and Drinking during Water Restriction and Rehydration. Nutrients. 2020; 12(9):2554. https://doi.org/10.3390/nu12092554
Chicago/Turabian StyleArmstrong, Lawrence E., Gabrielle E. W. Giersch, Leslie Dunn, Aidan Fiol, Colleen X. Muñoz, and Elaine C. Lee. 2020. "Inputs to Thirst and Drinking during Water Restriction and Rehydration" Nutrients 12, no. 9: 2554. https://doi.org/10.3390/nu12092554
APA StyleArmstrong, L. E., Giersch, G. E. W., Dunn, L., Fiol, A., Muñoz, C. X., & Lee, E. C. (2020). Inputs to Thirst and Drinking during Water Restriction and Rehydration. Nutrients, 12(9), 2554. https://doi.org/10.3390/nu12092554





