Protein Intake, Fatigue and Quality of Life in Stable Outpatient Kidney Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Protein Intake
2.3. Assessment of Fatigue
2.4. Assessment of Quality of Life
2.5. Assessment of Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Total Population and Across Categories of Fatigue
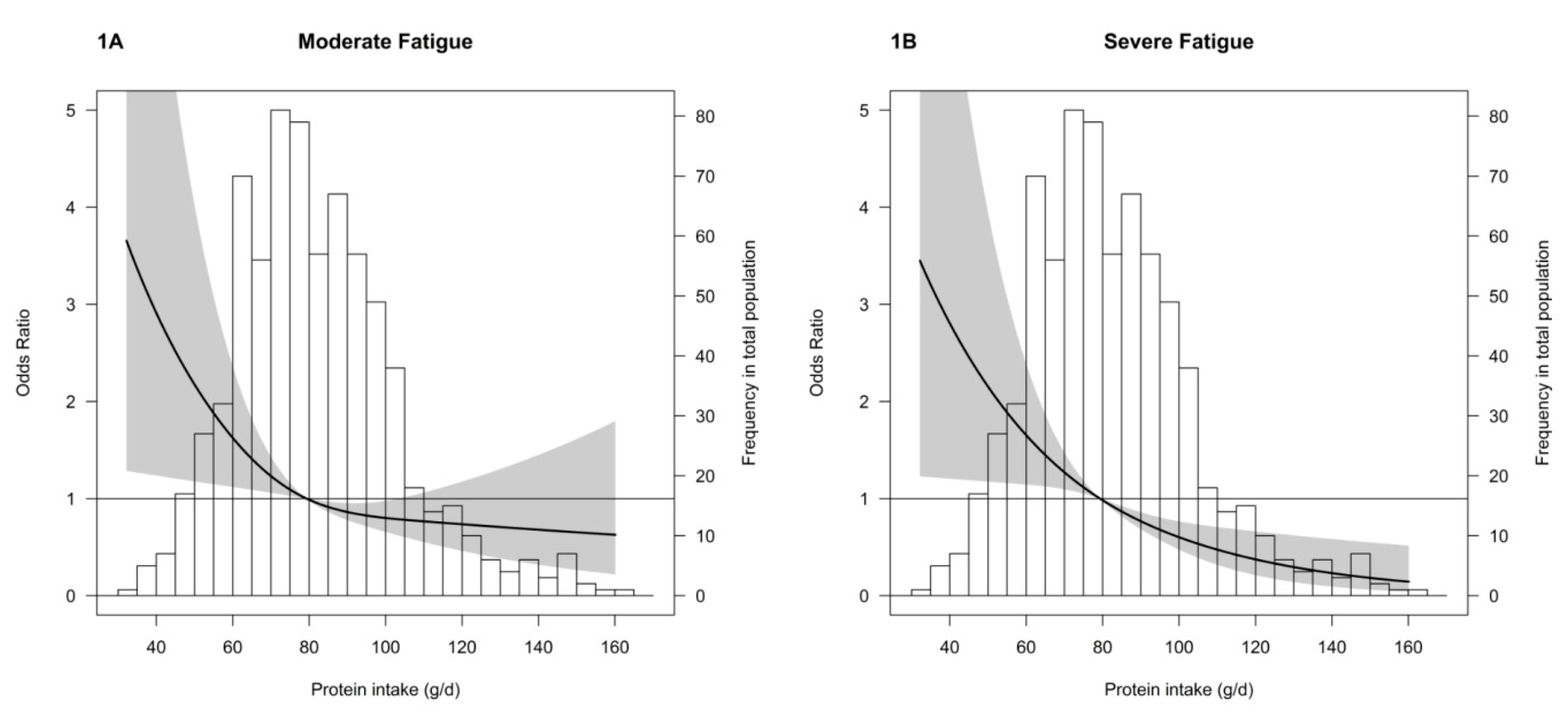
3.2. Fatigue
3.3. Quality of Life
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Jofré, R.; López-Gómez, J.M.; Moreno, F.; Sanz-Guajardo, D.; Valderrábano, F. Changes in quality of life after renal transplantation. Am. J. Kidney Dis. 1998, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kostro, J.Z.; Hellmann, A.; Kobiela, J.; Skóra, I.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Śledziński, Z. Quality of Life After Kidney Transplantation: A Prospective Study. Transplant. Proc. 2016, 48, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Van Sandwijk, M.S.; Al Arashi, D.; van de Hare, F.M.; van der Torren, J.M.R.; Kersten, M.J.; Bijlsma, J.A.; ten Berge, I.J.M.; Bemelman, F.J. Fatigue, anxiety, depression and quality of life in kidney transplant recipients, haemodialysis patients, patients with a haematological malignancy and healthy controls. Nephrol. Dial. Transplant. 2019, 34, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Goedendorp, M.M.; Hoitsma, A.J.; Bloot, L.; Bleijenberg, G.; Knoop, H. Severe fatigue after kidney transplantation: A highly prevalent, disabling and multifactorial symptom. Transpl. Int. 2013, 26, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Pepe, G.; Vulpio, C. Fatigue in kidney transplant recipients. Clin. Transplant. 2016, 30, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Bosch, J.A.; Jones, D.; Kaur, O.; Inston, N.; Moore, S.; McClean, A.; McTernan, P.G.; Harper, L.; Phillips, A.C.; et al. Predictors and consequences of fatigue in prevalent kidney transplant recipients. Transplantation 2013, 96, 987–994. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Carter, S.M.; Hall, B.; Harris, D.C.; Walker, R.G.; Hawley, C.M.; Chadban, S.; Craig, J.C. Patients’ priorities for health research: Focus group study of patients with chronic kidney disease. Nephrol. Dial. Transplant. 2008, 23, 3206–3214. [Google Scholar] [CrossRef]
- Artom, M.; Moss-Morris, R.; Caskey, F.; Chilcot, J. Fatigue in advanced kidney disease. Kidney Int. 2014, 86, 497–505. [Google Scholar] [CrossRef]
- Rodrigue, J.R.; Mandelbrot, D.A.; Hanto, D.W.; Johnson, S.R.; Karp, S.J.; Pavlakis, M. A cross-sectional study of fatigue and sleep quality before and after kidney transplantation. Clin. Transplant. 2011, 25, E13–E21. [Google Scholar] [CrossRef]
- Kovacs, A.Z.; Molnar, M.Z.; Szeifert, L.; Ambrus, C.; Molnar-Varga, M.; Szentkiralyi, A.; Mucsi, I.; Novak, M. Sleep disorders, depressive symptoms and health-related quality of life--a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol. Dial. Transplant. 2011, 26, 1058–1065. [Google Scholar] [CrossRef]
- Ujszaszi, A.; Czira, M.E.; Fornadi, K.; Novak, M.; Mucsi, I.; Molnar, M.Z. Quality of life and protein-energy wasting in kidney transplant recipients. Int. Urol. Nephrol. 2012, 44, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Jones, D.; Bosch, J.A.; McPhee, J.; Crabtree, N.; McTernan, P.G.; Kaur, O.; Inston, N.; Moore, S.; McClean, A.; et al. Cardiovascular, muscular and perceptual contributions to physical fatigue in prevalent kidney transplant recipients. Transpl. Int. 2016, 29, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Deetman, P.E.; Said, M.Y.; Kromhout, D.; Dullaart, R.P.F.; Kootstra-Ros, J.E.; Sanders, J.S.F.; Seelen, M.A.J.; Gans, R.O.B.; Navis, G.; Joosten, M.M.; et al. Urinary Urea Excretion and Long-term Outcome After Renal Transplantation. Transplantation 2015, 99, 1009–1015. [Google Scholar] [CrossRef]
- Said, M.Y.; Deetman, P.E.; de Vries, A.P.J.; Zelle, D.M.; Gans, R.O.B.; Navis, G.; Joosten, M.M.; Bakker, S.J.L. Causal path analyses of the association of protein intake with risk of mortality and graft failure in renal transplant recipients. Clin. Transplant. 2015, 29, 447–457. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and design of TransplantLines: A prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018, 8, e024502. [Google Scholar] [CrossRef]
- Maroni, B.; Steinman, T.; Mitch, W. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef]
- Weijs, P.J.; Sauerwein, H.P.; Kondrup, J. Protein Recommendations in the ICU: G Protein/Kg Body Weight—Which Body Weight for Underweight and Obese Patients? Clin. Nutr. 2012, 31, 774–775. [Google Scholar] [CrossRef]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef]
- Bültmann, U.; de Vries, M.; Beurskens, A.J.; Bleijenberg, G.; Vercoulen, J.H.; Kant, I. Measurement of prolonged fatigue in the working population: Determination of a cutoff point for the checklist individual strength. J. Occup. Health Psychol. 2000, 5, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Worm-Smeitink, M.; Gielissen, M.; Bloot, L.; van Laarhoven, H.W.M.; van Engelen, B.G.M.; van Riel, P.; Bleijenberg, G.; Nikolaus, S.; Knoop, H. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J. Psychosom. Res. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J.; Looijmans, M.; Prins, J.B.; Janssen, D.J.A.; Thong, M.S.Y.; Peters, J.B.; Burtin, C.; Meertens-Kerris, Y.; Coors, A.; Muris, J.W.M.; et al. Fatigue in patients with chronic obstructive pulmonary disease: Protocol of the Dutch multicentre, longitudinal, observational FAntasTIGUE study. BMJ Open 2018, 8, e021745. [Google Scholar] [CrossRef]
- Vercoulen, J.H.M.M.; Hommes, O.R.; Swanink, C.M.; Jongen, P.J.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. The Measurement of Fatigue in Patients With Multiple Sclerosis. Arch. Neurol. 1996, 53, 642–649. [Google Scholar] [CrossRef]
- Van Herck, M.; Spruit, M.; Burtin, C.; Djamin, R.; Antons, J.; Goërtz, Y.; Ebadi, Z.; Janssen, D.; Vercoulen, J.; Peters, J.; et al. Fatigue is Highly Prevalent in Patients with Asthma and Contributes to the Burden of Disease. J. Clin. Med. 2018, 7, 471. [Google Scholar] [CrossRef]
- Hays, R.D.; Morales, L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001, 33, 350–357. [Google Scholar] [CrossRef]
- Sealy, M.J.; Haß, U.; Ottery, F.D.; van der Schans, C.P.; Roodenburg, J.L.N.; Jager-Wittenaar, H. Translation and Cultural Adaptation of the Scored Patient-Generated Subjective Global Assessment. Cancer Nurs. 2018, 41, 450–462. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Trabal, J.; Leyes, P.; Forga, M.T.; Hervás, S. Quality of life, dietary intake and nutritional status assessment in hospital admitted cancer patients. Nutr. Hosp. 2006, 21, 505–510. [Google Scholar] [PubMed]
- Garonzik-Wang, J.M.; Govindan, P.; Grinnan, J.W.; Liu, M.; Ali, H.M.; Chakraborty, A.; Jain, V.; Ros, R.L.; James, N.T.; Kucirka, L.M.; et al. Frailty and delayed graft function in kidney transplant recipients. Arch. Surg. 2012, 147, 190–193. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Law, A.; King, E.; Orandi, B.; Salter, M.; Gupta, N.; Chow, E.; Alachkar, N.; Desai, N.; Varadhan, R.; et al. Frailty and Mortality in Kidney Transplant Recipients. Am. J. Transplant. 2015, 15, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Tsikas, D.; Bakker, S.J.L. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Keszei, A.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Haromszeki, B.; Kosa, J.P.; Lakatos, P.; Sarvary, E.; Beko, G.; et al. Evaluation of the Malnutrition-Inflammation Score in Kidney Transplant Recipients. Am. J. Kidney Dis. 2010, 56, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Oterdoom, L.H.; De Vries, A.P.J.; Gansevoort, R.T.; Van Son, W.J.; Van Der Heide, J.J.H.; Ploeg, R.J.; De Jong, P.E.; Gans, R.O.B.; Bakker, S.J.L. Determinants of insulin resistance in renal transplant recipients. Transplantation 2007, 83, 29–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siew, E.D.; Ikizler, T.A. Determinants of insulin resistance and its effects on protein metabolism in patients with advanced chronic kidney disease. Contrib. Nephrol. 2008, 161, 138–144. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Lindner, A.; Fornadi, K.; Kiss, I.; Remport, A.; Novak, M.; Kennedy, S.H.; et al. Association of the Malnutrition-Inflammation Score With Clinical Outcomes in Kidney Transplant Recipients. Am. J. Kidney Dis. 2011, 58, 101–108. [Google Scholar] [CrossRef]
- Boslooper-Meulenbelt, K.; Patijn, O.; Battjes-Fries, M.C.E.; Haisma, H.; Pot, G.K.; Navis, G.J. Barriers and facilitators of fruit and vegetable consumption in renal transplant recipients, family members and healthcare professionals—A focus group study. Nutrients 2019, 11, 2427. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Herpich, C.; Norman, K. Anti-inflammatory diets and fatigue. Nutrients 2019, 11, 2315. [Google Scholar] [CrossRef] [PubMed]
- Masud, T.; Manatunga, A.; Cotsonis, G.; Mitch, W.E. The precision of estimating protein intake of patients with chronic renal failure. Kidney Int. 2002, 62, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E. Dietary protein restriction in patients with chronic renal failure. Kidney Int. 1991, 40, 326–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, T.; Kato, H.; Suzuki, H.; Mizugaki, A.; Ezaki, T.; Ogita, F. Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase. Nutrients 2018, 10, 1809. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Kopple, J.D. National Kidney Foundation K/DOQI Work Group. The National Kidney Foundation K/DOQI clinical practice guidelines for dietary protein intake for chronic dialysis patients. Am. J. Kidney Dis. 2001, 38, S68–S73. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Shinaberger, C.S.; Kilpatrick, R.D.; Regidor, D.L.; McAllister, C.J.; Greenland, S.; Kopple, J.D.; Kalantar-Zadeh, K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 37–49. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Pedrollo, E.F.; Nicoletto, B.B.; Carpes, L.S.; de Freitas, J.D.M.C.; Buboltz, J.R.; Forte, C.C.; Bauer, A.C.; Manfro, R.C.; Souza, G.C.; Leitão, C.B. Effect of an intensive nutrition intervention of a high protein and low glycemic-index diet on weight of kidney transplant recipients: Study protocol for a randomized clinical trial. Trials 2017, 18, 413. [Google Scholar] [CrossRef] [PubMed][Green Version]
| CIS | |||||
|---|---|---|---|---|---|
| Total Population | <20 | 20–35 | ≥35 | p-Value | |
| Number of Subjects | 730 (100) | 231 (31) | 254 (35) | 245 (34) | - |
| 24-h Urea excretion, mmol | 377 ± 114 | 403 ± 119 | 373 ± 118 | 356 ± 101 | <0.001 |
| Daily protein intake, g/day | 82.2 ± 21.3 | 86.4 ± 14.7 | 81.4 ± 21.9 | 78.9 ± 19.3 | <0.001 |
| Quality of Life | |||||
| PCS | 45.0 ± 10.0 | 52.0 ± 5.3 | 45.6± 8.6 | 37.6 ± 9.9 | <0.001 |
| MCS | 50.6 ± 8.6 | 55.0 ± 4.9 | 50.9 ± 7.6 | 46.0 ± 10.2 | <0.001 |
| Demographics | |||||
| Sex, male | 423 (57) | 147 (64) | 142 (56) | 134 (55) | 0.10 |
| Age, years | 58 [48–65] | 58 [46–65] | 59 [48–66] | 57 [49–65] | 0.53 |
| Education | 0.30 | ||||
| Low | 265 (36) | 73 (32) | 104 (41) | 88 (36) | |
| Intermediate | 239 (33) | 83 (36) | 72 (28) | 84 (34) | |
| High | 175 (24) | 61 (26) | 61 (24) | 53 (22) | |
| Unknown/PNA | 51 (7) | 14 (6) | 17 (7) | 20 (8) | |
| Employment | <0.001 | ||||
| Paid employment | 274 (38) | 115 (50) | 91 (36) | 68 (28) | |
| Medically unfit for work | 143 (20) | 18 (8) | 47 (18) | 78 (32) | |
| Unemployed | 74 (10) | 21 (9) | 32 (13) | 21 (8) | |
| Retired | 186 (25) | 61 (26) | 67 (26) | 58 (24) | |
| Unknown | 53 (7) | 16 (7) | 17 (7) | 20 (8) | |
| Kidney Transplant Characteristics | |||||
| Primary kidney disease | 0.29 | ||||
| Glomerulonephritis | 166 (23) | 48 (21) | 55 (22) | 63 (25) | |
| Interstitial Nephritis | 67 (9) | 18 (8) | 25 (10) | 24 (10) | |
| Cystic Kidney Disease | 147 (20) | 55 (24) | 48 (19) | 44 (18) | |
| Other congenital and hereditary kidney disease | 44 (6) | 14 (6) | 12 (5) | 18 (7) | |
| Renal vascular disease | 93 (13) | 35 (15) | 37 (14) | 21 (9) | |
| Diabetes Mellitus | 44 (6) | 9 (4) | 14 (5) | 21 (9) | |
| Other multisystem diseases | 31 (4) | 8 (3) | 11 (4) | 12 (5) | |
| Other | 17 (2) | 6 (2) | 4 (2) | 7 (3) | |
| Unknown | 121 (17) | 38 (17) | 48 (19) | 35 (14) | |
| Time since Tx, years | 4.0 [1.0–11.0] | 2.0 [1.0–7.9] | 4.0 [1.0–10.8] | 6.4 [1.3–13.0] | <0.001 |
| History of combined organ Tx | 20 (3) | 1 (0.4) | 8 (3) | 11 (5) | 0.02 |
| eGFR, mL/min × 1.73 m2 | 51.2 ± 17.9 | 53.1 ± 17.7 | 51.9 ± 17.0 | 48.6 ± 18.8 | 0.01 |
| Proteinuria | 110 (15) | 34 (15) | 27 (11) | 49 (20) | 0.01 |
| Pre-emptive Tx | 283 (39) | 110 (48) | 98 (39) | 75 (31) | 0.001 |
| Donor sex, male | 382 (52) | 114 (50) | 130 (52) | 138 (59) | 0.19 |
| Donor age, years | 52 [43–60] | 54 [45–62] | 52 [43–60] | 49 [37–58] | 0.003 |
| Living donor | 412 (56) | 143 (62) | 150 (59) | 119 (49) | 0.008 |
| Body Composition and Nutritional Status | |||||
| Weight, kg | 81.8 ± 16.3 | 80.2 ± 14.7 | 81.4 ± 15.0 | 83.7 ± 18.6 | 0.06 |
| Height, cm | 173 ± 10 | 173 ± 11 | 172 ± 9 | 173 ± 10 | 0.41 |
| BMI, kg/m2 | 27.3 ± 4.7 | 26.6 ± 4.3 | 27.4 ± 4.5 | 27.9 ± 5.3 | 0.01 |
| 24-h CER, mmol | 12.2 ± 3.8 | 12.9 ± 3.9 | 12.1 ± 3.7 | 11.7 ± 3.7 | 0.002 |
| PGSGA stage B or C | 47 (8) | 4 (2) | 11 (6) | 32 (17) | <0.001 |
| Cardio-Metabolic Parameters | |||||
| SBP, mm Hg | 135 ± 16 | 135 ± 15 | 134 ± 16 | 134 ± 16 | 0.64 |
| DBP, mm Hg | 79 ± 10 | 79 ± 10 | 78 ± 10 | 78 ± 11 | 0.42 |
| Use of antihypertensive drugs | 582 (80) | 180 (78) | 201 (79) | 201 (82) | 0.51 |
| Total cholesterol, mmol/L | 4.7 ± 1.0 | 4.7 ± 1.0 | 4.6 ± 0.9 | 4.8 ± 1.1 | 0.07 |
| LDL-cholesterol, mmol/L | 2.9 ± 0.9 | 2.9 ± 0.8 | 2.8 ± 0.8 | 2.9 ± 1.0 | 0.15 |
| Statin use | 425 (58) | 134 (58) | 157 (62) | 134 (55) | 0.27 |
| Diabetes | 208 (29) | 48 (21) | 70 (34) | 90 (37) | 0.001 |
| HbA1c, mmol/mol | 5.8 [5.4–6.3] | 5.7 [5.4–6.1] | 5.8 [5.4–6.3] | 5.9 [5.6–6.5] | 0.003 |
| Inflammatory and Hematological Parameters | |||||
| Albumin, g/L | 43.5 ± 3.0 | 44.2 ± 2.7 | 43.6 ± 2.8 | 42.9 ± 3.4 | <0.001 |
| CRP, mg/L | 2.0 [0.8–4.9] | 1.6 [0.7–3.7] | 2.4 [0.8–5.0] | 2.5 [0.8–6.0] | 0.02 |
| Hemoglobin, mmol/L | 8.3 ± 1.1 | 8.4 ± 1.2 | 8.4 ± 1.0 | 8.1 ± 1.1 | 0.008 |
| Iron, µg/dL | 13.8 ± 5.6 | 14.3 ± 5.1 | 13.6 ± 5.5 | 13.6 ± 6.0 | 0.34 |
| Ferritin, µg/L | 89 [41–189] | 90 [41–193] | 88 [39–198] | 89 [44–178] | 0.9 |
| Vitamin B12, pmol/L | 289 [219–391] | 288 [226–375] | 291 [221–391] | 288 [214–411] | 0.84 |
| Immunosuppressive Drugs | |||||
| Tacrolimus | 483 (66) | 160 (69) | 168 (66) | 155 (63) | 0.39 |
| Cyclosporin | 109 (15) | 29 (13) | 30 (12) | 50 (20) | 0.01 |
| Mycophenolic acid | 545 (75) | 181 (78) | 201 (79) | 163 (67) | 0.002 |
| Azathioprine | 78 (11) | 21 (9) | 28 (11) | 29 (12) | 0.61 |
| Prednisolone | 711 (97) | 225 (97) | 247 (97) | 293 (98) | 0.9 |
| Lifestyle Parameters | |||||
| Smoking status | 0.36 | ||||
| Yes | 83 (11) | 23 (10) | 25 (10) | 35 (14) | |
| No | 617 (85) | 198 (86) | 221 (87) | 198 (81) | |
| Unknown | 30 (4) | 10 (4) | 8 (3) | 12 (5) | |
| Alcohol use | 0.37 | ||||
| Yes | 364 (50) | 124 (54) | 130 (52) | 110 (45) | |
| No | 229 (31) | 68 (29) | 75 (39) | 86 (35) | |
| Unknown | 137 (19) | 39 (17) | 49 (19) | 49 (20) | |
| No-Mild Fatigue | Moderate Fatigue | Severe Fatigue | ||||
|---|---|---|---|---|---|---|
| CIS | <20 | 20–34 | ≥35 | |||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Crude | Reference | (-) | 0.89 (0.83–0.98) | 0.01 | 0.85 (0.78–0.92) | <0.001 |
| Model 1 | Reference | (-) | 0.87 (0.79–0.96) | 0.005 | 0.79 (0.72–0.88) | <0.001 |
| Model 2 | Reference | (-) | 0.86 (0.78–0.95) | 0.004 | 0.78 (0.70–0.87) | <0.001 |
| Model 3 | Reference | (-) | 0.87 (0.79–0.96) | 0.006 | 0.80 (0.72–0.90) | <0.001 |
| Model 4 | Reference | (-) | 0.88 (0.79–0.97) | 0.01 | 0.80 (0.72–0.90) | <0.001 |
| Model 5 | Reference | (-) | 0.90 (0.80–1.00) | 0.06 | 0.80 (0.70–0.90) | <0.001 |
| Model 6 | Reference | (-) | 0.89 (0.80–1.00) | 0.06 | 0.80 (0.71–0.91) | <0.001 |
| PCS | MCS | |||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Crude | 0.74 (0.39–1.09) | <0.001 | 0.36 (0.06–0.66) | 0.02 |
| Model 1 | 0.97 (0.60–1.34) | <0.001 | 0.25 (−0.09–0.59) | 0.15 |
| Model 2 | 0.97 (0.60–1.34) | <0.001 | 0.30 (−0.04–0.64) | 0.09 |
| Model 3 | 0.83 (0.46–1.21) | <0.001 | 0.25 (−0.10–0.59) | 0.16 |
| Model 4 | 0.74 (0.36–1.12) | <0.001 | 0.23 (−0.12–0.58) | 0.20 |
| Model 5 | 0.65 (0.24–1.06) | 0.002 | 0.26 (−0.12–0.64) | 0.19 |
| Model 6 | 0.64 (0.23–1.05) | 0.002 | 0.26 (−0.12–0.65) | 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, A.W.G.; Boslooper-Meulenbelt, K.; Geelink, M.; van Vliet, I.M.Y.; Post, A.; Joustra, M.L.; Knoop, H.; Berger, S.P.; Navis, G.J.; Bakker, S.J.L. Protein Intake, Fatigue and Quality of Life in Stable Outpatient Kidney Transplant Recipients. Nutrients 2020, 12, 2451. https://doi.org/10.3390/nu12082451
Neto AWG, Boslooper-Meulenbelt K, Geelink M, van Vliet IMY, Post A, Joustra ML, Knoop H, Berger SP, Navis GJ, Bakker SJL. Protein Intake, Fatigue and Quality of Life in Stable Outpatient Kidney Transplant Recipients. Nutrients. 2020; 12(8):2451. https://doi.org/10.3390/nu12082451
Chicago/Turabian StyleNeto, Antonio W. Gomes, Karin Boslooper-Meulenbelt, Marit Geelink, Iris M. Y. van Vliet, Adrian Post, Monica L. Joustra, Hans Knoop, Stefan P. Berger, Gerjan J. Navis, and Stephan J. L. Bakker. 2020. "Protein Intake, Fatigue and Quality of Life in Stable Outpatient Kidney Transplant Recipients" Nutrients 12, no. 8: 2451. https://doi.org/10.3390/nu12082451
APA StyleNeto, A. W. G., Boslooper-Meulenbelt, K., Geelink, M., van Vliet, I. M. Y., Post, A., Joustra, M. L., Knoop, H., Berger, S. P., Navis, G. J., & Bakker, S. J. L. (2020). Protein Intake, Fatigue and Quality of Life in Stable Outpatient Kidney Transplant Recipients. Nutrients, 12(8), 2451. https://doi.org/10.3390/nu12082451







