Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study
Abstract
1. Introduction
2. Materials and Methods
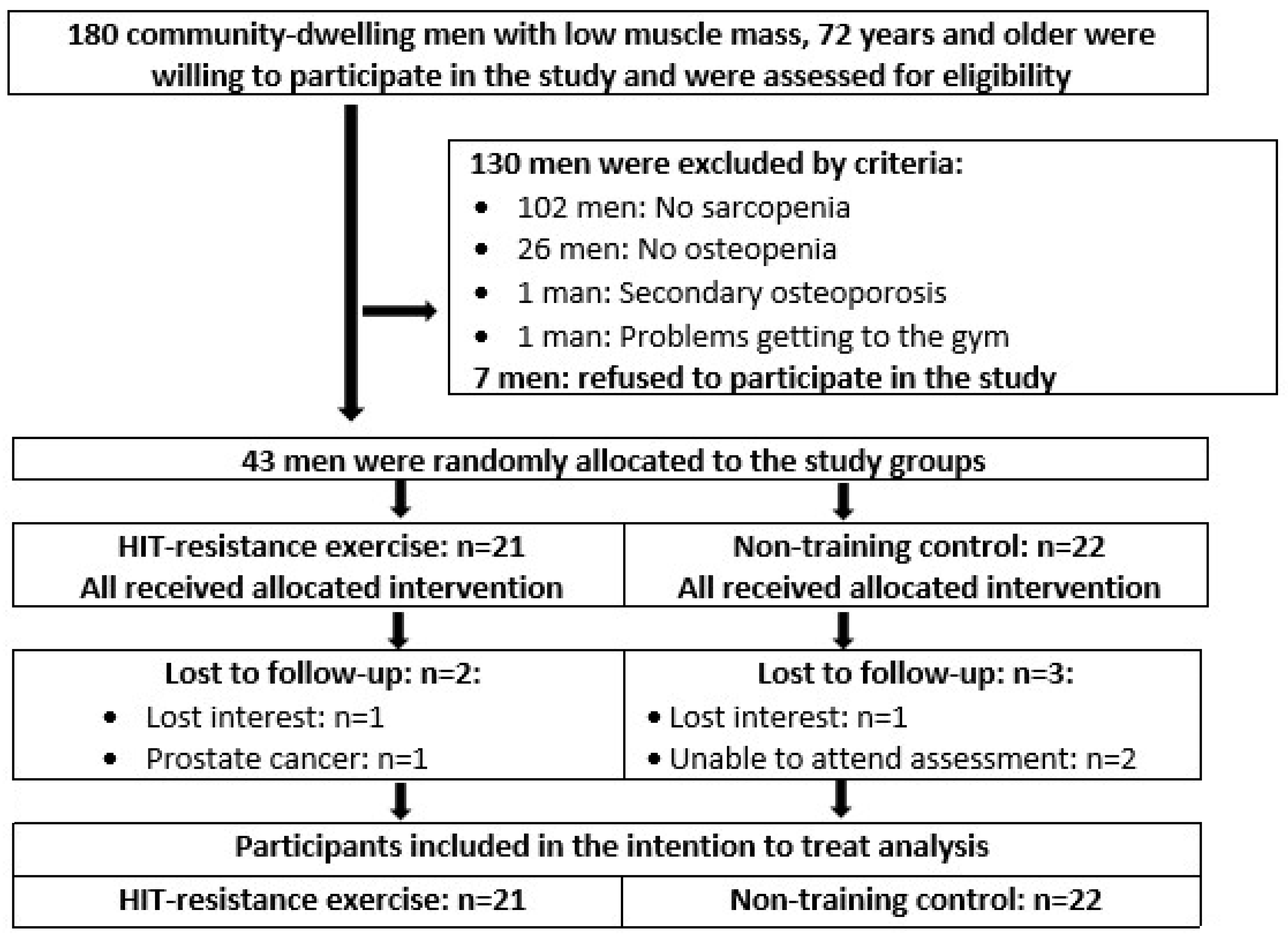
2.1. Participants
2.2. Randomization Procedures
2.3. Blinding
2.4. Study Procedure
2.5. Interventions
2.5.1. Dietary Supplementation
2.5.2. Vitamin D and Calcium Supplementation
2.5.3. Resistance Exercise
2.5.4. Compliance with the Intervention
2.6. Study Outcomes
2.6.1. Primary Study Outcomes
- Sarcopenia Z-Score according to EWGSOP I [19] at baseline and immediately post-intervention (18 months).
- Areal bone mineral density (aBMD) at the Lumbar Spine (LS) as determined by Dual Energy X-ray absorptiometry (DXA) at baseline and immediately post-intervention.
- Areal bone mineral density (aBMD) at the total hip as determined by DXA at baseline and immediately post-intervention.
2.6.2. Secondary (i.e., Explanatory) Study Outcomes Related to Sarcopenia
- Skeletal Muscle Mass Index (SMI) as determined by Dual-Energy Absorptiometry (DXA) at baseline and immediately post-intervention.
- Handgrip strength at baseline and immediately post-intervention.
- Gait velocity at baseline and immediately post-intervention.
2.6.3. Changes in Trial Outcomes after Trial Commencement
2.7. Assessments
2.8. Sample Size Analysis
2.9. Statistical Analysis
3. Results
3.1. Participant and Exercise Characteristics
3.2. Primary Study Outcomes
3.3. Secondary Study Outcomes
3.4. Confounding Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2019, 67, 831–840. [Google Scholar] [CrossRef]
- Sánchez, A.; Vidal, M.; Vidal Neira, L.F.; Messina, O.D. Sarcopenia, Osteoporosis and Frailty a Triad Comes of Age. J. Ann. Arthritis Clin. Rheumatol. 2020, 3, 2017. [Google Scholar]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Muller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone-Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef]
- Pagnotti, G.M.; Styner, M.; Uzer, G.; Patel, V.S.; Wright, L.E.; Ness, K.K.; Guise, T.A.; Rubin, J.; Rubin, C.T. Combating osteoporosis and obesity with exercise: Leveraging cell mechanosensitivity. Nat. Rev. Endocrinol. 2019, 15, 339–355. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Benichou, O.; Lord, S.R. Rationale for Strengthening Muscle to Prevent Falls and Fractures: A Review of the Evidence. Calcif. Tissue Int. 2016, 98, 531–545. [Google Scholar] [CrossRef]
- Shojaa, M.; von Stengel, V.; Kohl, M.; Schoene, D.; Kemmler, W. Effects of dynamic resistance exercise on Bone Mineral Density in postmenopausal women- A systematic review and meta-analysis with special emphasis to exercise parameters. Osteo. Int. 2020, 31, 1427–1444. [Google Scholar] [CrossRef]
- Helge, E.W.; Andersen, T.R.; Schmidt, J.F.; Jorgensen, N.R.; Hornstrup, T.; Krustrup, P.; Bangsbo, J. Recreational football improves bone mineral density and bone turnover marker profile in elderly men. Scand. J. Med. Sci. Sports 2014, 24, 98–104. [Google Scholar] [CrossRef]
- McCartney, N.; Hicks, A.L.; Martin, J.; Webber, C.E. Long-term resistance training in the elderly: Effects on dynamic strength, exercise capacity, muscle, and bone. J. Gerontol. 1995, 50, B97–B104. [Google Scholar] [CrossRef]
- Whiteford, J.; Ackland, T.R.; Dhaliwal, S.S.; James, A.P.; Woodhouse, J.J.; Price, R.; Prince, R.L.; Kerr, D.A. Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos. Int. 2010, 21, 1529–1536. [Google Scholar] [CrossRef]
- Woo, J.; Hong, A.; Lau, E.; Lynn, H. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing 2007, 36, 262–268. [Google Scholar] [CrossRef]
- World_Medical_Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Lichtenberg, T.; von Stengel, S.; Sieber, C.; Kemmler, W. The favorable effects of a high-intensity resistance training on sarcopenia in older community-dwelling men with osteosarcopenia: The randomized controlled FrOST study. Clin. Interv. Aging 2019, 14, 2173–2186. [Google Scholar] [CrossRef]
- Kemmler, W.; Weineck, M.; Kohl, M.; von Stengel, S.; Giessing, J.; Fröhlich, M.; Schoene, D. High Intensity Resistance Exercise Training to Improve Body Composition and Strength in Older Men With Osteosarcopenia. Results of the Randomized Controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). Front. Sports Active Living 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Frohlich, M.; Jakob, F.; Engelke, K.; von Stengel, S.; Schoene, D. Effects of High Intensity Resistance Training on Osteopenia and Sarcopenia parameters in Older Men with Osteosarcopenia-One-year results of the randomized controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). J. Bone Miner. Res. 2020. [Google Scholar] [CrossRef]
- Kemmler, W.; von Stengel, S.; Schoene, D. Longitudinal changes in muscle mass and function in older men at increased risk for sarcopenia–the FrOST-study. JOFA 2019, 8, 57–61. [Google Scholar]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- WHO. Assessment of Osteoporotic Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis; Technical Report Series no. 843; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- DVO. Prophylaxe, Diagnostik und Therapie der Osteoporse bei Postmenopausalen Frauen und bei Männern; Schattauer: Stuttgart, Germany, 2017. [Google Scholar]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. J. Int. Soc. Sports Nutr. 2013, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Gießing, J. HIT-Hochintensitätstraining; Novagenics-Verlag: Arnsberg, Germany, 2008. [Google Scholar]
- Steele, J.; Fisher, J.; Giessing, J.; Gentil, P. Clarity in Reporting Terminology and Definitions of Set End Points in Resistance Training. Muscle Nerve 2017, 56, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Wittke, A.; Bebenek, M.; Fröhlich, M.; von Stengel, S. High intensity resistance training methods with and without protein supplementation to fight cardiometabolic risk in middle-aged males A randomized controlled trial. BioMed Res. Int. 2016. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Fröhlich, M.; Kohl, M.; von Stengel, S. Ganzkörper-Elektromyostimulationst versus HIT-Krafttraining-Effekte auf Körperzusammensetzung und Muskelkraft. Dtsch. Z. Sportmed. 2015, 66, 321–327. [Google Scholar] [CrossRef]
- Zourdos, M.C.; Klemp, A.; Dolan, C.; Quiles, J.M.; Schau, K.A.; Jo, E.; Helms, E.; Esgro, B.; Duncan, S.; Garcia Merino, S.; et al. Novel Resistance Training-Specific Rating of Perceived Exertion Scale Measuring Repetitions in Reserve. J. Strength Cond. Res. 2016, 30, 267–275. [Google Scholar] [CrossRef]
- Kemmler, W.; Lauber, D.; Mayhew, J.; Wassermann, F. Repetition to Fatigue to Predict 1RM Performance. Looking behind the Covariates; Cuvillier Verlag: Göttingen, Germany, 2008; Volume 2, pp. 79–92. [Google Scholar]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Strom, O.; Borgstrom, F. Development and use of FRAX in osteoporosis. Osteoporos. Int. 2010, 21, S407–S413. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older, P.; the Extended Group for, E., Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body Electromyostimulation to Fight Sarcopenic Obesity in Community-Dwelling Older Women at Risk. Results of the Randomized Controlled FORMOsA-Sarcopenic Obesity Study. Osteo. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef]
- Kemmler, W.; Weissenfels, A.; Teschler, M.; Willert, S.; Bebenek, M.; Shojaa, M.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Whole-body Electromyostimulation and protein supplementation favorably affect Sarcopenic Obesity in community-dwelling older men at risk. The Randomized Controlled FranSO Study. Clin. Interv. Aging 2017, 12, 1503–1513. [Google Scholar] [CrossRef]
- Kressig, R.W.; Beauchet, O.; European, G.N.G. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin. Exp. Res. 2006, 18, 174–176. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Weber, K.; Volland, G.; Kashman, N. Reliability and validity of grip and pinch strength evaluations. J. Hand Surg. 1984, 9, 222–226. [Google Scholar] [CrossRef]
- Kemmler, W.; Lauber, D.; Weineck, J.; Hensen, J.; Kalender, W.; Engelke, K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: Results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch. Intern. Med. 2004, 164, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Weineck, J.; Kalender, W.A.; Engelke, K. The effect of habitual physical activity, non-athletic exercise, muscle strength, and VO2max on bone mineral density is rather low in early postmenopausal osteopenic women. J. Musculoskelet. Neuronal Interact. 2004, 4, 325–334. [Google Scholar]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Honaker, J.; King, G.; Blackwell, M. Amelia II: A program for missing data. JSS 2011, 45, 1–47. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associate: Hillsdale, NJ, USA, 1988; pp. 8–16. [Google Scholar]
- Li, G.; Taljaard, M.; Van den Heuvel, E.R.; Levine, M.A.; Cook, D.J.; Wells, G.A.; Devereaux, P.J.; Thabane, L. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int. J. Epidemiol. 2017, 46, 746–755. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bauer, J.M.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cruz-Jentoft, A.J.; Dicker, D.; Fruhbeck, G.; Giustina, A.; et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin. Nutr. 2019, 39, 2368–2388. [Google Scholar] [CrossRef]
- Bauer, J.M.; Cruz-Jentoft, A.J.; Fielding, R.A.; Kanis, J.A.; Reginster, J.Y.; Bruyere, O.; Cesari, M.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; et al. Is There Enough Evidence for Osteosarcopenic Obesity as a Distinct Entity? A Critical Literature Review. Calcif. Tissue Int. 2019, 105, 109–124. [Google Scholar] [CrossRef]
- Schafer, I.; von Leitner, E.C.; Schon, G.; Koller, D.; Hansen, H.; Kolonko, T.; Kaduszkiewicz, H.; Wegscheider, K.; Glaeske, G.; van den Bussche, H. Multimorbidity patterns in the elderly: A new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS ONE 2010, 5, e15941. [Google Scholar] [CrossRef]
- Binkley, N.; Buehring, B. Beyond FRAX: It’s time to consider “sarco-osteopenia”. J. Clin. Densitom. 2009, 12, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Hita-Contreras, F.; Bueno-Notivol, J.; Martinez-Amat, A.; Cruz-Diaz, D.; Hernandez, A.V.; Perez-Lopez, F.R. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: A meta-analysis of randomized controlled trials. Maturitas 2018, 116, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Shojaa, M.; Kohl, M.; von Stengel, S. Exercise effects on bone mineral density in older men: A systematic review with special emphasis on study interventions. Osteoporos. Int. 2018, 29, 1493–1504. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Kohrt, W.M. Exercise and bone mineral density in premenopausal women: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2013, 2013, 741639. [Google Scholar]
- DiGirolamo, D.J.; Kiel, D.P.; Esser, K.A. Bone and skeletal muscle: Neighbors with close ties. J. Bone Miner. Res. 2013, 28, 1509–1518. [Google Scholar] [CrossRef]
- Laurent, M.R.; Dubois, V.; Claessens, F.; Verschueren, S.M.; Vanderschueren, D.; Gielen, E.; Jardi, F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]
- Beck, B.R.; Daly, R.M.; Singh, M.A.; Taaffe, D.R. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 2016, 20, 438–445. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef]
- Turner, C.H.; Owan, I.; Takano, Y. Mechanotransduction in bone: Role of strain rate. Am. J. Physiol. Endocrinol. Metab. 1995, 269, E438–E442. [Google Scholar] [CrossRef] [PubMed]
- Von Stengel, S.; Kemmler, W.; Lauber, D.; Weineck, J.; Kalender, W.A.; Engelke, K. Power Training is more Effective than Strength Training to Maintain Bone Mineral Density in Postmenopausal Woman. J. Appl. Physiol. 2005, 99, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Forwood, M.R.; Otter, M.W. Mechanotransduction in bone: Do bone cells act as sensors of fluid flow? Faseb. J. 1994, 8, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Robling, A.G. Exercise as an anabolic stimulus for bone. Curr. Pharm. Des. 2004, 10, 2629–2641. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.T.; Villanueva, M.; West, D.D.; Phillips, S.M. Are acute post-resistance exercise increases in testosterone, growth hormone, and IGF-1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Med. Sci. Sports Exerc. 2013, 45, 2044–2051. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Turner, C.H. Homeostatic control of bone structure: An application of feedback theory. Bone 1991, 12, 203–217. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef]
- Marsh, A.P.; Miller, M.E.; Rejeski, W.J.; Hutton, S.L.; Kritchevsky, S.B. Lower extremity muscle function after strength or power training in older adults. J. Aging Phys. Act. 2009, 17, 416–443. [Google Scholar] [CrossRef]
- Antoniak, A.E.; Greig, C.A. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: A systematic review and meta-analysis. BMJ Open 2017, 7, e014619. [Google Scholar] [CrossRef]
- Borde, R.; Hortobagyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Csapo, R.; Alegre, L.M. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand. J. Med. Sci Sports 2016, 26, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Finger, D.; Goltz, F.R.; Umpierre, D.; Meyer, E.; Rosa, L.H.; Schneider, C.D. Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis. Sports Med. 2015, 45, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Wu, Y.T.; Cheng, C.P.; Chen, H.C.; Huang, Y.C.; Chen, H.C.; Liou, T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef]
- Tieland, M.; Verdijk, L.B.; de Groot, L.C.; van Loon, L.J. Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 27–36. [Google Scholar] [CrossRef]
- Clark, D.J.; Manini, T.M.; Fielding, R.A.; Patten, C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp. Gerontol. 2013, 48, 358–363. [Google Scholar] [CrossRef]
- Sakari, R.; Era, P.; Rantanen, T.; Leskinen, E.; Laukkanen, P.; Heikkinen, E. Mobility performance and its sensory, psychomotor and musculoskeletal determinants from age 75 to age 80. Aging Clin. Exp. Res. 2010, 22, 47–53. [Google Scholar] [CrossRef]
- Smith-Ray, R.L.; Hughes, S.L.; Prohaska, T.R.; Little, D.M.; Jurivich, D.A.; Hedeker, D. Impact of Cognitive Training on Balance and Gait in Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015, 70, 357–366. [Google Scholar] [CrossRef]
- Lemke, M.R.; Wendorff, T.; Mieth, B.; Buhl, K.; Linnemann, M. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J. Psychiatr. Res. 2000, 34, 277–283. [Google Scholar] [CrossRef]
- Kukuljan, S.; Nowson, C.A.; Sanders, K.M.; Nicholson, G.C.; Seibel, M.J.; Salmon, J.; Daly, R.M. Independent and combined effects of calcium-vitamin D3 and exercise on bone structure and strength in older men: An 18-month factorial design randomized controlled trial. J. Clin. Endocrinol. Metab. 2011, 96, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Bebenek, M.; Kohl, M.; Von Stengel, S. Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporos. Int. 2015, 26, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 3, CD002759. [Google Scholar] [CrossRef] [PubMed]
- Mangione, K.K.; Miller, A.H.; Naughton, I.V. Cochrane review: Improving physical function and performance with progressive resistance strength training in older adults. Phys. Ther. 2010, 90, 1711–1715. [Google Scholar] [CrossRef]
- Teschler, M.; Weissenfels, A.; Bebenek, M.; Frohlich, M.; Kohl, M.; von Stengel, S.; Kemmler, W. Very high creatine kinase CK levels after WB_EMS. Are there implications for health. Int. J. Clin. Exp. Med. 2016, 9, 22841–22850. [Google Scholar]
- Cuenca-Sanchez, M.; Navas-Carrillo, D.; Orenes-Pinero, E. Controversies surrounding high-protein diet intake: Satiating effect and kidney and bone health. Adv. Nutr. 2015, 6, 260–266. [Google Scholar] [CrossRef]
- Kemmler, W.; von Stengel, S.; Kohl, M.; Rohleder, N.; Bertsch, T.; Sieber, C.C.; Freiberger, E.; Kob, R. Safety of a Combined WB-EMS and High-Protein Diet Intervention in Sarcopenic Obese Elderly Men. Clin. Interv. Aging 2020, 15, 953–967. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C.; et al. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef]
- Beckwee, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Gade, J.; Pedersen, R.; M, B. Effect of protein or essential amino acid supplementation during prolonged exercise training in older adults on body composition, muscle strength, and physical performance parameters: A systematic review. Rehabil. Process Outcome 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Carlson, S.A.; Fulton, J.E.; Schoenborn, C.A.; Loustalot, F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am. J. Prev. Med. 2010, 39, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kob, R.; Bollheimer, L.C.; Bertsch, T.; Fellner, C.; Djukic, M.; Sieber, C.C.; Fischer, B.E. Sarcopenic obesity: Molecular clues to a better understanding of its pathogenesis? Biogerontology 2015, 16, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef]
| Variable | CG (n = 22) MV ± SD | HIT-RT (n = 21) MV ± SD | p |
|---|---|---|---|
| Age [years] | 79.2 ± 4.7 | 77.8 ± 3.6 | 0.262 |
| Body Mass Index (kg/m2) | 24.5 ± 1.9 | 25.0 ± 3.0 | 0.515 |
| Total Body Fat (DXA) (%) | 33.6 ± 4.0 | 34.5 ± 6.1 | 0.563 |
| Osteoporosis (n) a | 8 | 7 | 0.757 |
| More than two diseases (n) b | 12 | 10 | 0.826 |
| Lower limb arthritis (n) b | 2 | 2 | 0.959 |
| Diabetes Mellitus type II (n) | 1 | 1 | 0.960 |
| Habitual gait velocity (m/s) | 1.26 ± 0.15 | 1.25 ± 0.17 | 0.703 |
| Handgrip strength (kg) | 30.0 ± 4.3 | 30.7 ± 5.1 | 0.675 |
| Physical activity (Index)c | 4.15 ± 1.53 | 4.45 ± 1.32 | 0.490 |
| Exercise ≥1x week (n) | 5 | 5 | 0.931 |
| 25-OHD (ng/mL) d | 17.5 ± 7.0 | 21.6 ± 8.4 | 0.126 |
| Calcium intake (mg/d) e | 833 ± 282 | 802 ± 226 | 0.636 |
| Energy intake (kcal/d) f | 2291 ± 590 | 2155 ± 416 | 0.407 |
| Protein intake (g/kg/d) f | 1.29 ± 0.34 | 1.10 ± 0.25 | 0.043 |
| CG MV (95% CI) | HIT-RT MV (95% CI) | Difference MV (95% CI) | p-Value | |
|---|---|---|---|---|
| Sarcopenia Z-Score | ||||
| Baseline | −2.14 (−1.45 to −2.83) | −2.51 (−1.45 to −3.65) | ------------- | 0.558 |
| Changes | 0.48 (0.13 to 0.82) | −0.83 (−0.49 to −1.17) | 1.31 (0.74 to 1.89) | <0.001 * |
| Areal bone mineral density at the lumbar spine (mg/cm2) | ||||
| Baseline | 0.987 (0.916 to 1.060) | 1.054 (0.981 to 1.122) | ------------ | 0.140 |
| Changes | −0.001 (−0.008 to 0.005) | 0.011 (0.004 to 0.017) | 0.012 (0.001 to −0.020) | 0.024 * |
| Areal bone mineral density at the total hip (mg/cm2) | ||||
| Baseline | 0.869 (0.826 to 0.911) | 0.894 (0.856 to 0.932) | ------------ | 0.364 |
| Changes | −0.013 (−0.021 to −0.007) | −0.000 (−0.008 to 0.006) | 0.013 (0.002 to 0.022) | 0.025 * |
| CG MV (95% CI) | HIT-RT MV (95% CI) | Difference MV (95% CI) | p-Value | |
|---|---|---|---|---|
| Skeletal Muscle Mass Index (SMI) (kg/m2) | ||||
| Baseline | 6.89 (6.74 to 7.02) | 7.01 (6.85 to 7.16) | ------------- | 0.671 |
| Changes | −0.09 (−0.16 to −0.02) | 0.26 (0.18 to 0.33) | 0.34 (0.23 to 0.45) | <0.001 * |
| Handgrip strength (kg) | ||||
| Baseline | 30.0 ± 4.3 | 30.7 ± 5.1 | ------------- | 0.675 |
| Changes | −0.52 (−1.60 to 0.53) | 2.13 (0.91 to 3.39) | 2.65 (0.75 to 4.56) | 0.008 * |
| Gait velocity (m/s) | ||||
| Baseline | 1.26 ± 0.15 | 1.25 ± 0.17 | ------------- | 0.803 |
| Changes | −0.03 (−0.05 to −0.01) | 0.00 (−0.02 to 0.02) | 0.02 (−0.06 to 0.01) | 0.209 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemmler, W.; Kohl, M.; Jakob, F.; Engelke, K.; von Stengel, S. Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients 2020, 12, 2341. https://doi.org/10.3390/nu12082341
Kemmler W, Kohl M, Jakob F, Engelke K, von Stengel S. Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients. 2020; 12(8):2341. https://doi.org/10.3390/nu12082341
Chicago/Turabian StyleKemmler, Wolfgang, Matthias Kohl, Franz Jakob, Klaus Engelke, and Simon von Stengel. 2020. "Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study" Nutrients 12, no. 8: 2341. https://doi.org/10.3390/nu12082341
APA StyleKemmler, W., Kohl, M., Jakob, F., Engelke, K., & von Stengel, S. (2020). Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients, 12(8), 2341. https://doi.org/10.3390/nu12082341






