Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life—A Birth Cohort Study
Abstract
1. Introduction
2. Materials and Methods
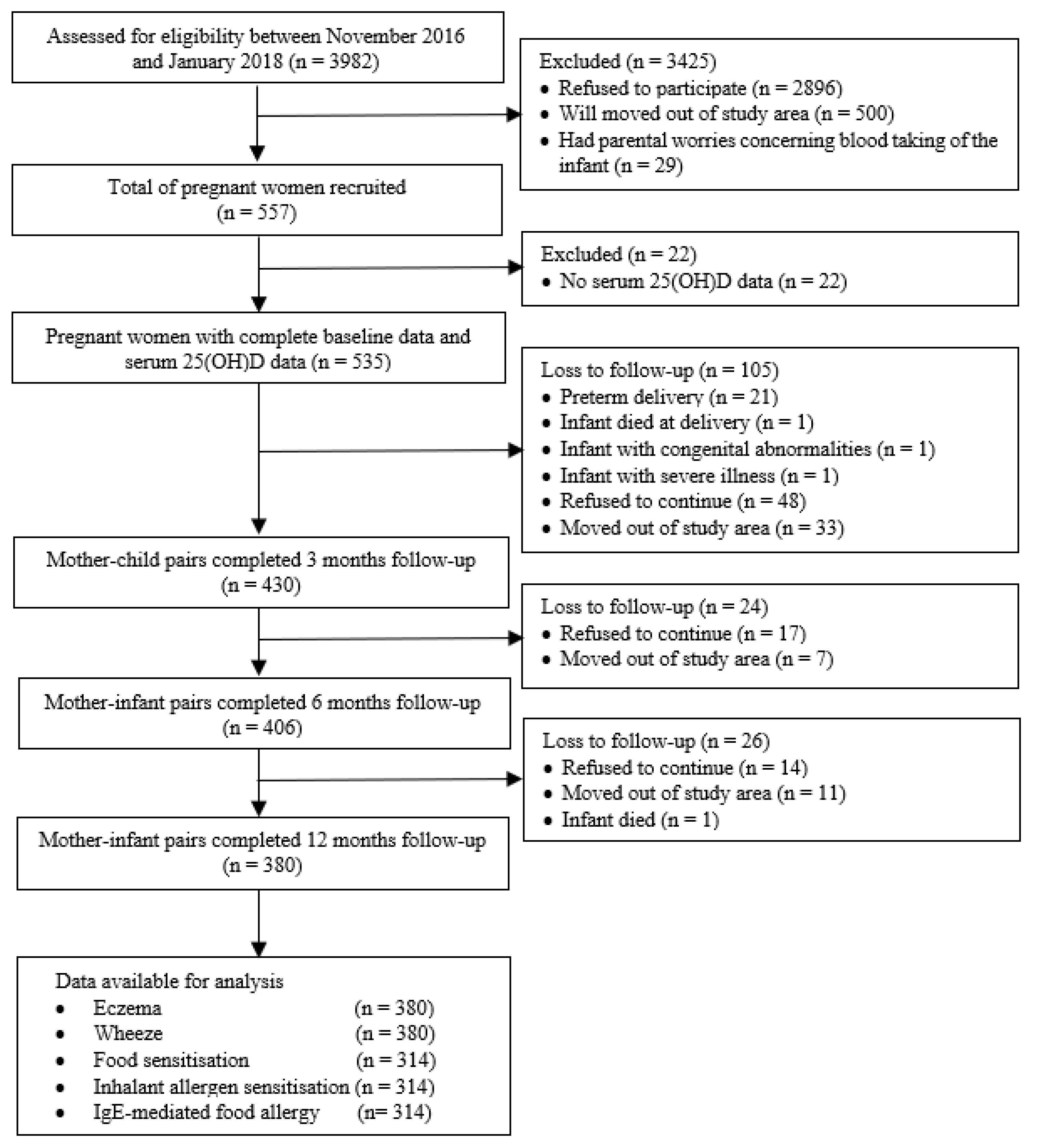
2.1. Study Design and Study Population
2.2. Serum 25-Hydroxyvitamin D [25(OH)D] Analysis
2.3. Allergic Sensitization
2.4. Allergic Outcomes
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Mother–Child Pairs
3.2. Allergic Outcomes in Infants
3.3. Associations between Maternal Vitamin D Levels and Allergic Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, T.; Yu, J.; Oh, M.H.; Zhu, Z. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. J. Clin. Cell. Immunol. 2011, 3, 67–73. [Google Scholar] [CrossRef]
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F.; Blaiss, M.S. World Allergy Organisation (WAO) White Book on Allergy: Update 2013; World Allergy Organization: Milwaukee, WI, USA, 2013. [Google Scholar]
- Deckers, I.A.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: A systematic review of epidemiological studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef]
- Wennergren, G. The prevalence of asthma has reached a plateau. Acta Paediatr. 2011, 100, 938–939. [Google Scholar] [CrossRef]
- Malik, G.; Tagiyeva, N.; Aucott, L.; McNeill, G.; Turner, S.W. Changing trends in asthma in 9–12 year olds between 1964 and 2009. Arch. Dis. Child. 2011, 96, 227–321. [Google Scholar] [CrossRef]
- Leung, A.S.Y.; Wong, G.W.K.; Tang, M.L.K. Food allergy in the developing world. J. Allergy Clin. Immunol. 2018, 141, 76–78. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Fiscaletti, M.; Stewart, P.; Munns, C.F. The importance of vitamin D in maternal and child health: A global perspective. Public Health Rev. 2017, 38, 19. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Al-Garawi, A.; Weiss, S.T.; Litonjua, A.A. Vitamin D and the development of allergic disease: How important is it? Clin. Exp. Allergy 2015, 45, 114–125. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Huang, S.Y.; Peng, Y.C.; Tsai, M.H.; Hua, M.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatric Allergy Immunol. 2015, 26, 337–343. [Google Scholar] [CrossRef]
- Weisse, K.; Winkler, S.; Hirche, F.; Herberth, G.; Hinz, D.; Bauer, M.; Roder, S.; Rolle-Kampczyk, U.; von Bergen, M.; Olek, S.; et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 2013, 68, 220–228. [Google Scholar] [CrossRef]
- Baïz, N.; Dargent-Molina, P.; Wark, J.D.; Souberbielle, J.C.; Annesi-Maesano, I.; EDEN Mother-Child Cohort Study Group. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J. Allergy Clin. Immunol. 2014, 133, 147–153. [Google Scholar] [CrossRef]
- Rueter, K.; Siafarikas, A.; Prescott, S.L.; Palmer, D.J. In utero and postnatal vitamin D exposure and allergy risk. Expert Opin. Drug Saf. 2014, 13, 1601–2611. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Kassim Javaid, M.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C.; the Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008, 62, 68–77. [Google Scholar] [CrossRef]
- Hennessy, Á.; Hourihane, J.O.; Malvisi, L.; Irvine, A.D.; Kenny, L.C.; Murray, D.M.; Kiely, M.E. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork BASELINE Birth Cohort Study. Allergy 2018, 73, 2182–2191. [Google Scholar] [CrossRef]
- Goldring, S.T.; Griffiths, C.J.; Martineau, A.R.; Robinson, S.; Yu, C.; Poulton, S.; Kirkby, J.C.; Stocks, J.; Hooper, R.; Shaheen, S.O.; et al. Prenatal vitamin D supplementation and child respiratory health: A randomised controlled trial. PLoS ONE 2013, 8, e66627. [Google Scholar] [CrossRef]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: A randomized clinical trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson Jr, R.E.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Chawes, B.L.; Bønnelykke, K.; Jensen, P.F.; Schoos, A.M.; Heickendorff, L.; Bisgaard, H. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: The COPSAC2000 birth cohort study. PLoS ONE 2014, 9, e99856. [Google Scholar] [CrossRef]
- Woon, F.C.; Chin, Y.S.; Intan Hakimah, I.; Batterham, M.; Amir Hamzah, A.L.; Gan, W.Y.; Geeta, A.; Siti Huzaifah, M.H.; Muliana, E.; Tan, M.L.; et al. Vitamin D deficiency during pregnancy and its associated factors among third trimester Malaysian pregnant women. PLoS ONE 2019, 14, e0216439. [Google Scholar] [CrossRef]
- Woon, F.C.; Chin, Y.S.; Intan Hakimah, I.; Chan, Y.M.; Batterham, M.; Amir Hamzah, A.L.; Gan, W.Y.; Geeta, A. Contribution of early nutrition on the development of malnutrition and allergic diseases in the first year of life: A study protocol for the Mother and Infant Cohort Study (MICOS). BMC Pediatrics 2018, 18, 233. [Google Scholar] [CrossRef]
- Freeman, J.; Sibley, P.; Parker, N.; Spears, R.; Wilson, K.; Levy, H. Standardization and certification of the ADVIA Centaur Vitamin D Total assay. Med. Res. Arch. 2017, 5, 1–19. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Han, M.; Shin, S.; Park, H.; Park, K.U.; Park, M.H.; Song, E.Y. Comparison of three multiple allergen simultaneous tests: RIDA allergy screen, MAST optigen, and polycheck allergy. BioMed Res. Int. 2013, 340513. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Hay, R.J.; Archer, C.B.; Shipley, M.J.; Hunter, J.J.; Bingham, E.A.; Finlay, A.Y.; Pembroke, A.C.; Graham-Brown, R.A.; et al. The U.K. working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br. J. Dermatol. 1994, 131, 383–396. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Nurmatov, U.; Nwaru, B.I.; Devereux, G.; Sheikh, A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy 2012, 67, 1041–1059. [Google Scholar] [CrossRef]
- Loo, E.X.L.; Sim, J.Z.T.; Loy, S.L.; Goh, A.; Chan, Y.H.; Tan, K.H.; Yap, F.; Gluckman, P.D.; Godfrey, K.M.; Van Bever, H.; et al. Associations between caesarean delivery and allergic outcomes: Results from the GUSTO study. Ann. Allergy Asthma Immunol. 2017, 118, 636–638. [Google Scholar] [CrossRef]
- Loo, E.X.; Sim, J.Z.; Goh, A.; Teoh, O.H.; Chan, Y.H.; Saw, S.M.; Kwek, K.; Gluckman, P.D.; Godfrey, K.M.; Van Bever, H.; et al. Predictors of allergen sensitization in Singapore children from birth to 3 years. Allergy Asthma Clin. Immunol. 2016, 12, 56. [Google Scholar] [CrossRef]
- Tham, E.H.; Lee, B.W.; Chan, Y.H.; Loo, E.X.L.; Toh, J.Y.; Goh, A.; Teoh, O.H.; Yap, F.; Tan, K.H.; Godfrey, K.M.; et al. Low food allergy prevalence despite delayed introduction of allergenic foods-data from the GUSTO cohort. J. Allergy Clin. Immunol. 2018, 6, 466–475. [Google Scholar] [CrossRef]
- Loo, E.X.L.; Tham, E.H.; Phang, K.W.; Goh, A.; Teoh, O.H.; Chong, Y.S.; Shek, L.P. Associations between maternal vitamin D levels during pregnancy and allergic outcomes in the offspring in the first 5 years of life. Pediatric Allergy Immunol. 2019, 30, 117–122. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Hong, X.; Wang, D.; Tsai, H.J.; Zhang, S.; Arguelles, L.; Kumar, R.; Wang, H.; Liu, R.; et al. Gene-vitamin D interactions on food sensitization: A prospective birth cohort study. Allergy 2011, 66, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, M.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Gold, D.R.; Asgari, M.M.; Thyssen, J.P.; Litonjua, A.A.; Gillman, M.W.; Oken, E. Low maternal prenatal 25-hydroxyvitamin D blood levels are associated with childhood atopic dermatitis. J. Investig. Dermatol. 2017, 137, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Gazibara, T.; Elbert, N.J.; den Dekker, H.T.; de Jongste, J.C.; Reiss, I.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Tiemeier, H.; Jaddoe, V.W.V.; et al. Associations of maternal and fetal 25-hydroxyvitamin D levels with childhood eczema: The Generation R Study. Pediatric Allergy Immunol. 2016, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 2008, 121, 1203–1209. [Google Scholar] [CrossRef]
- Jones, A.P.; Palmer, D.; Zhang, G.; Prescott, S.L. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics 2012, 130, e1128–e1135. [Google Scholar] [CrossRef]
- Pike, K.C.; Inskip, H.M.; Robinson, S.; Lucas, J.S.; Cooper, C.; Harvey, N.C.; Godfrey, K.M.; Roberts, G.; Southampton Women’s Survey Study Group. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax 2012, 67, 950–956. [Google Scholar] [CrossRef]
- Rothers, J.; Wright, A.L.; Stern, D.A.; Halonen, M.; Camargo, C.A., Jr. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J. Allergy Clin. Immunol. 2011, 128, 1093–1099. [Google Scholar] [CrossRef]
- Agudelo-Zapata, Y.; Maldonado-Acosta, L.M.; Sandoval-Alzate, H.F.; Poveda, N.E.; Garcés, M.F.; Cortés-Vásquez, J.A.; Linares-Vaca, A.F.; Mancera-Rodríguez, C.A.; Perea-Ariza, S.A.; Ramírez-Iriarte, K.Y.; et al. Serum 25hydroxyvitamin D levels throughout pregnancy: A longitudinal study in healthy and preeclamptic pregnant women. Endocr. Connect. 2018, 7, 698–707. [Google Scholar] [CrossRef]
- Thomas, S.D.C.; Fudge, A.N.; Whiting, M.; Coates, P.S. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ Open 2011, 1, e000236. [Google Scholar] [CrossRef]
- Nicolaidou, P.; Hatzistamatiou, Z.; Papadopoulou, A.; Kaleyias, J.; Floropoulou, E.; Lagona, E.; Tsagris, V.; Costalos, C.; Antsaklis, A. Low vitamin D status in mother-newborn pairs in Greece. Calcif. Tissue Int. 2006, 78, 337–342. [Google Scholar] [CrossRef] [PubMed]
| Total | Maternal 25(OH)D Levels | |||||
|---|---|---|---|---|---|---|
| Characteristics | Included in Age 12 Month Analysis (n = 380) | Loss to Follow-Up (n = 155) | p-Value | Deficient < 30 nmol/L (n = 164) | Nondeficient ≥ 30 nmol/L (n = 216) | p-Value |
| Maternal 25(OH)D levels | ||||||
| Deficient (<30 nmol/L) | 164 (43.2) | 63 (40.6) | 0.594 | |||
| Nondeficient (≥30 nmol/L) | 216 (56.8) | 92 (59.4) | ||||
| Gestational age at blood withdrawal (weeks) | ||||||
| Median (IQR) | 32 (29, 36) | 31 (28–35) | 0.013 | |||
| Family characteristics | ||||||
| Maternal age (years) | 30.1 ± 4.2 | 29.6 ± 4.0 | 0.225 | 30.0 ± 4.0 | 30.2 ± 4.3 | 0.591 |
| Maternal ethnicity, Malay (%) | 350 (92.1) | 143 (92.3) | 0.952 | 161 (98.2) | 189 (87.5) | 0.001 |
| Maternal educational level, higher (%) | 312 (82.1) | 126 (81.3) | 0.824 | 129 (78.7) | 183 (84.7) | 0.127 |
| Monthly household income | ||||||
| Low (< RM 2300) | 52 (13.7) | 40 (25.8) | 0.003 | 26 (15.9) | 26 (12.0) | 0.062 |
| Moderate (RM 2300–5599) | 209 (55.0) | 72 (46.5) | 97 (59.1) | 112 (51.9) | ||
| High (≥RM 5600) | 119 (31.3) | 43 (27.7) | 41 (25.0) | 78 (36.1) | ||
| Maternal work status, working (%) | 267 (70.3) | 103 (66.5) | 0.387 | 118 (72.0) | 149 (69.0) | 0.530 |
| Parity, multiparous (%) | 226 (59.5) | 83 (53.5) | 0.208 | 101 (61.6) | 125 (57.9) | 0.465 |
| Family history of allergic disease, yes (%) | 257 (67.6) | 98 (63.2) | 0.328 | 109 (66.5) | 148 (68.5) | 0.672 |
| Maternal antibiotics use during pregnancy, yes (%) | 44 (11.6) | 6 (12.0) a | 0.930 | 37 (22.6) | 56 (25.9) | 0.450 |
| Pet keeping, yes (%) | 93 (24.5) | − | − | 13 (7.9) | 31 (14.4) | 0.053 |
| Infant characteristics | ||||||
| Gestational age at birth (weeks) | 38.9 ± 1.1 | 38.8 ± 1.0 a | 0.579 | 38.8 ± 1.1 | 38.9 ± 1.2 | 0.867 |
| Birth weight (kg) | 3.1 ± 0.4 | 3.1 ± 0.4 a | 0.845 | 3.1 ± 0.4 | 3.1 ± 0.4 | 0.620 |
| Mode of delivery, vaginal (%) | 278 (73.2) | 36 (72.0) a | 0.862 | 119 (72.6) | 159 (73.6) | 0.819 |
| Sex, male (%) | 190 (50.0) | 31 (62.0) a | 0.110 | 77 (47.0) | 113 (52.3) | 0.300 |
| Older siblings, yes (%) | 226 (59.5) | 83 (53.5) | 0.208 | 101 (61.6) | 125 (57.9) | 0.465 |
| Daycare attendance, yes (%) | 207 (54.5) | − | − | 82 (50.0) | 125 (57.9) | 0.127 |
| Antibiotic use, yes (%) | 224 (58.9) | − | − | 93 (56.7) | 131 (60.6) | 0.439 |
| Exclusive breastfeeding till 6 months (%) | 177 (46.6) | 13 (50.0) b | 0.735 | 74 (45.1) | 103 (47.7) | 0.620 |
| Allergic Diseases | N (%) |
|---|---|
| Eczema in the past 12 months (n = 380) | 105 (27.6) |
| Wheeze in the past 12 months (n = 380) | 23 (6.1) |
| Food sensitization at 12 months (n = 314) 1 | 86 (27.4) |
| Beef (n = 314) | 45 (14.3) |
| Peanut (n = 314) | 34 (10.8) |
| Egg white (n = 314) | 22 (7.0) |
| Egg yolk (n = 314) | 10 (3.2) |
| Soya (n = 314) | 14 (4.5) |
| Cow’s milk (n = 314) | 7 (2.2) |
| Shrimp (n = 314) | 6 (1.9) |
| Crab (n = 314) | 6 (1.9) |
| Clam (n = 314) | 4 (1.3) |
| Codfish (n = 314) | 4 (1.3) |
| Wheat (n = 314) | 4 (1.3) |
| Salmon (n = 314) | 3 (1.0) |
| Chocolate (n = 314) | 2 (0.6) |
| Rice (n = 314) | 2 (0.6) |
| Tuna (n = 314) | 2 (0.6) |
| Chicken (n = 314) | 1 (0.3) |
| Orange (n = 314) | 1 (0.3) |
| Inhalant allergen sensitization at 12 months (n = 314) 1 | 34 (10.8) |
| Dermatophagoides farinae (n = 314) | 20 (6.4) |
| Dermatophagoides pteronyssinus (n = 314) | 17 (5.4) |
| Blomia tropicalis (n = 314) | 13 (4.1) |
| Candida (n = 314) | 7 (2.2) |
| Cat dander (n = 314) | 7 (2.2) |
| House dust (n = 314) | 6 (1.9) |
| Dog dander (n = 314) | 4 (1.3) |
| Cockroach mix (n = 314) | 4 (1.3) |
| Penicillium (n = 314) | 3 (1.0) |
| Cladosporium (n = 314) | 2 (0.6) |
| Aspergillus (n = 314) | 1 (0.3) |
| Bermuda grass (n = 314) | 1 (0.3) |
| IgE-mediated food allergy at 12 months (n = 314) | 12 (3.8) |
| Eggs (n = 314) | 10 (3.2) |
| Cow’s milk (n = 314) | 3 (1.0) |
| Wheat (n = 314) | 2 (0.6) |
| Soy (n = 314) | 1 (0.3) |
| Allergic Outcomes | Crude | Adjusted 1 | Adjusted 2 | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-Value | RR (95% CI) | p-Value | RR (95% CI) | p-Value | |
| Eczema (n = 380) | ||||||
| Nondeficient (≥30 nmol/L) | 1 | 1 | 1 | |||
| Deficient (<30 nmol/L) | 1.02 (0.77–1.35) | 0.884 | 1.04 (0.79–1.38) | 0.770 | 1.10 (0.83–1.46) | 0.495 |
| Wheeze (n = 380) | ||||||
| Nondeficient (≥30 nmol/L) | 1 | 1 | 1 | |||
| Deficient (<30 nmol/L) | 1.01 (0.48–2.13) | 0.973 | 1.04 (0.50–2.18) | 0.915 | 1.10 (0.61–2.00) | 0.755 |
| Food allergen sensitization (n = 314) | ||||||
| Nondeficient (≥30 nmol/L) | 1 | 1 | 1 | |||
| Deficient (<30 nmol/L) | 1.22 (0.85–1.75) | 0.282 | 1.08 (0.76–1.54) | 0.650 | 1.05 (0.75–1.48) | 0.782 |
| Inhalant allergen sensitization (n = 314) | ||||||
| Nondeficient (≥30 nmol/L) | 1 | 1 | 1 | |||
| Deficient (<30 nmol/L) | 0.58 (0.29–1.16) | 0.122 | 0.58 (0.29–1.15) | 0.121 | 0.59 (0.29–1.19) | 0.137 |
| IgE-mediated food allergy (n = 314) | ||||||
| Nondeficient (≥30 nmol/L) | 1 | 1 | 1 | |||
| Deficient (<30 nmol/L) | 0.54 (0.18–1.62) | 0.269 | 0.64 (0.30–1.40) | 0.268 | 0.68 (0.31–1.53) | 0.355 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woon, F.C.; Chin, Y.S.; Ismail, I.H.; Abdul Latiff, A.H.; Batterham, M.; Chan, Y.M.; on behalf of the MICOS Research Group. Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life—A Birth Cohort Study. Nutrients 2020, 12, 2418. https://doi.org/10.3390/nu12082418
Woon FC, Chin YS, Ismail IH, Abdul Latiff AH, Batterham M, Chan YM, on behalf of the MICOS Research Group. Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life—A Birth Cohort Study. Nutrients. 2020; 12(8):2418. https://doi.org/10.3390/nu12082418
Chicago/Turabian StyleWoon, Fui Chee, Yit Siew Chin, Intan Hakimah Ismail, Amir Hamzah Abdul Latiff, Marijka Batterham, Yoke Mun Chan, and on behalf of the MICOS Research Group. 2020. "Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life—A Birth Cohort Study" Nutrients 12, no. 8: 2418. https://doi.org/10.3390/nu12082418
APA StyleWoon, F. C., Chin, Y. S., Ismail, I. H., Abdul Latiff, A. H., Batterham, M., Chan, Y. M., & on behalf of the MICOS Research Group. (2020). Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life—A Birth Cohort Study. Nutrients, 12(8), 2418. https://doi.org/10.3390/nu12082418







