An Open Pilot Study of the Effect and Tolerability of Add-On Multivitamin Therapy in Patients with Intractable Focal Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment, Evaluation and Follow up
2.3. Outcome Criteria
2.4. Serum Vitamin Levels
2.5. Metabolic Profile
2.6. Compliance
2.7. Statistical Analysis
3. Results
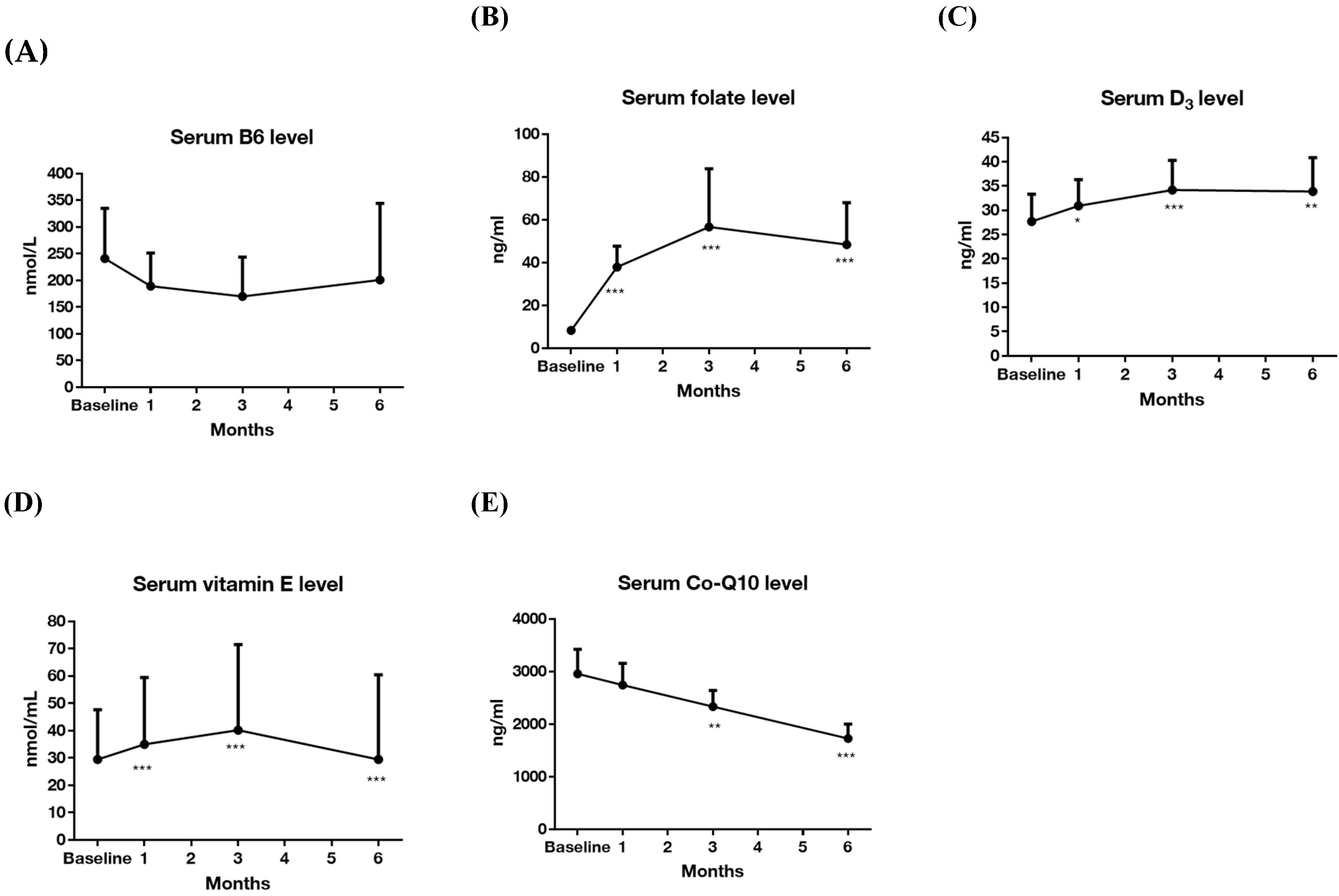
3.1. Effectiveness on Seizure Frequency (Primary Outcome) and Serum Levels of Vitamins
3.2. Body and Metabolic Profiles
3.3. Safety and Tolerability (Secondary Outcome)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwan, P.; Brodie, M.J. Refractory epilepsy: Mechanisms and solutions. Expert Rev. Neurother. 2006, 6, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Carter, J.; Vahle, V.; Gilliam, F.G. Adverse antiepileptic drug effects: Toward a clinically and neurobiologically relevant taxonomy. Neurology 2009, 72, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Kook, D.H.; Cho, S.Y.; Lee, K.Y.; Lee, I.G.; Lee, J.S. A case of acute isoniazid intoxication in childhood. J. Korean Child Neurol. Soc. 2006, 14, 358–362. [Google Scholar]
- Dave, H.N.; Ramsay, R.E.; Khan, F.; Sabharwal, V.; Irland, M. Pyridoxine deficiency in adult patients with status epilepticus. Epilepsy Behav. 2015, 52, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Pillai, K.; Pal, S.N. Effects of folic acid and lamotrigine therapy in some rodent models of epilepsy and behaviour. J. Pharm. Pharmacol. 2003, 55, 387–391. [Google Scholar] [CrossRef]
- Rasic-Markovic, A.; Hrncic, D.; Krstic, D.; Colovic, M.; Djuric, E.; Rankov-Petrovic, B.; Susic, V.; Stanojlovic, O.; Djuric, D. The effect of subchronic supplementation with folic acid and l-arginine on homocysteine-induced seizures. Can. J. Psychophysiol. Pharmacol. 2016, 94, 1083–1089. [Google Scholar] [CrossRef]
- Holló, A.; Clemens, Z.; Kamondi, A.; Lakatos, P.; Szűcs, A. Correction of vitamin D deficiency improves seizure control in epilepsy: A pilot study. Epilepsy Behav. 2012, 24, 131–133. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B. What is the role of vitamin D in autism? Derm. Endocrinol. 2013, 5, 199–204. [Google Scholar] [CrossRef]
- Borowicz, K.K.; Morawska, D.; Morawska, M. Effect of cholecalciferol on the anticonvulsant action of some second generation antiepileptic drugs in the mouse model of maximal electroshock. Pharmacol. Rep. 2015, 67, 875–880. [Google Scholar] [CrossRef]
- Ogunmekan, A.O.; Hwang, P.A. A randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopheryl acetate (vitamin E), as add-on therapy, for epilepsy in children. Epilepsia 1989, 30, 84–89. [Google Scholar] [CrossRef]
- Mehvari, J.; Motlagh, F.G.; Najafi, M.; Ghazvini, M.R.A.; Naeini, A.A.; Zare, M. Effects of Vitamin E on seizure frequency, electroencephalogram findings, and oxidative stress status of refractory epileptic patients. Adv. Biomed. Res. 2016, 5. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Coenzyme q10 ameliorates neurodegeneration, mossy fiber sprouting, and oxidative stress in intrahippocampal kainate model of temporal lobe epilepsy in rat. J. Mol. Neurosci. 2013, 49, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Sattarinezhad, E.; Shafaroodi, H.; Sheikhnouri, K.; Mousavi, Z.; Moezi, L. The effects of coenzyme Q10 on seizures in mice: The involvement of nitric oxide. Epilepsy Behav. 2014, 37, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.K. Coenzyme Q10 enhances the anticonvulsant effect of phenytoin in pilocarpine-induced seizures in rats and ameliorates phenytoin-induced cognitive impairment and oxidative stress. Epilepsy Behav. 2011, 22, 671–677. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Gutch, M.; Kumar, S.; Razi, S.; Gupta, K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Menik, L.; Palangasinghe, S. Comparison of Insulin Resistance by Indirect Methods-HOMA, QUICKI and McAuley-with Fasting Insulin in Patients with Type 2 Diabetes in Galle, Sri Lanka: A Pilot Study. Available online: http://cogprints.org/5000/1/2006-1-2.pdf (accessed on 16 July 2006).
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Ho, Y.; Hu, C.-J.; Su, W.-W.; Hsu, K.-Y.; Shen, W.W.; Chiueh, C.C.; Yuan, R.-Y. Development of a Taiwan version of the eight-item Morisky Medication Adherence Scale and factors influencing patients’ comprehension. J. Exp. Clin. Med. 2013, 5, 77–80. [Google Scholar] [CrossRef]
- Tang, F.; Hartz, A.; Bauer, B. Drug-resistant epilepsy: Multiple hypotheses, few answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- LoVecchio, F.; Curry, S.C.; Graeme, K.A.; Wallace, K.L.; Suchard, J. Intravenous pyridoxine-induced metabolic acidosis. Ann. Emerg. Med. 2001, 38, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Rainwater, D.L.; Mahaney, M.C.; Stocker, R. Cosupplementation with vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am. J. Clin. Nutr. 2004, 80, 649–655. [Google Scholar] [CrossRef]
- Huang, C.W.; Huang, C.C.; Wu, S.N. The Opening Effect of Pregabalin on ATP-Sensitive Potassium Channels in Differentiated Hippocampal Neuron–derived H19-7 Cells. Epilepsia 2006, 47, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Huang, C.C.; Cheng, J.T.; Tsai, J.J.; Wu, S.N. Glucose and hippocampal neuronal excitability: Role of ATP-sensitive potassium channels. J. Neurosci. Res. 2007, 85, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Tsai, J.-J.; Ou, H.-Y.; Wang, S.-T.; Cheng, J.-T.; Wu, S.-N.; Huang, C.-C. Diabetic hyperglycemia is associated with the severity of epileptic seizures in adults. Epilepsy Res. 2008, 79, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Cheng, J.-T.; Tsai, J.-J.; Wu, S.-N.; Huang, C.-C. Diabetic hyperglycemia aggravates seizures and status epilepticus-induced hippocampal damage. Neurotox. Res. 2009, 15, 71–81. [Google Scholar] [CrossRef]
- Huang, C.-W.; Wu, S.-N.; Cheng, J.-T.; Tsai, J.-J.; Huang, C.-C. Diazoxide reduces status epilepticus neuron damage in diabetes. Neurotox. Res. 2010, 17, 305–316. [Google Scholar] [CrossRef]
- Effoe, V.S.; Wagenknecht, L.E.; Echouffo Tcheugui, J.B.; Chen, H.; Joseph, J.J.; Kalyani, R.R.; Bell, R.A.; Wu, W.C.H.; Casanova, R.; Bertoni, A.G. Sex differences in the association between insulin resistance and incident coronary heart disease and stroke among blacks without diabetes mellitus: The Jackson Heart Study. J. Am. Heart Assoc. 2017, 6, e004229. [Google Scholar] [CrossRef]
- Alissa, E.M. Relationship between serum gamma-glutamyltransferase activity and cardiometabolic risk factors in metabolic syndrome. J. Fam. Med Prim. Care 2018, 7, 430–434. [Google Scholar] [CrossRef]
- Panayiotou, A.; Kouis, P.; Griffin, M.; Nicolaides, A. Comparison between insulin resistance indices and carotid and femoral atherosclerosis: A cross-sectional population study. Int. Angiol. J. Int. Union Angiol. 2015, 34, 437. [Google Scholar]
- Li, J.; Goh, C.E.; Demmer, R.T.; Whitcomb, B.W.; Du, P.; Liu, Z. Association between serum folate and insulin resistance among US nondiabetic adults. Sci. Rep. 2017, 7, 1–7. [Google Scholar]
- Setola, E.; Monti, L.D.; Galluccio, E.; Palloshi, A.; Fragasso, G.; Paroni, R.; Magni, F.; Sandoli, E.P.; Lucotti, P.; Costa, S. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: Relationship between homocysteine levels and hyperinsulinemia. Eur. J Endocrinol. 2004, 151, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.A.; Ashraf Aminorroaya, B.I.; Amini, M. The effects of oral vitamin D on insulin resistance in pre-diabetic patients. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 47–51. [Google Scholar]
- Yu, L.; Zhai, Y.; Shen, S. Association between vitamin D and prediabetes: A PRISMA-compliant meta-analysis. Medicine 2020, 99. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Nascimento, C.M.C.; Costa-Guarisco, L.P.; Gomes, G.A.d.O.; Gramani-Say, K.; Orlandi, F.d.S.; Gratão, A.C.M.; Orlandi, A.A.d.S.; Pavarini, S.C.I.; Vasilceac, F.A. Vitamin D Deficient Older Adults Are More Prone to Have Metabolic Syndrome, but Not to a Greater Number of Metabolic Syndrome Parameters. Nutrients 2020, 12, 748. [Google Scholar] [CrossRef]
- Manning, P.J.; Sutherland, W.H.F.; Walker, R.J.; Williams, S.M.; de Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of High-Dose Vitamin E on Insulin Resistance and Associated Parameters in Overweight Subjects. Diabetes Care 2004, 27, 2166. [Google Scholar] [CrossRef]
- Sanchez-Lugo, L.; Mayer-Davis, E.J.; Howard, G.; Selby, J.V.; Ayad, M.F.; Rewers, M.; Haffner, S. Insulin sensitivity and intake of vitamins E and C in African American, Hispanic, and non-Hispanic white men and women: The Insulin Resistance and Atherosclerosis Study (IRAS). Am. J. Clin. Nutr. 1997, 66, 1224–1231. [Google Scholar] [CrossRef]
- Schwetz, V.; Scharnagl, H.; Trummer, C.; Stojakovic, T.; Pandis, M.; Gruebler, M.R.; Verheyen, N.; Gaksch, M.; Zittermann, A.; Aberer, F. Vitamin D supplementation and lipoprotein metabolism: A randomized controlled trial. J. Clin. Lipidol. 2018, 12, 588.e584–596.e584. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; LaBree, L.; Mahrer, P.R.; Sevanian, A.; Liu, C.-r.; Liu, C.-h.; Hwang, J.; Selzer, R.H.; Azen, S.P. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 2002, 106, 1453–1459. [Google Scholar] [CrossRef]
- Asbaghi, O.; Choghakhori, R.; Abbasnezhad, A. Effect of Omega-3 and vitamin E co-supplementation on serum lipids concentrations in overweight patients with metabolic disorders: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, C.; Guo, H.; Wang, J.; Lin, S.; Li, H.; Yang, Y.; Ling, W. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J. Clin. Lipidol. 2018, 12, 417.e415–427.e415. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 26) | ||
|---|---|---|
| Female, n (%) | 17 | 65.38% |
| Age (mean, SD), years | 42.37 | 9.40 |
| Age at onset of epilepsy (mean, SD), years | 29.92 | 9.32 |
| Type of seizure, n (%) | FIAS | 6 (23.07%) |
| FIAS and FAS | 12 (46.15%) | |
| FIAS and FBTCS | 8 (30.77%) | |
| Negative MRI abnormality, n (%) | 15 (57.70%) | |
| Epilepsy syndrome, n (%) | Temporal lobe epilepsy | 15 (57.70%) |
| Extratemporal lobe epilepsy | 11 (42.30%) | |
| Side of EEG focus, n (%) | Left/right | 10/5 (38.46%/19.23%) |
| Bilateral | 5 (19.23%) | |
| Unknown | 6 (23.08%) | |
| Patients with ≥3 AEDs,n (%) | 18 (69.23%) | |
| Concomitant AEDs, n (%) | Levetiracetam | 14 (53.84%) |
| Valproate | 11 (42.31%) | |
| Perampanel | 11 (42.31%) | |
| Clobazam | 9 (34.62%) | |
| Topiramate | 8 (30.77%) | |
| Lamotrigine | 8 (30.77%) | |
| Clonazepam | 5 (19.23%) | |
| Zonisamide | 4 (15.38%) | |
| Carbamazepine | 4 (15.38%) | |
| Vigabatrin | 4 (15.38%) | |
| Phenytoin | 2 (7.69%) | |
| Oxcarbazepine | 1 (3.85%) |
| Characteristics | Normal Range (Adult) | Baseline | After 6 Months of Supplementation | Comparison | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t/χ2 | 95% CI | p-Value | ||
| Seizure frequency, per 28 days | 9.04 ± 18.16 | 2.06 ± 3.89 | 2.13 | (0.18–13.78) | 0.045 * | |
| Serum vitamin level | ||||||
| B6, nmol/L | 20–202 | 207.09 ± 215.64 | 200.75 ± 237.34 | 0.11 | (−115.09–−127.76) | 0.911 |
| B9, ng/mL | 4.6–34.8 | 8.39 ± 4.12 | 48.40 ± 32.38 | −4.31 | (−60.24–−19.77) | 0.001 ** |
| D, ng/mL | 30–100 | 26.21 ± 13.64 | 33.90 ± 11.55 | −3.05 | (−13.18–−2.20) | 0.010 ** |
| E, nmol/mL | 11.6–39.5 | 38.16 ± 51.19 | 29.47 ± 51.44 | 2.01 | (−0.73–18.13) | 0.068 |
| Q10, ng/mL | 360–1590 | 2947.61 ± 856.08 | 1725.32 ± 450.91 | 5.98 | (777.25–1667.33) | <0.001 *** |
| Body profile | ||||||
| Body weight, kg | 62.42 ± 14.17 | 63.20 ± 14.52 | −1.45 | (−1.89–0.33) | 0.161 | |
| BMI, kg/m2 | 23.59 ± 4.51 | 23.92 ± 4.89 | −1.65 | (−0.76–0.09) | 0.113 | |
| Waist circumference, cm | 85.14 ± 8.16 | 85.34 ± 8.59 | −0.23 | (−2.08–1.67) | 0.822 | |
| Lipid profile | ||||||
| Cholesterol, mg/dL | <200 | 185.82 ± 28.79 | 180.91 ± 29.35 | 1.33 | (−2.75–12.57) | 0.197 |
| HDL, mg/dL | >40 | 66.27 ± 17.59 | 67.18 ± 18.42 | −0.49 | (−4.78–2.96) | 0.630 |
| LDL, mg/dL | <100 | 115.82 ± 31.15 | 111.45 ± 32.61 | 1.77 | (−0.76–9.48) | 0.091 |
| TG, mg/dL | <150 | 87.62 ± 43.97 | 104.05 ± 42.66 | −1.94 | (−34.08–1.22) | 0.066 |
| Sugar profile | ||||||
| AC glucose, mg/dL | 60–99 | 86.59 ± 7.03 | 90.09 ± 13.47 | −1.72 | (−7.73–0.73) | 0.100 |
| Insulin, uIU/mL | 4–16 | 9.16 ± 6.15 | 12.50 ± 9.57 | −2.05 | (−6.73–0.05) | 0.053 |
| HOMA-IR | <1.9 | 1.98 ± 1.37 | 2.89 ± 2.40 | −2.14 | (−1.79–−0.03) | 0.044 * |
| McAuley Index | <5.8 | 8.31 ± 2.18 | 7.27 ± 1.76 | 2.47 | (0.16–1.93) | 0.023 * |
| HbA1c, % | 4.8–5.9 | 5.24 ± 0.43 | 5.08 ± 0.43 | 2.29 | (0.01–0.30) | 0.034 * |
| Characteristics | Baseline | After 6 Months of Supplementation | Comparison | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t | 95% CI | p-Value | |
| Serum vitamin level | |||||
| B6, nmol/L | 247.87 ± 228.00 | 163.93 ± 205.43 | 1.60 | (−26.00–193.87) | 0.126 |
| B9, ng/mL | 8.58 ± 4.23 | 32.93 ± 9.27 | −10.20 | (−29.36–−19.33) | <0.001 *** |
| D, ng/mL | 26.53 ± 15.68 | 32.43 ± 11.05 | −2.02 | (−12.04–0.24) | 0.059 |
| E, nmol/mL | 30.24 ± 42.73 | 17.64 ± 29.07 | 2.39 | (1.51–23.69) | 0.028 * |
| Q10, ng/mL | 2812.49 ± 842.07 | 1885.57 ± 514.14 | 4.90 | (529.38–1324.47) | <0.001 *** |
| Seizure Frequency (Per 28 Days) ∀ (n = 26) | Treatment Response Rate ※ (n = 26) | |||||
|---|---|---|---|---|---|---|
| Parameters | Estimate (SE) | Wald χ2 | p-Value | Estimate (SE) | Wald χ2 | p-Value |
| Intercept | 8.12 ± 2.36 | 11.87 | 0.001 *** | 0.00 ± 10.35 | 0.00 | 1.000 |
| Visit | ||||||
| 6 months | −6.59 ± 2.21 | 8.88 | 0.003 ** | −39.29 ± 13.07 | 9.04 | 0.003 ** |
| 3 months | −5.45 ± 2.21 | 6.06 | 0.014 * | −38.45 ± 13.07 | 8.66 | 0.003 ** |
| 1 month | −2.21 ± 2.18 | 1.03 | 0.310 | −19.29 ± 12.89 | 2.24 | 0.135 |
| Baseline (reference) | 0 | 0 | ||||
| Seizure Frequency (Per 28 Days) ∀ (n = 26) | Treatment Response Rate ※ (n = 26) | |||||
|---|---|---|---|---|---|---|
| Parameters | Estimate (SE) | Wald χ2 | p-Value | Estimate (SE) | Wald χ2 | p-Value |
| Intercept | 10.99 ± 3.23 | 11.56 | 0.001 *** | 5.08 ± 14.93 | 0.116 | 0.734 |
| Visit | ||||||
| 6 months | −8.16 ± 3.14 | 6.76 | 0.009 ** | −50.78 ± 17.48 | 8.44 | 0.004 ** |
| 3 months | −8.25 ± 3.15 | 6.86 | 0.009 ** | −39.36 ± 17.61 | 5.00 | 0.025 ** |
| 1 month | −3.47 ± 3.03 | 1.31 | 0.253 | −16.91 ± 17.02 | 0.99 | 0.320 |
| Baseline (reference) | 0 | 0 | ||||
| Compliance # | −3.66 ± 3.15 | 1.35 | 0.246 | −8.12 ± 16.26 | 0.25 | 0.617 |
| Visit Compliance | ||||||
| 6 months | 1.68 ± 3.49 | 0.23 | 0.631 | 22.68 ± 19.35 | 1.37 | 0.241 |
| 3 months | 3.99 ± 3.37 | 1.41 | 0.236 | 1.80 ± 18.72 | 0.01 | 0.923 |
| 1 month | 0.75 ± 3.64 | 0.04 | 0.836 | −18.39 ± 20.44 | 0.81 | 0.368 |
| Baseline (reference) | 0 | 0 | ||||
| MMAS (0~1 Month) | MMAS (1~3 Month) | MMAS (3~6 Month) | |
|---|---|---|---|
| Vitamin usage (0~1 month) | −0.999 (p < 0.001 ***) | - | - |
| Vitamin usage (1~3 month) | - | −0.798 (p < 0.001 ***) | - |
| Vitamin usage (3~6 month) | - | - | −0.633 (p < 0.001 ***) |
| Adverse Event | Patients, n (%) | Comedications |
|---|---|---|
| Dizziness a | 1 (3.33) | PER, LEV, OXC |
| Insomnia a | 1 (3.33) | CBZ, PER, VGB, ZNS |
| Skin rashes a | 2 (6.67) | VPA, LTG |
| CBZ, PER, TPM, VPA | ||
| Seizure worsening b | 3 (10) | LEV, PER |
| LEV, LTG | ||
| LEV |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.H.; Sung, P.-S.; Liao, W.C.; Chang, A.Y.W.; Hsiao, Y.-H.; Fu, T.-F.; Huang, C.-Y.; Huang, C.-W. An Open Pilot Study of the Effect and Tolerability of Add-On Multivitamin Therapy in Patients with Intractable Focal Epilepsy. Nutrients 2020, 12, 2359. https://doi.org/10.3390/nu12082359
Chang HH, Sung P-S, Liao WC, Chang AYW, Hsiao Y-H, Fu T-F, Huang C-Y, Huang C-W. An Open Pilot Study of the Effect and Tolerability of Add-On Multivitamin Therapy in Patients with Intractable Focal Epilepsy. Nutrients. 2020; 12(8):2359. https://doi.org/10.3390/nu12082359
Chicago/Turabian StyleChang, Hui Hua, Pi-Shan Sung, Wei Chen Liao, Alice Y. W. Chang, Ya-Hsin Hsiao, Tzu-Fun Fu, Chin-Ying Huang, and Chin-Wei Huang. 2020. "An Open Pilot Study of the Effect and Tolerability of Add-On Multivitamin Therapy in Patients with Intractable Focal Epilepsy" Nutrients 12, no. 8: 2359. https://doi.org/10.3390/nu12082359
APA StyleChang, H. H., Sung, P.-S., Liao, W. C., Chang, A. Y. W., Hsiao, Y.-H., Fu, T.-F., Huang, C.-Y., & Huang, C.-W. (2020). An Open Pilot Study of the Effect and Tolerability of Add-On Multivitamin Therapy in Patients with Intractable Focal Epilepsy. Nutrients, 12(8), 2359. https://doi.org/10.3390/nu12082359






