Preliminary Investigation to Review If a Glycomacropeptide Compared to L-Amino Acid Protein Substitute Alters the Pre- and Postprandial Amino Acid Profile in Children with Phenylketonuria
Abstract
1. Introduction
2. Ethical Permission
3. Materials and Methods
3.1. Inclusion Criteria
3.2. Study Design
3.3. Protein Substitutes (PHE-FREE AA and CGMP1, CGMP2)
3.4. Measurement of Quantitative Plasma Amino Acids
3.5. Statistics
4. Results
4.1. Subjects
4.2. Quantitative Plasma Amino Acid Results
Individual Amino Acids
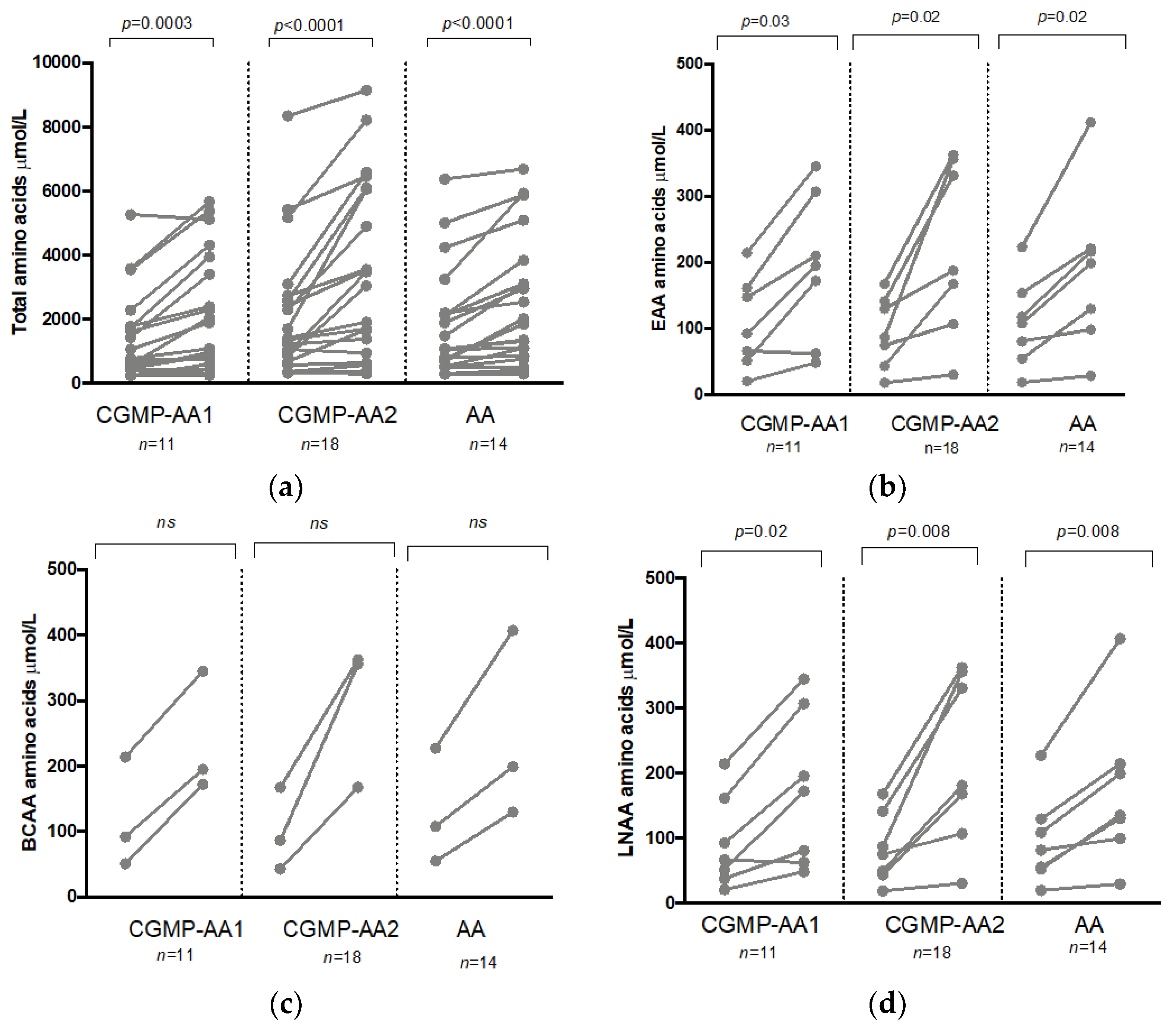
4.3. Total Amino Acids, LNAA, BCAA, and EAA
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation; Food and Agriculture Organization of the United Nations; United Nations University. Protein and Amino Acid Requirements in Human Nutition. In Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series 935; WHO: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Macdonald, A.; Daly, A.; Davies, P.; Asplin, D.; Hall, K.; Rylance, G.; Chakrapani, A. Protein substitutes for PKU: What’s new? J. Inherit. Metab. Dis. 2004, 27, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Woolf, L.I.; Griffiths, R.; Moncrieff, A. Treatment of phenylketonuria with a diet low in phenylalanine. Br. Med. J. 1995, 1, 57–64. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, D.; Bruinenberg, V.M.; Mazzola, P.N.; van Faassen, M.H.; de Blaauw, P.; Kema, I.P.; Fokkema, M.R.H.; van Anholt, R.D.; van der Zee, E.A.; van Spronsen, F.J. Large Neutral Amino Acid Supplementation Exerts Its Effect through Three Synergistic Mechanisms: Proof of Principle in Phenylketonuria Mice. PLoS ONE 2015, 10, e0143833. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef]
- Neelima, S.R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef]
- Usuda, Y.; Hara, Y.; Kojima, H. Toward Sustainable Amino Acid Production. Adv. Biochem. Eng. Biotechnol. 2017, 159, 289–304. [Google Scholar]
- Macleod, E.L.; Ney, D.M. Nutritional Management of Phenylketonuria. Ann. Nestle Eng. 2010, 68, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ney, D.M.; Gleason, S.T.; van Calcar, S.C.; MacLeod, E.L.; Nelson, K.L.; Etzel, M.R.; Rice, G.M.; Wolff, J.A. Nutritional management of PKU with glycomacropeptide from cheese whey. J. Inherit. Metab. Dis. 2009, 32, 32–39. [Google Scholar] [CrossRef]
- O’Riordan, N.; Kane, M.; Joshi, L.; Hickey, R.M. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology 2014, 24, 220–236. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballevre, O.; Beufrere, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–348. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, H.; Mariotti, F.; Gaudichon, C.; Bos, C.; Tome, D. Peripheral and splanchnic metabolism of dietary nitrogen are differently affected by the protein source in humans as assessed by compartmental modeling. J. Nutr. 2002, 132, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Pennings, B.; Groen, B.B.L.; Wall, B.T.; Churchward, T.A.-V.; Horstman, A.M.H.; Koopman, R.; et al. Protein Type, Protein Dose, and Age Modulate Dietary Protein Digestion and Phenylalanine Absorption Kinetics and Plasma Phenylalanine Availability in Humans. J. Nutr. 2020, 150, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Church, D.D.; Azhar, G.; Schutzler, S.E.; Ferrando, A.A.; Wolfe, R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E.; Jakel, M.; Soeters, P.B. Casein and soy protein meals differentially affect whole-body and splanchnic protein metabolism in healthy humans. J. Nutr. 2005, 135, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Cabero, A.I.; Torcello-Gomez, A.; Saha, S.; Mackie, A.R.; Wilde, P.J.; Brodkorb, A. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem. 2020, 319, 126514. [Google Scholar] [CrossRef]
- Roth, E.; Druml, W. Plasma amino acid imbalance: Dangerous in chronic diseases? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 67–74. [Google Scholar] [CrossRef]
- Macdonald, A.; Rylance, G.; Davies, P.; Asplin, D.; Hall, S.K.; Booth, I.W. Administration of protein substitute and quality of control in phenylketonuria: A randomized study. J. Inherit. Metab. Dis. 2003, 26, 319–326. [Google Scholar] [CrossRef]
- Mönch, E.; Herrmann, M.E.; Brösicke, H.; Schöffer, A.; Keller, M. Utilisation of amino acid mixtures in adolescents with phenylketonuria. Eur. J. Nucl. Med. Mol. Imaging 1996, 155, S115–S120. [Google Scholar] [CrossRef]
- Weigel, C.; Rauh, M.; Kiener, C.; Rascher, W.; Knerr, I. Effects of Various Dietary Amino Acid Preparations for Phenylketonuric Patients on the Metabolic Profiles along with Postprandial Insulin and Ghrelin Responses. Ann. Nutr. Metab. 2007, 51, 352–358. [Google Scholar] [CrossRef]
- Macdonald, A.; Rylance, G.; Hall, S.K.; Asplin, D.; Booth, I.W. Factors affecting the variation in plasma phenylalanine in patients with phenylketonuria on diet. Arch. Dis. Child. 1996, 74, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.; Murali, S.G.; Brinkman, A.S.; Nelson, D.W.; Clayton, M.K.; Yen, C.-L.E.; Ney, D.M. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am. J. Physiol. Metab. 2012, 302, E885–E895. [Google Scholar] [CrossRef] [PubMed]
- Van Calcar, S.C.; MacLeod, E.L.; Gleason, S.T.; Etzel, M.R.; Clayton, M.K.; Wolff, J.; Ney, D.M. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am. J. Clin. Nutr. 2009, 89, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.A.; Fairclough, P.D.; Clark, M.L.; Hegarty, J.E.; Addison, J.M.; Burston, D.; Clegg, K.M.; Matthews, D.M. Use of a Peptide Rather Than Free Amino Acid Nitrogen Source in Chemically Defined “Elemental” Diets. J. Parenter. Enter. Nutr. 1980, 4, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Van Wegberg, A.M.J.; Macdonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.B.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Chahal, S.; Santra, S.; Macdonald, A. Glycomacropeptide in children with phenylketonuria: Does its phenylalanine content affect blood phenylalanine control? J. Hum. Nutr. Diet. 2017, 30, 515–523. [Google Scholar] [CrossRef]
- Ney, D.M.; Murali, S.G.; Stroup, B.M.; Nair, N.; Sawin, E.A.; Rohr, F.; Levy, H.L. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol. Genet. Metab. 2017, 121, 96–103. [Google Scholar] [CrossRef]
- Pinto, A.; Almeida, M.F.; Ramos, P.C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; Macdonald, A.; Rocha, J.C. Nutritional status in patients with phenylketonuria using glycomacropeptide as their major protein source. Eur. J. Clin. Nutr. 2017, 71, 1230–1234. [Google Scholar] [CrossRef]
- MacLeod, E.L.; Clayton, M.K.; Van Calcar, S.C.; Ney, D.M. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol. Genet. Metab. 2010, 100, 303–308. [Google Scholar] [CrossRef]
- Ahring, K.K.; Lund, A.M.; Jensen, E.; Jensen, T.G.; Brøndum-Nielsen, K.; Pedersen, M.; Bardow, A.; Holst, J.J.; Rehfeld, J.F.; Møller, L.B. Comparison of Glycomacropeptide with Phenylalanine Free-Synthetic Amino Acids in Test Meals to PKU Patients: No Significant Differences in Biomarkers, Including Plasma Phe Levels. J. Nutr. Metab. 2018, 2018, 6352919. [Google Scholar] [CrossRef]
- Gropper, S.S.; Gropper, D.M.; Acosta, P.B. Plasma Amino Acid Response to Ingestion of L-Amino Acids and Whole Protein. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A.; Morse, E.L. Intestinal transport of dipeptides in man: Relative importance of hydrolysis and intact absorption. J. Clin. Investig. 1971, 50, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A.; Allen, E.R. Impaired Jejunal Absorption Rates of Essential Amino Acids Induced by Either Dietary Caloric or Protein Deprivation in Man. Gastroenterol. 1970, 59, 404–413. [Google Scholar] [CrossRef]
- Marrs, T.C.; Addison, J.M.; Burston, D.; Matthews, D.M. Changes in plasma amino acid concentrations in man after ingestion of an amino acid mixture simulating casein, and a tryptic hydrolysate of casein. Br. J. Nutr. 1975, 34, 259–265. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.; Doi, S.; Sellitti, D.; Procopio, J.; Pithon-Curi, T.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef]
- Nilsson, M.; Holst, J.J.; Björck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [CrossRef]
| Protein Substitute | CGMP-AA1 | CGMP-AA2 | PHE-FREE AA | |
|---|---|---|---|---|
| Nutrients | Units | Per 20 g PE Sachet | Per 20 g PE Sachet | Per 20 g PE Pouch |
| Calories | Kcal | 120 | 120 | 124 |
| Protein equivalent | g | 20 | 20 | 20 |
| Total Carbohydrate | g | 6.5 | 6.5 | 9.4 |
| Sugars | g | 2.2 | 2.2 | 7.8 |
| Total Fat | g | 1.5 | 1.5 | 0.7 |
| Docosahexaenoic Acid | mg | 84 | 84 | 134 |
| Arachidonic Acid | mg | - | - | - |
| Fiber | g | 0.1 | 0.1 | - |
| Comprehensive amino acid profile | ||||
| L-amino acids | CGMP-AA1 | CGMP-AA2 | PHE-FREE AA | |
| 20 g PE | 20 g PE | 20 g PE | ||
| L-Alanine | g | 0.76 | 0.83 | 0.92 |
| L-Arginine | g | 1.00 | 0.96 | 1.5 |
| L-Aspartic Acid | g | 2.04 | 1.31 | 2.37 |
| L-Cystine | g | 0.01 | 0.24 | 0.61 |
| L-Glutamine | g | 2.49 | 2.70 | - |
| Glycine | g | 2.77 | 1.20 | 2.35 |
| L-Histidine | g | 0.42 | 0.70 | 0.92 |
| L-Isoleucine | g | 1.37 | 1.35 | 1.62 |
| L-Leucine | g | 1.30 | 3.00 | 2.54 |
| L-Lysine | g | 1.07 | 0.80 | 1.67 |
| L-Methionine | g | 0.54 | 0.28 | 0.45 |
| L-Phenylalanine | g | 0.03 | 0.03 | - |
| L-Proline | g | 1.51 | 1.52 | 1.69 |
| L-Serine | g | 0.98 | 0.96 | 1.04 |
| L-Threonine | g | 2.17 | 2.20 | 1.62 |
| L-Tryptophan | g | 0.17 | 0.40 | 0.5 |
| L-Tyrosine | g | 1.01 | 2.24 | 2.38 |
| Taurine | g | - | - | 0.04 |
| L-Valine | g | 1.13 | 1.09 | 1.86 |
| Median L-Amino Acids μmol/L (range) | CGMP-AA1 (n = 11) | CGMP-AA 2 (n = 18) | PHE-FREE AA (n = 14) | p Value | |||
|---|---|---|---|---|---|---|---|
| Pre- Prandial | Post- Prandial | Pre- Prandial | Post- Prandial | Pre- Prandial | Post- Prandial | ||
| Alanine | 320 ^ (171–425) | 482 ^,** (266–685) | 277 ^^,§ (182–566) | 457 ^^ (304–701) | 356 ^^^,§ (153–463) | 412 ^^^,** (319–612) | ^ 0.007, ^^ <0.0001, ^^^ <0.002 § 0.012, ** 0.036 |
| Arginine | 44 ^ (27–56) | 77 ^ (28–138) | 38 ^^ (14–56) | 94 ^^ (23–130) | 38 ^^^ (16–57) | 74 ^^^ (33–168) | ^ 0.003, ^^ <0.0001, ^^^ 0.0001 |
| Aspartic acid | 21 T (12–35) | 19 (12–36) | 17 T (11–28) | 15 (10–31) | 21 (6–31) | 18 (11–42) | T 0.03 |
| Citrulline | 39 T (30–51) | 39 (28–55) | 29 T (23–46) | 33 (24–49) | 37 (16–48) | 35 (17–47) | T 0.02 |
| Cystine | 36 ^,T (18–45) | 28 ^ (12–44) | 16 ^^,T,§ (4–35) | 21 ^^,§§ (5–35) | 30 § (19–57) | 31 §§ (20–171) | ^ 0.02, ^^ 0.001 T 0.003, § <0.001, §§ <0.001 |
| Glutamine | 495 ^ (406–542) | 476 ^,TT (258–578) | 472 ^^ (350–606) | 513 ^^,TT (396–675) | 461 (339–583) | 455 (345–613) | ^ 0.001, ^^ 0.001 TT 0.035 |
| Glutamic acid | 62 ^ (40–79) | 71 ^ (45–104) | 63 ^^ (31–133) | 70 ^^ (41–155) | 75 (22–110) | 78 (33–111) | ^ 0.04, ^^ 0.02 |
| Glycine | 323 ^ (227–429) | 476 ^,TT,** (291–767) | 306 ^^ (195–446) | 342 ^^,TT (227–526) | 303 ^^^ (189–380) | 368 ^^^,** (199–642) | ^ 0.003, ^^ <0.0001, ^^^ 0.004 TT <0.001, ** 0.001 |
| Histidine | 66 (56–83) | 62 TT,** (39–83) | 75 ^^ (62–119) | 107 ^^,TT (70–151) | 81 ^^^ (46–99) | 99 ^^^,** (53–181) | ^^ <0.0001, ^^^ 0.003 TT <0.001, ** 0.005 |
| Isoleucine | 51^ (39–71) | 172 ^,** (97–270) | 43 ^^ (34–62) | 168 ^^ (96–230) | 55 ^^^ (30–72) | 130 ^^^,** (68–311) | ^ 0.001, ^^ <0.0001, ^^^ 0.0001 ** 0.008 |
| Leucine | 92 ^ (73–116) | 195 ^,TT (99–296) | 87 ^^ (72–130) | 356 ^^,TT,§§ (227–440) | 108 ^^^ (66–127) | 199 ^^^,§§ (108–474) | ^ 0.001, ^^ <0.0001, ^^^ 0.0001 TT <0.001, §§ <0.001 |
| Lysine | 147 ^ (102–179) | 210 ^ (128–314) | 130 ^^,§ (94–172) | 188 ^^ (96–332) | 155 ^^^,§ (109–193) | 216 ^^^ (148–385) | ^ 0.002, ^^ <0.0001, ^^^ 0.0001 § 0.04 |
| Methionine | 20 ^ (13–23) | 48 ^,TT,** (28–82) | 18 ^^ (15–27) | 30 ^^,TT (15–42) | 19 ^^^ (14–27) | 29 ^^^,** (19–52) | ^ 0.001, ^^ <0.0001, ^^^ 0.002 TT <0.001, ** <0.001 |
| Ornithine | 66 ^ (53–114) | 87 ^ (55–175) | 72 ^^ (37–96) | 91 ^^ (69–136) | 83 ^^^ (37–99) | 99 ^^^ (51–141) | ^ 0.004, ^^ 0.0004, ^^^ 0.004 |
| Proline | 116 ^ (93–183) | 282 ^ (174–522) | 106 ^^ (80–284) | 275 ^^ (198–530) | 141 ^^^ (83–322) | 258 ^^^ (161–447) | ^0.002, ^^ <0.0001, ^^^ 0.0001 |
| Serine | 156 ^ (123–227) | 202 ^ (131–321) | 146 ^^ (119–196) | 193 ^^ (119–251) | 155 (71–228) | 169 (132–325) | ^ 0.008, ^^ <0.0001 |
| Taurine | 54 (35–73) | 53 (36–326) | 55 (26–92) | 47 (33–74) | 56 (39–98) | 56 (47–100) | |
| Threonine | 161 ^ (90–241) | 307 ^,** (200–614) | 141 ^^ (80–215) | 331 ^^,§§ (240–440) | 129 ^^^ (67–193) | 214 ^^^,**,§§ (122–358) | ^ 0.002, ^^ <0.0001, ^^^ 0.0001 ** 0.001, §§ <0.001 |
| Tyrosine | 37 ^ (29–68) | 80 ^,TT,** (34–149) | 49 ^^ (36–84) | 181 ^^,TT,§§ (126–327) | 47 ^^^ (31–73) | 136 ^^^,**,§§ (52–206) | ^ 0.002, ^^ <0.0001, ^^^ 0.0001 TT <0.001,** 0.005, §§ 0.003 |
| Valine | 214 ^,T (157–278) | 345 ^ (206–580) | 168 ^^,§,T (131–243) | 363 ^^ (248–538) | 227 ^^^,§ (136–345) | 407 ^^^ (240–628) | ^ 0.002, ^^ <0.0001,^^^ 0.0001 T 0.031, § <0.001 |
| (a) | |||||||
| CGMP-AA1 n= 11 | CGMP-AA2 n = 18 | PHE-FREE AA n = 14 | p Value | ||||
| Total AA μmol/L (range) | Pre- Prandial | Post- Prandial | PRE- PRANDIAL | Pre- Prandial | Pre- Prandial | Pre- Prandial | |
| 753 * (219–5257) | 1473 * (237–5659) | 1375 ** (317–8344) | 3249 ** (291–9139) | 1067 *** (336–8513) | 1922 *** (396–9064) | * 0.0003, ** <0.0001, *** <0.0001 | |
| Amino acids: alanine, arginine, aspartic acid, cystine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, proline, serine, threonine, tyrosine, valine (excluding phenylalanine, tryptophan, citrulline) | |||||||
| (b) | |||||||
| EAA μmol/L (range) | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | |
| 92 * (20–214) | 195 * (48–345) | 87 ** (18–168) | 188 ** (30–363) | 108 *** (17–223) | 199 *** (30–415) | * 0.03 ** 0.02 *** 0.02 | |
| Amino acids: histidine, isoleucine, leucine, lysine, methionine, threonine, valine (excluding phenylalanine, tryptophan) | |||||||
| (c) | |||||||
| BCAA μmol/L (range) | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | |
| 92 (51–214) | 195 (172–345) | 87 (43–168) | 356 (168–363) | 98 (46–223) | 214 (131–415) | ns | |
| Amino acids: isoleucine, leucine, valine | |||||||
| (d) | |||||||
| LNAA μmol/L (range) | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | Pre- Prandial | |
| 66 * (20–214) | 172 * (48–345) | 75 ** (18–167) | 180 ** (30–363) | 67 *** (17–223) | 138 *** (30–415) | * 0.03, ** 0.02, *** 0.02 | |
| Amino acids: histidine, isoleucine, leucine, methionine, threonine, tyrosine, valine | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daly, A.; Evans, S.; Pinto, A.; Jackson, R.; Ashmore, C.; Rocha, J.C.; MacDonald, A. Preliminary Investigation to Review If a Glycomacropeptide Compared to L-Amino Acid Protein Substitute Alters the Pre- and Postprandial Amino Acid Profile in Children with Phenylketonuria. Nutrients 2020, 12, 2443. https://doi.org/10.3390/nu12082443
Daly A, Evans S, Pinto A, Jackson R, Ashmore C, Rocha JC, MacDonald A. Preliminary Investigation to Review If a Glycomacropeptide Compared to L-Amino Acid Protein Substitute Alters the Pre- and Postprandial Amino Acid Profile in Children with Phenylketonuria. Nutrients. 2020; 12(8):2443. https://doi.org/10.3390/nu12082443
Chicago/Turabian StyleDaly, Anne, Sharon Evans, Alex Pinto, Richard Jackson, Catherine Ashmore, Júlio César Rocha, and Anita MacDonald. 2020. "Preliminary Investigation to Review If a Glycomacropeptide Compared to L-Amino Acid Protein Substitute Alters the Pre- and Postprandial Amino Acid Profile in Children with Phenylketonuria" Nutrients 12, no. 8: 2443. https://doi.org/10.3390/nu12082443
APA StyleDaly, A., Evans, S., Pinto, A., Jackson, R., Ashmore, C., Rocha, J. C., & MacDonald, A. (2020). Preliminary Investigation to Review If a Glycomacropeptide Compared to L-Amino Acid Protein Substitute Alters the Pre- and Postprandial Amino Acid Profile in Children with Phenylketonuria. Nutrients, 12(8), 2443. https://doi.org/10.3390/nu12082443







