Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status
Abstract
:1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
3.1. Iodine Status
3.2. Dietary Habits
3.2.1. Iodized Salt
3.2.2. Cow’s Milk
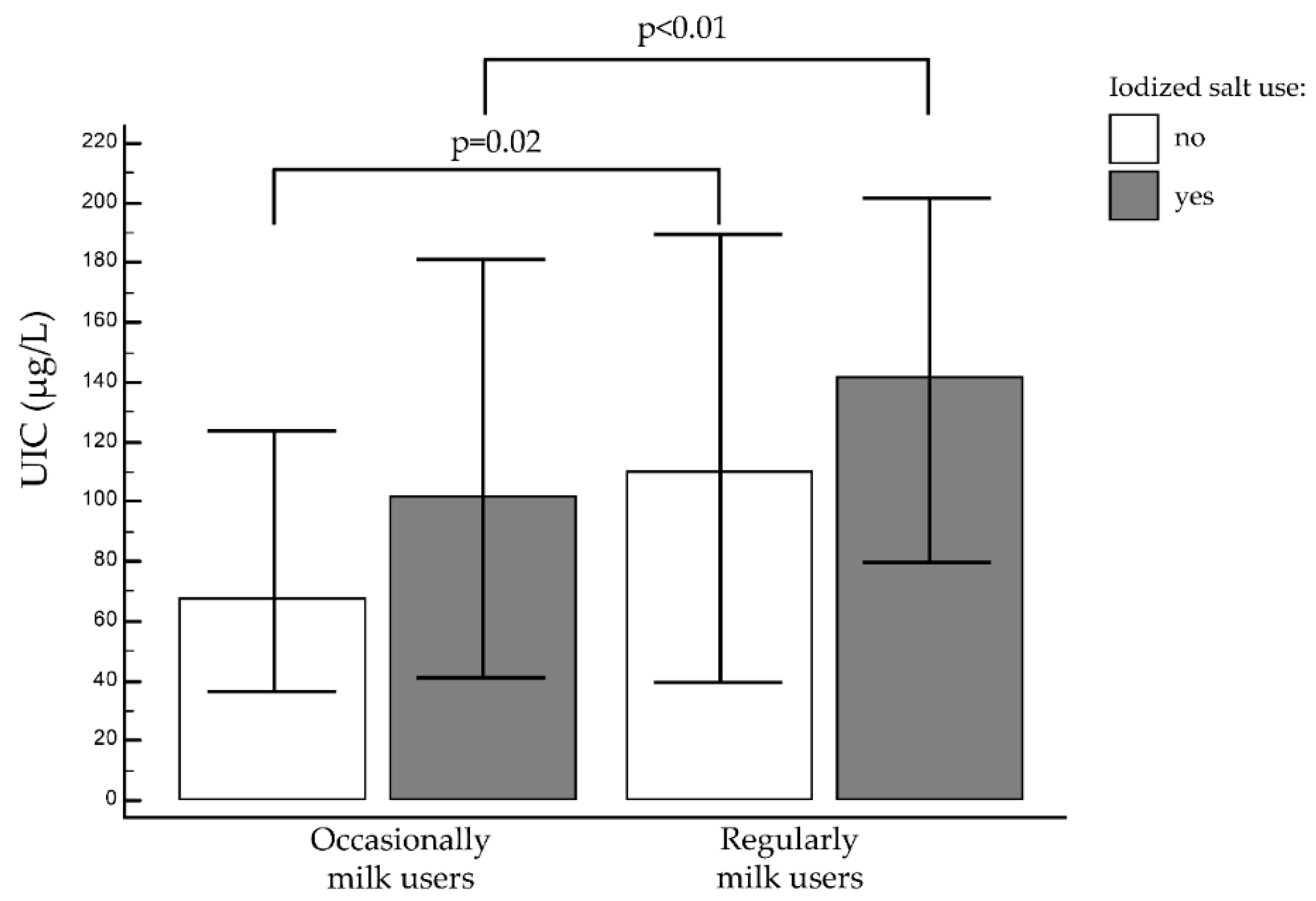
3.2.3. Cow’s Milk and Iodized Salt
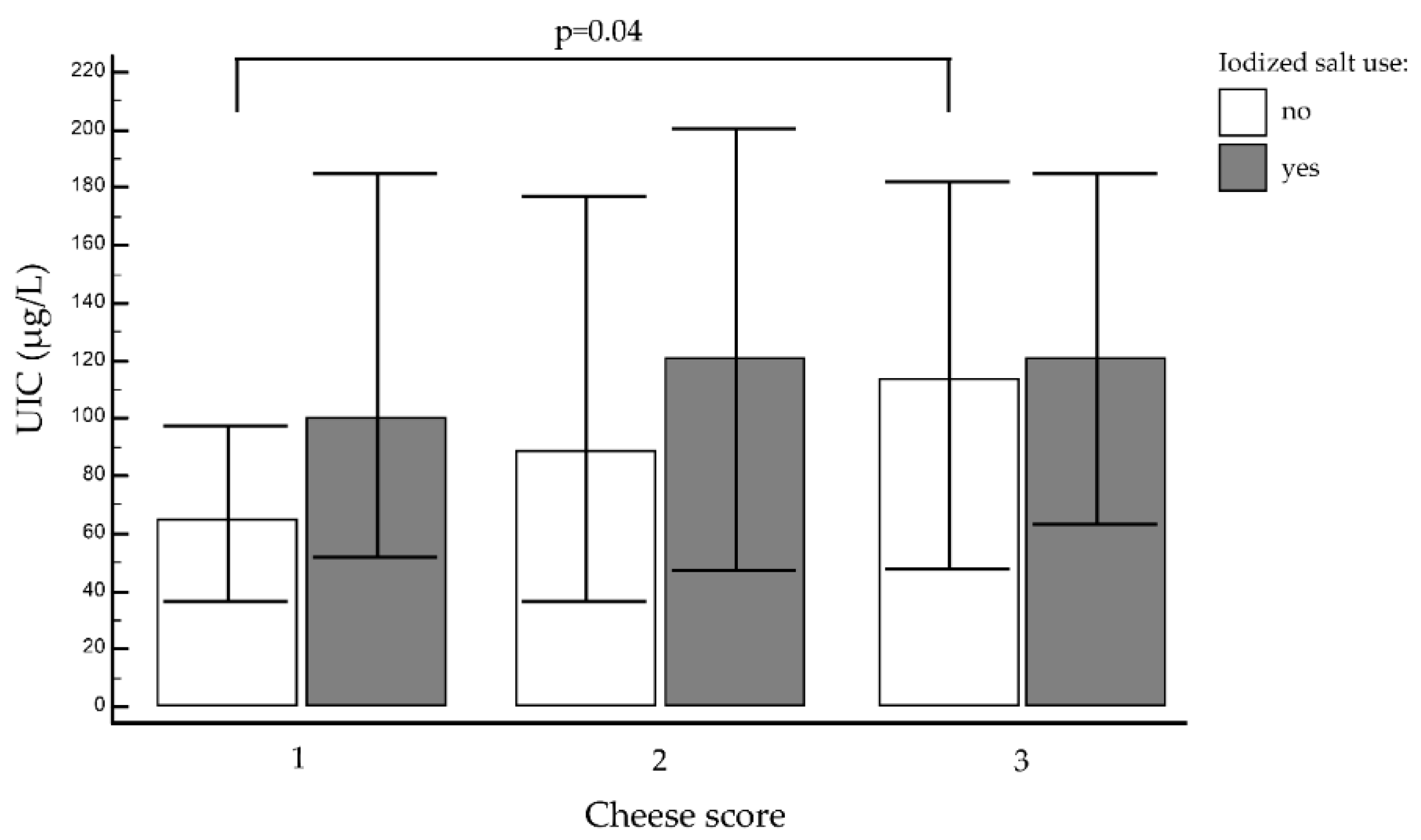
3.2.4. Other Foods, Cheese, and Yogurt
3.2.5. Univariate Analysis of Dietary Factors
3.3. Perception of the Importance of Iodine for Human Health and Relationship with Parent’s Educational Level
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO; UNICEF; International Council for Control of Iodine Deficienc Disorders. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide for Programme Managers. Available online: http://www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en/ (accessed on 21 August 2017).
- Engle, P.L.; Black, M.M.; Behrman, J.R.; Cabral de Mello, M.; Gertler, P.J.; Kapiriri, L.; Martorell, R.; Young, M.E. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet 2007, 369, 229–242. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levie, D.; Korevaar, T.I.M.; Bath, S.C.; Murcia, M.; Dineva, M.; Llop, S.; Espada, M.; van Herwaarden, A.E.; de Rijke, Y.B.; Ibarluzea, J.M.; et al. Association of maternal iodine status with child IQ: A meta-analysis of individual-participant data. J. Clin. Endocrinol. Metab. 2019, 104, 5957–5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, D.I.W. Iodine, milk, and the elimination of endemic goitre in Britain: The story of an accidental public health triumph. J. Epidemiol. Community Health 1997, 51, 391–393. [Google Scholar] [CrossRef] [Green Version]
- Girelli, M.E.; Coin, P.; Mian, C.; Nacamulli, D.; Zambonin, L.; Piccolo, M.; Vianello-Dri, A.; Gottardo, F.; Busnardo, B. Milk represents an important source of iodine in schoolchildren of the Veneto region, Italy. J. Endocrinol. Investig. 2004, 27, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Delange, F. Iodine deficiency in Europe. Cas. Lek. Cesk. 1995, 134, 35–43. [Google Scholar] [PubMed]
- Zimmermann, M.B.; Molinari, L.; Spehl, M.; Weidinger-Toth, J.; Podoba, J.; Hess, S.; Delange, F. Toward a consensus on reference values for thyroid volume in iodine-replete schoolchildren: Results of a workshop on interobserver and inter-equipment variation in sonographic measurement of thyroid volume. Eur. J. Endocrinol. 2001, 144, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watutantrige-Fernando, S.; Barollo, S.; Bertazza, L.; Cavedon, E.; Censi, S.; Manso, J.; Vianello, F.; Mian, C.; Andrighettoni, S.; Chiamenti, A.M.; et al. Efficacy of educational intervention to improve awareness of the importance of iodine, use of iodized salt, and dietary iodine intake in northeastern Italian schoolchildren. Nutrition 2018, 53, 134–139. [Google Scholar] [CrossRef]
- Busnardo, B.; Nacamulli, D.; Frigato, F.; Vianello-Dri, A.; De Vido, D.; Milan, C.; Candiani, F.; Tomasella, G.; Zambonin, L.; Piccolo, M.; et al. Normal values for thyroid ultrasonography, goiter prevalence and urinary iodine concentration in schoolchildren of the Veneto Region, Italy. J. Endocrinol. Investig. 2003, 26, 991–996. [Google Scholar] [CrossRef]
- Watutantrige Fernando, S.; Barollo, S.; Nacamulli, D.; Pozza, D.; Giachetti, M.; Frigato, F.; Redaelli, M.; Zagotto, G.; Girelli, M.E.; Mantero, F.; et al. Iodine status in schoolchildren living in northeast Italy: The importance of iodized-salt use and milk consumption. Eur. J. Clin. Nutr. 2013, 67, 366–370. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Dairy product intake in children and adolescents in developed countries: Trends, nutritional contribution, and a review of association with health outcomes. Nutr. Rev. 2014, 72, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Jooste, P.; Upson, N.; Charlton, K. Knowledge of iodine nutrition in the South African adult population. Public Health Nutr. 2005, 8, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; De Pee, S.; Hess, S.Y.; Sun, K.; Sari, M.; Bloem, M.W. Child malnutrition and mortality among families not utilizing adequately iodized salt in Indonesia. Am. J. Clin. Nutr. 2008, 87, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völzke, H.; Craesmeyer, C.; Nauck, M.; Below, H.; Kramer, A.; John, U.; Baumeister, S.; Ittermann, T. Association of socioeconomic status with iodine supply and thyroid disorders in northeast Germany. Thyroid 2013, 23, 348–353. [Google Scholar] [CrossRef]
- Navia, B.; Ortega, R.M.; Requejo, A.M.; Perea, J.M.; López-Sobaler, A.M.; Faci, M. Influence of Maternal Education on Food Consumption and Energy and Nutrient Intake in a Group of Pre-School Children from Madrid. Int. J. Vitam. Nutr. Res. 2003, 73, 439–445. [Google Scholar] [CrossRef]
- Olivieri, A.; Andò, S.; Bagnasco, M.; Meringolo, D.; Mian, C.; Moleti, M.; Puxeddu, E.; Regalbuto, C.; Taccaliti, A.; Tanda, M.L.; et al. The iodine nutritional status in the Italian population: Data from the Italian National Observatory for Monitoring Iodine Prophylaxis (OSNAMI) (period 2015-2019). Am. J. Clin. Nutr. 2019, 110, 1265–1266. [Google Scholar] [CrossRef]
- Garry, P.J.; Lashley, D.W.; Owen, G.M. Automated Measurement of Urinary Iodine. Clin. Chem. 1973, 19, 950–953. [Google Scholar] [CrossRef]
- Istisan, R.; Cura Di, A.; Olivieri, A.; Vitti, P. Attività di Monitoraggio del Programma Nazionale per la Prevenzione dei Disordini da Carenza Iodica. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2375_allegato.pdf (accessed on 24 March 2020).
- Pastorelli, A.A.; Stacchini, P.; Olivieri, A. Daily iodine intake and the impact of salt reduction on iodine prophylaxis in the Italian population. Eur. J. Clin. Nutr. 2015, 69, 211–215. [Google Scholar] [CrossRef]
- Watutantrige Fernando, S.; Cavedon, E.; Nacamulli, D.; Pozza, D.; Ermolao, A.; Zaccaria, M.; Girelli, M.E.; Bertazza, L.; Barollo, S.; Mian, C. Iodine status from childhood to adulthood in females living in North-East Italy: Iodine deficiency is still an issue. Eur. J. Nutr. 2016, 55, 335–340. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle. Subcommittee on Dairy Cattle Nutrition, Committee on Animal, Nutrition Board on Agriculture and Natural Resources, National Research Council; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Nicklas, T.A. Calcium intake trends and health consequences from childhood through adulthood. J. Am. Coll. Nutr. 2003, 22, 340–356. [Google Scholar] [CrossRef]
- Astrup, A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: Epidemiologic and experimental studies. Am. J. Clin. Nutr. 2014, 99, 1235S–1242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschini, M.; Battaglia, M.; Beone, G.M.; Piva, G.; Masoero, F. Iodine and selenium carry over in milk and cheese in dairy cows: Effect of diet supplementation and milk yield. Animal 2010, 4, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Reijden, O.L.; Galetti, V.; Herter-Aeberli, I.; Zimmermann, M.B.; Zeder, C.; Krzystek, A.; Haldimann, M.; Barmaz, A.; Kreuzer, M.; Berard, J.; et al. Effects of feed iodine 353 concentrations and milk processing on iodine concentrations of cow’s milk and dairy products, andpotential impact oniodine intake in Swiss adults. Br. J. Nutr. 2019, 122, 172–185. [Google Scholar] [CrossRef]
- Haldimann, M.; Walther, B.; Dudler, V.; Aubert, R.; Wechsler, D. Increase of iodine content in brine-salted soft, semi-hard and hard cheeses by diffusion of iodide. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1787–1799. [Google Scholar] [CrossRef] [Green Version]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]


| Total | 11–12 Years | 13–14 Years | 15–16 Years | p * | ||
|---|---|---|---|---|---|---|
| (n = 100/474, 13.4%) | (n = 624/747, 83.6%) | (n = 23/474, 3%) | ||||
| Median (μg/L) (IQR) | 111.0 | 112.4 | 111.6 | 130.8 | 0.98 | |
| (55.9–198.0) | (37.8–194.7) | (48.0–188.4) | (41.7–205.5) | |||
| UIC < 50 μg/L n (%) | 193/747 | 32/100 | 154/624 | 7/23 | 0.81 | |
| (26.0) | (32.0) | (24.8) | (30.4) | |||
| M | Median (μg/L) | 121.0 | 130.8 | 114.0 | 130.8 | 0.79 |
| (IQR) | (56.4–199.2) | (56.1–2262) | (55.2–199.5) | (77.1–188.1) | ||
| UIC < 50 μg/L n (%) | 97/411 | 12/54 | 82/342 | 3/15 | 0.82 | |
| (23.6) | (22.2) | (23.9) | (20.0) | |||
| Iodized salt use n (%) | 336/411 | 45/54 | 277/342 | 12/15 | 0.66 | |
| (81.8) | (83.3) | (80.9) | (80.0) | |||
| Regular milk use n (%) | 221/411 | 24/54 | 189/342 | 8/15 | 0.42 | |
| (54.0) | (44.4) | (55.3) | (53.3) | |||
| F | Median (μg/L) | 103.2 | 85.2 | 106.8 | 55.8 | 0.53 |
| (IQR) | (37.2–174.0) | (36.0–171.6) | (41.1–176.1) | (40.8–248.4) | ||
| UIC < 50 μg/L n (%) | 102/336, | 20/46 | 78/282 | 4/8 | 0.22 | |
| (30.3) | (42.5) | (27.6) | (50.0) | |||
| Iodized salt use n (%) | 279/336 | 41/46 | 230/282 | 8/8 | 0.26 | |
| (83.0) | (89.0) | (81.5) | (100.0) | |||
| Regular milk use n (%) | 126/336 | 21/46 | 100/282 | 5/8 | 0.14 | |
| (38.0) | (45.6) | (35.4) | (62.5) |
| Product | Total | Median UIC (μg/L) (Interquartile Range) | p ** | Percentage of UIC < 50 ug/L 193/747 (26%) | Percentage of UIC ≥ 50 and < 100 ug/L 138/747 (18%) | Percentage of UIC ≥ 100 ug/L 416/747 (56%) | p * | |
|---|---|---|---|---|---|---|---|---|
| Iodized salt | yes | 610/747 (81.6%) | 117.0 (54.0–192.0) | 0.01 | 147/610 (24.0%) | 112/610 (18.4%) | 351/610 (57.6%) | 0.02 |
| no | 137/747 (18.4%) | 90.0 (36.6–174.6) | 51/137 (37.2%) | 23/137 (16.7%) | 63/137 (45.9%) | |||
| Cow’s milk | regularly | 349/747 (47%) | 132.0 (72.0–198.9) | < 0.01 | 68/349 (19.6%) | 56/349 (16.1%) | 225/349 (64.3%) | < 0.01 |
| occasionally | 398/747 (53%) | 96.0 (36.0–177.3) | 127/398 (31.9%) | 77/398 (19.3%) | 194/398 (48.8%) | |||
| Cow’s milk | 1 | 143/747 (19.12%) | 96.0 (36.0–162.6) | < 0.01 | 45/143 (31.1%) | 28/143 (19.7%) | 70/143 (49.2%) | < 0.01 |
| 2 | 255/747 (34.1%) | 94.8 (36.0–183.6) | 82/255 (32.3%) | 49/255 (19.1%) | 124/255 (48.5%) | |||
| 3 | 321/747 (43%) | 130.8 (69.6–192.6) | 65/321 (20.3%) | 53/321 (16.6%) | 203/321 (63.2%) | |||
| 4 | 28/74 (3.8%) | 223.2 (115.8–273.0) | 3/28 (11.5%) | 3/2 (11.5%) | 22/28 (76.9%) | |||
| Yogurt | 1 | 205/747 (27.4%) | 110.4 (40.2–188.4) | 0.22 | 60/205 (29.3%) | 32/205 (15.4%) | 113/205 (55.3%) | 0.26 |
| 2 | 329/747 (44%) | 109.2 (44.4–183.6) | 89/329 (27.2%) | 58/329 (17.5%) | 182/329 (55.3%) | |||
| 3 | 213/747 (28.6%) | 126.6 (62.4–195.6) | 43/213 (20.4%) | 45/213 (20.9%) | 125/213 (58.7%) | |||
| Cheese | 1 | 89/747 (12%) | 87.6 (39.6–165.6) | 0.16 | 26/89 (29.3%) | 25/89 (28%) | 38/89 (42.7%) | 0.02 |
| 2 | 328/747 (43.9%) | 112.8 (39.3–199.5) | 96/328 (29.2%) | 51/328 (15.6%) | 181/328 (55.21%) | |||
| 3 | 330/747 (44.1%) | 116.4 (57.9–184.8) | 74/330 (22.4%) | 57/330 (17.2%) | 199/330 (60.4%) | |||
| Beef | 1 | 33/747 (4.4%) | 135.0 (48.0–244.8) | 0.19 | 9/33 (26.7%) | 3/33 (10%) | 21/33 (63.3%) | 0.52 |
| 2 | 395/747 (52.9%) | 116.4 (54.9–194.4) | 230/395 (58.2%) | 69/395 (17.5%) | 96/395 (24.3%) | |||
| 3 | 319/747 (42.7%) | 105.6 (44.7–176.4) | 169/319 (53.0%) | 61/319 (19.1%) | 89/319 (27.9%) | |||
| Chicken | 1 | 10/747 (1.3%) | 130.8 (75.6-257.4) | 0.56 | 2/10 (22.2%) | 1/10 (11.1%) | 7/10 (66.7%) | 0.56 |
| 2 | 410/747 (54.9%) | 114.0 (51.6–188.0) | 100/410 (24.4%) | 75/410 (18.3%) | 235/410 (57.3%) | |||
| 3 | 327/74 (43.8%) | 110.4 (40.8–183.9) | 93/327 (28.5%) | 57/327 (17.4%) | 177/327 (54.1%) | |||
| Eggs | 1 | 78/747 (10.4%) | 96.0 (40.8–192.6) | 0.77 | 23/78 (29.2%) | 17/78 (22.2%) | 38/78 (48.6%) | 0.68 |
| 2 | 591/747 (79.2%) | 114.0 (48.0–186.0) | 152/591 (25.7%) | 102/591 (17.3%) | 337/591 (57.0%) | |||
| 3 | 78/747 (10.4%) | 112.8 (36.6–112.4) | 22/78 (27.8%) | 12/78 (15.3%) | 44/78 (56.9%) | |||
| Fish | 1 | 156/747 (20.9%) | 110.4 (39.6–195.6) | 0.89 | 47/156 (30.1%) | 26/156 (16.7%) | 83/156 (53.2%) | 0.82 |
| 2 | 524/747 (70.1%) | 112.8 (39.8–188.4) | 131/524 (25.0%) | 96/524 (18.4%) | 297/524 (56.6%) | |||
| 3 | 67/747 (9%) | 121.2 (48.0–176.4) | 18/67 (27.4%) | 11/67 (16.1%) | 38/67 (56.5%) | |||
| Chips | 1 | 107/747 (14.3%) | 115.2 (40.8–186.0) | 0.77 | 28/107 (26.2%) | 18/107 (16.8%) | 61/107 (57.0%) | 0.98 |
| 2 | 512/747 (68.5%) | 114.0 (48.0–191.7) | 135/512 (26.3%) | 90/512 (17.6%) | 287/512 (56.1%) | |||
| 3 | 128/747 (17.2%) | 110.4 (49.2–176.4) | 32/128 (25.0%) | 25/128 (19.5%) | 71/128 (55.5%) | |||
| Pizza | 1 | 17/747 (2.3%) | 85.2 (36.0-125.4) | 0.28 | 5/17 (29.4%) | 5/17 (29.4%) | 7/17 (41.2%) | 0.38 |
| 2 | 681/747 (91.1%) | 112.8 (48.0–188.4) | 178/681 (26.1%) | 121/681 (17.8%) | 382/681 (56.1%) | |||
| 3 | 49/747 (6.6%) | 112.8 (45.0–188.1) | 13/49 (26.7%) | 5/49 (11.1%) | 31/49 (62.2%) | |||
| Cold Cuts | 1 | 49/747 (6.5%) | 115.2 (47.1–214.5) | 0.87 | 13/49 (26.5%) | 8/49 (16.3%) | 28/49 (57.2%) | 0.99 |
| 2 | 330/747 (44.2%) | 110.4 (48.0–189.3) | 88/330 (26.7%) | 59/330 (17.9%) | 183/330 (55.4%) | |||
| 3 | 368/747 (49.3%) | 112.8 (48.0–184.8) | 94/368 (25.6%) | 66/368 (17.9%) | 208/368 (56.5%) | |||
| Canned Food | 1 | 374/747 (50.0%) | 114.0 (45.3–188.7) | 0.58 | 101/374 (27.0%) | 58/374 (15.5%) | 215/374 (57.5%) | 0.32 |
| 2 | 329/747 (44.1%) | 110.4 (49.8–187.8) | 82/329 (25.0%) | 64/329 (19.3%) | 183/329 (55.7%) | |||
| 3 | 44/747 (5.9%) | 84.6 (40.8–145.8) | 12/44 (27.5%) | 12/44 (27.5%) | 20/44 (45%) | |||
| Soy Milk | regularly | 738/747 (98.8%) | 119.4 (44.8–278.5) | 0.81 | 193/738 (26.1%) | 134/738 (18.2%) | 411/738 (55.7%) | 0.90 |
| occasionally | 9/747 (1.2%) | 110.4 (104.4–122.4) | 2/9 (25.0%) | 1/9 (12.5%) | 6/9 (62.5%) | |||
| Soft Drinks | 1 | 331/747 (44.3%) | 104.4 (44.4–183.6) | 0.19 | 91/331 (27.5%) | 65/331 (19.6%) | 175/331 (52.9%) | 0.40 |
| 2 | 382/747 (51.2%) | 122.4 (51.6–193.5) | 93/382 (24.4%) | 64/382 (16.7%) | 225/382 (58.9%) | |||
| 3 | 34/747 (4.5%) | 110.4 (36.0–174.9) | 12/34 (35.5%) | 4/34 (12.9%) | 18/34 (51.6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Censi, S.; Manso, J.; Barollo, S.; Mondin, A.; Bertazza, L.; De Marchi, M.; Mian, C.; on behalf of the Food and Nutrition Hygiene Services. Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status. Nutrients 2020, 12, 2399. https://doi.org/10.3390/nu12082399
Censi S, Manso J, Barollo S, Mondin A, Bertazza L, De Marchi M, Mian C, on behalf of the Food and Nutrition Hygiene Services. Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status. Nutrients. 2020; 12(8):2399. https://doi.org/10.3390/nu12082399
Chicago/Turabian StyleCensi, Simona, Jacopo Manso, Susi Barollo, Alberto Mondin, Loris Bertazza, Massimo De Marchi, Caterina Mian, and on behalf of the Food and Nutrition Hygiene Services (SIAN). 2020. "Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status" Nutrients 12, no. 8: 2399. https://doi.org/10.3390/nu12082399
APA StyleCensi, S., Manso, J., Barollo, S., Mondin, A., Bertazza, L., De Marchi, M., Mian, C., & on behalf of the Food and Nutrition Hygiene Services. (2020). Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status. Nutrients, 12(8), 2399. https://doi.org/10.3390/nu12082399







