A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females—Effect on Fatigue, Sleepiness, Mood and Body Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Endpoints
2.3. Sample Size Calculation
2.4. Statistics Evaluation
3. Results
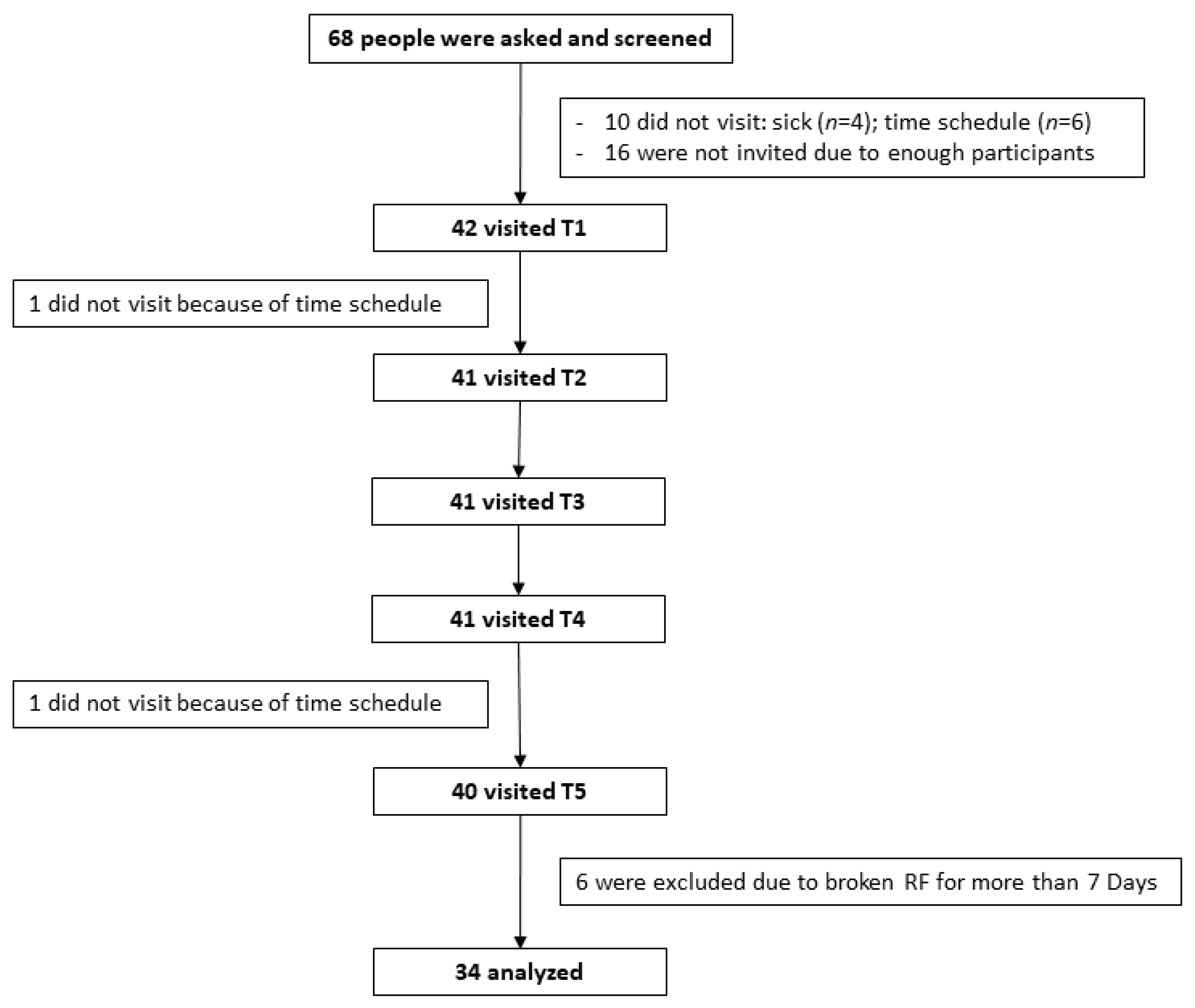
3.1. Recruitment of the Participants
3.2. Baseline Characteristics of Participants
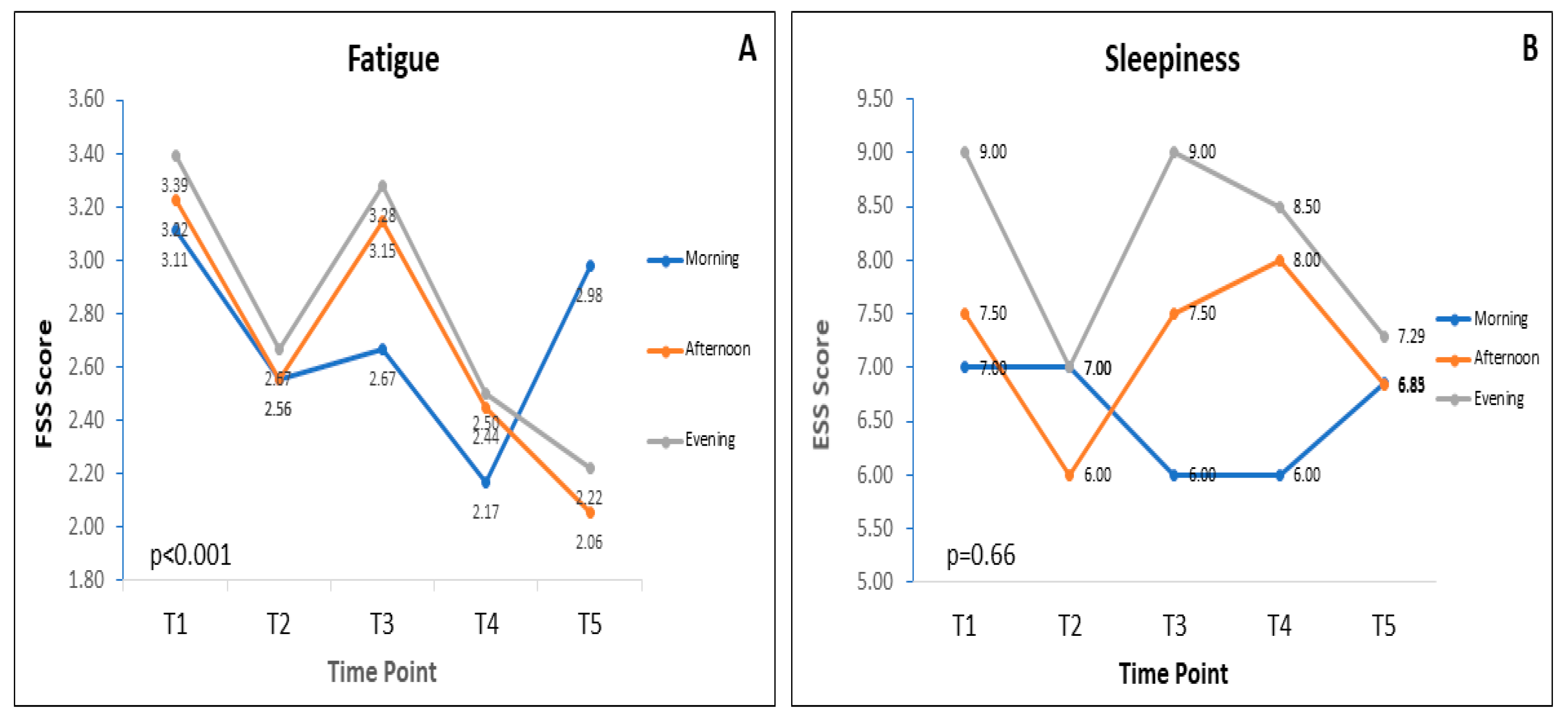
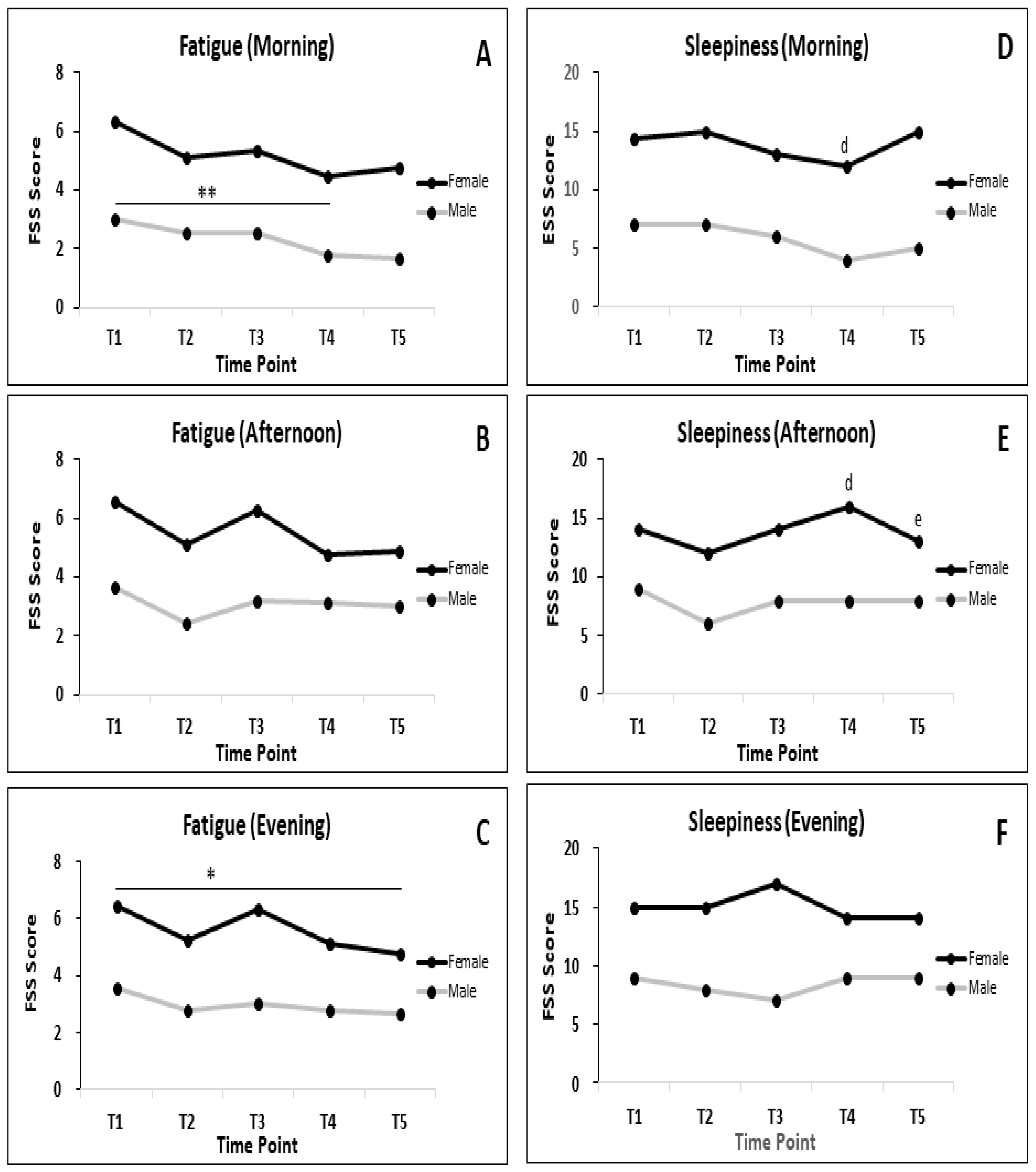
3.3. Fatigue and Sleepiness during RF
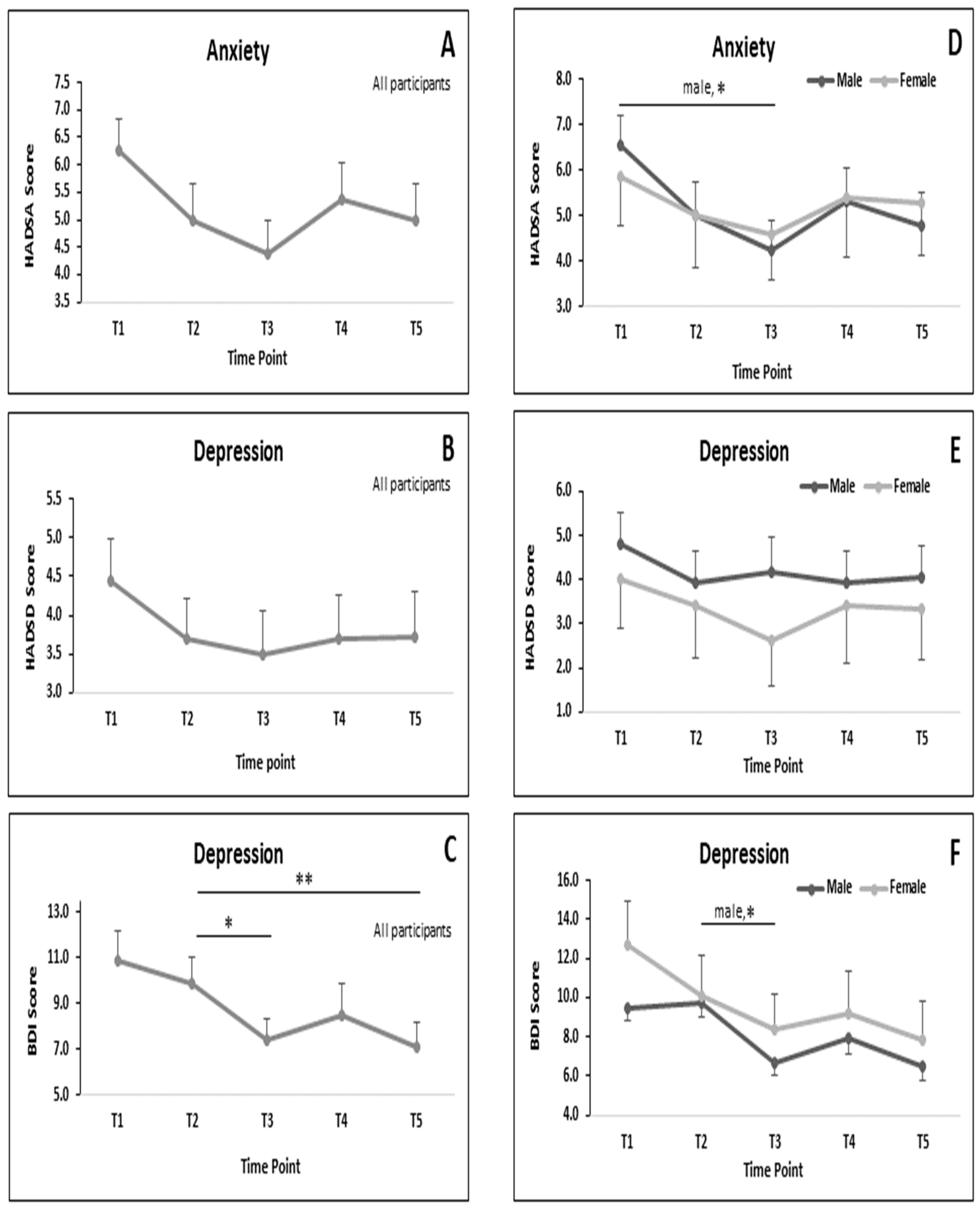
3.4. MRSs during RF
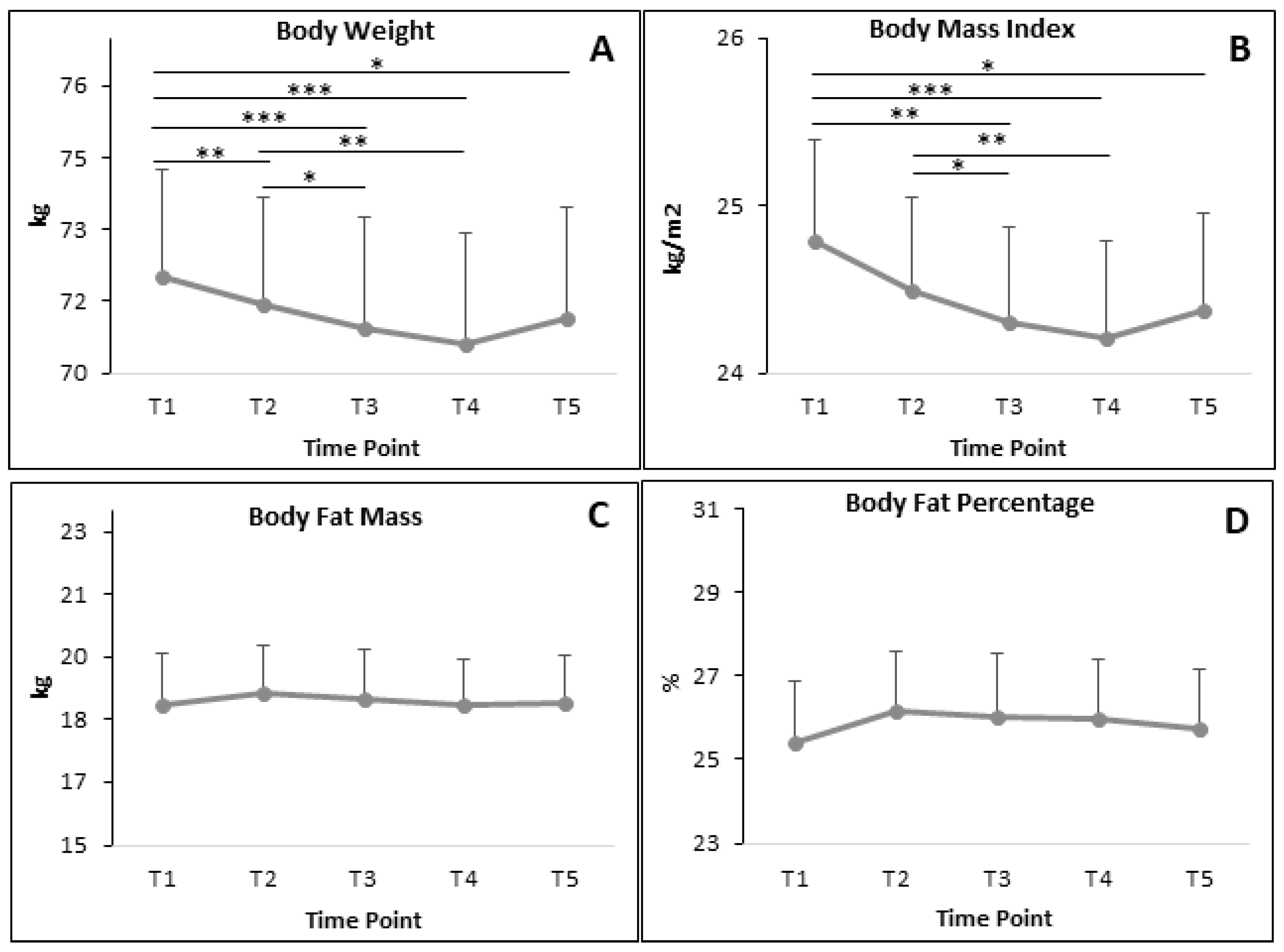
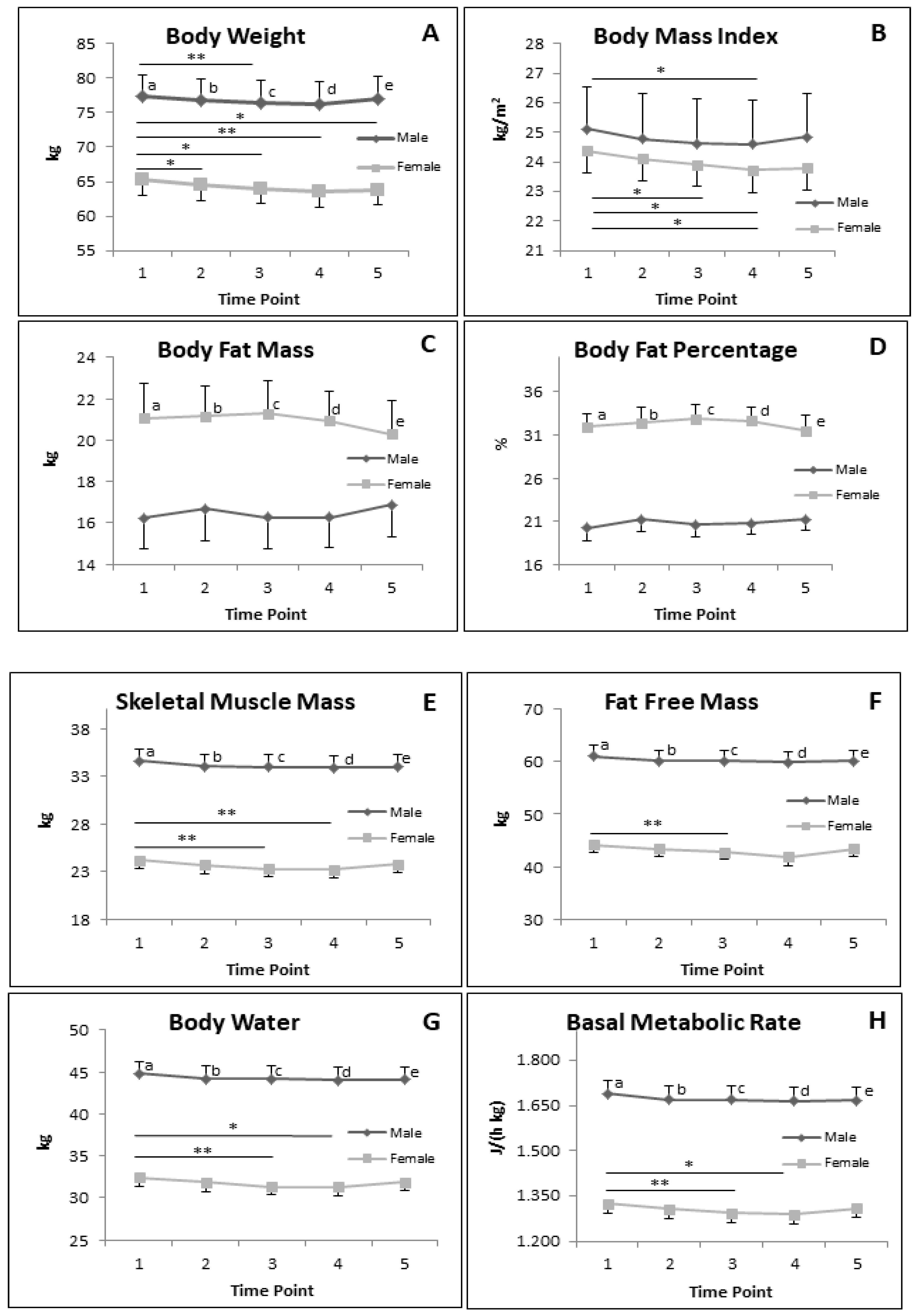
3.5. BC during RF
3.5.1. All Participants
3.5.2. BC Parameters in Subgroup of Male and Female during RF
4. Discussion
4.1. Fatigue and Sleepiness
4.2. Mood-Related Symptoms
4.3. BC Parameters
4.4. Effect Size
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martinez, M.E.; Villasenor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.W.; Cho, S.J.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Domaszewski, P.; Konieczny, M.; Pakosz, P.; Baczkowicz, D.; Sadowska-Krepa, E. Effect of a Six-Week Intermittent Fasting Intervention Program on the Composition of the Human Body in Women over 60 Years of Age. Int. J. Environ. Res. Public Health 2020, 17, 4138. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef]
- Malinowski, B.; Zalewska, K.; Wesierska, A.; Sokolowska, M.M.; Socha, M.; Liczner, G.; Pawlak-Osinska, K.; Wicinski, M. Intermittent Fasting in Cardiovascular Disorders-An Overview. Nutrients 2019, 11, 673. [Google Scholar] [CrossRef]
- Eldeeb, A.A.; Mahmoud, M.A.; Ibrahim, A.B.; Yousef, E.A.; Sabry, A.A. Effect of Ramadan fasting on arterial stiffness parameters among Egyptian hypertensive patients with and without chronic kidney disease. Saudi J. Kidney Dis. Transpl. 2020, 31, 582–588. [Google Scholar] [CrossRef]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 1–25. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Mattson, M.P.; de Cabo, R. Effects of Intermittent Fasting on Health, Aging, and Disease. Reply. N. Engl. J. Med. 2020, 382, 1773–1774. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Khedkar, P.H. Intermittent fasting-The new lifestyle? Acta Physiol. (Oxf.) 2020, e13518–e13520. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Alghamdi, K.A.; Jenkins, R.O.; Alghamdi, M.N.; Haris, P.I. Impact of Ramadan on Physical Activity and Sleeping Patterns in Individuals with Type 2 Diabetes: The First Study Using Fitbit Device. Diabetes Ther. 2020, 11, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Akif Buyukbese, M.; Malek, R.; Pilorget, V.; Naqvi, M.; Berthou, B.; Shaltout, I.; Kumar Sahay, R. Real-world safety and effectiveness of insulin glargine 300 U/mL in participants with type 2 diabetes who fast during Ramadan: The observational ORION study. Diabetes Res. Clin. Pract. 2020, 108113–108189. [Google Scholar] [CrossRef] [PubMed]
- Khazneh, E.; Qaddumi, J.; Hamdan, Z.; Qudaimat, F.; Sbitany, A.; Jebrin, K.; Sawalmeh, O.; Abuiram, Y.; Shraim, M. The effects of Ramadan fasting on clinical and biochemical markers among hemodialysis patients: A prospective cohort study. PLoS ONE 2019, 14, e0218745. [Google Scholar] [CrossRef]
- Adawi, M.; Watad, A.; Brown, S.; Aazza, K.; Aazza, H.; Zouhir, M.; Sharif, K.; Ghanayem, K.; Farah, R.; Mahagna, H.; et al. Ramadan Fasting Exerts Immunomodulatory Effects: Insights from a Systematic Review. Front. Immunol. 2017, 8, 1144–1157. [Google Scholar] [CrossRef]
- Nugraha, B.; Ghashang, S.K.; Hamdan, I.; Gutenbrunner, C. Effect of Ramadan fasting on fatigue, mood, sleepiness, and health-related quality of life of healthy young men in summer time in Germany: A prospective controlled study. Appetite 2017, 111, 38–45. [Google Scholar] [CrossRef]
- Ghashang, S.K.; Hamdan, I.; Lichtinghagen, R.; Gutenbrunner, C.; Nugraha, B. Alterations of Brain-Derived Neurotrophic Factor and Creatinine During Ramadan Fasting: A Prospective, Controlled Clinical Trial. Iran. Red Crescent Med. 2019, 21, e88324–e88328. [Google Scholar] [CrossRef]
- Al-Barha, N.S.; Aljaloud, K.S. The Effect of Ramadan Fasting on Body Composition and Metabolic Syndrome in Apparently Healthy Men. Am. J. Mens. Health 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Ovayolu, O.; Ovayolu, N.; Tasan, E. Does Ramadan Fasting Affect Fatigue in Nurses? Holist. Nurs. Pract. 2016, 30, 222–226. [Google Scholar] [CrossRef]
- Fekih, S.; Zguira, M.S.; Koubaa, A.; Masmoudi, L.; Bragazzi, N.L.; Jarraya, M. Effects of Motor Mental Imagery Training on Tennis Service Performance during the Ramadan Fasting: A Randomized, Controlled Trial. Nutrients 2020, 12, 1035. [Google Scholar] [CrossRef]
- Pak, I.E.; Cug, M.; Volpe, S.L.; Beaven, C.M. The effect of carbohydrate and caffeine mouth rinsing on kicking performance in competitive Taekwondo athletes during Ramadan. J. Sports Sci. 2020, 38, 795–800. [Google Scholar] [CrossRef]
- Madkour, M.I.; El-Serafi, A.T.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S.; Faris, M.A.E. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019, 155, 107801. [Google Scholar] [CrossRef] [PubMed]
- Ajabnoor, G.M.; Bahijri, S.; Shaik, N.A.; Borai, A.; Alamoudi, A.A.; Al-Aama, J.Y.; Chrousos, G.P. Ramadan fasting in Saudi Arabia is associated with altered expression of CLOCK, DUSP and IL-1alpha genes, as well as changes in cardiometabolic risk factors. PLoS ONE 2017, 12, e0174342. [Google Scholar] [CrossRef] [PubMed]
- Sadeghniiat-Haghighi, K.; Yazdi, Z. Fatigue management in the workplace. Ind. Psychiatry J. 2015, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Avlund, K.; Damsgaard, M.T.; Schroll, M. Tiredness as determinant of subsequent use of health and social services among nondisabled elderly people. J. Aging Health 2001, 13, 267–286. [Google Scholar] [CrossRef]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef]
- Valet, M.; Stoquart, G.; Glibert, Y.; Hakizimana, J.C.; Lejeune, T. Is fatigue associated with cardiorespiratory endurance among patients suffering from multiple sclerosis? Ann. Phys. Rehabil. Med. 2016, 59S, e41. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, X.J.; Nisi, R.A.; Martin, K.R.; Karaman, M.M.; Cai, K.; Weaver, T.E. Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness. JMRI 2016, 1371–1378. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Revista brasileira de psiquiatria 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Chaouachi, A.; Coutts, A.J.; Chamari, K.; Wong del, P.; Chaouachi, M.; Chtara, M.; Roky, R.; Amri, M. Effect of Ramadan intermittent fasting on aerobic and anaerobic performance and perception of fatigue in male elite judo athletes. J. Strength. Cond. Res. 2009, 23, 2702–2709. [Google Scholar] [CrossRef]
- Bahammam, A.S.; Almushailhi, K.; Pandi-Perumal, S.R.; Sharif, M.M. Intermittent fasting during Ramadan: Does it affect sleep? J. Sleep Res. 2014, 23, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Koushali, A.N.; Hajiamini, Z.; Ebadi, A.; Bayat, N.; Khamseh, F. Effect of Ramadan fasting on emotional reactions in nurses. Iran. J. Nurs. Midwifery Res. 2013, 18, 232–236. [Google Scholar] [PubMed]
- Smarr, K.L.; Keefer, A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res. 2011, 63, 454–466. [Google Scholar] [CrossRef]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, V.; Razaei, M.; Ahmadinejad, Z.; Shaikh, H.; Yousefi, R.; Yarmohammadi, L.; Bozorgi, F.; Behjati, M.J. The changes of metabolic profile and weight during Ramadan fasting. Singap. Med. J. 2006, 47, 409–414. [Google Scholar]
- Norouzy, A.; Salehi, M.; Philippou, E.; Arabi, H.; Shiva, F.; Mehrnoosh, S.; Mohajeri, S.M.; Mohajeri, S.A.; Motaghedi Larijani, A.; Nematy, M. Effect of fasting in Ramadan on body composition and nutritional intake: A prospective study. J. Hum. Nutr. Diet. 2013, 26, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rashid, M.; Basher, S.; Sultana, S.; Nomani, M.Z. Improved serum HDL cholesterol profile among Bangladeshi male students during Ramadan fasting. East Mediterr. Health J. 2004, 10, 131–137. [Google Scholar]
- Ramadan, J. Does fasting during Ramadan alter body composition, blood constituents and physical performance? Med. Princ. Pract. 2002, 11, 41–46. [Google Scholar] [CrossRef]
- Yucel, A.; Degirmenci, B.; Acar, M.; Albayrak, R.; Haktanir, A. The effect of fasting month of Ramadan on the abdominal fat distribution: Assessment by computed tomography. Tohoku J. Exp. Med. 2004, 204, 179–187. [Google Scholar] [CrossRef]
- Fahrial Syam, A.; Suryani Sobur, C.; Abdullah, M.; Makmun, D. Ramadan Fasting Decreases Body Fat but Not Protein Mass. Int. J. Clin. Endocrinol. Metab. 2016, 14, e29687–e29692. [Google Scholar] [CrossRef]
- Bak, A.M.; Moller, A.B.; Vendelbo, M.H.; Nielsen, T.S.; Viggers, R.; Rungby, J.; Pedersen, S.B.; Jorgensen, J.O.; Jessen, N.; Moller, N. Differential regulation of lipid and protein metabolism in obese vs. lean subjects before and after a 72-h fast. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E224–E235. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Fujita, S.; Glynn, E.L.; Drummond, M.J.; Volpi, E.; Rasmussen, B.B. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol. (Oxf.) 2010, 199, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Rasmussen, B.B.; Bell, J.A.; Cadenas, J.G.; Volpi, E. Basal muscle intracellular amino acid kinetics in women and men. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 77–83. [Google Scholar] [CrossRef] [PubMed]
- BaHammam, A.; Alrajeh, M.; Albabtain, M.; Bahammam, S.; Sharif, M. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite 2010, 54, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Abizari, A.R. Ramadan fasting alters food patterns, dietary diversity and body weight among Ghanaian adolescents. Nutr. J. 2018, 17, 75. [Google Scholar] [CrossRef]
- Leiper, J.B.; Molla, A.M.; Molla, A.M. Effects on health of fluid restriction during fasting in Ramadan. Eur. J. Clin. Nutr. 2003, 57, 30–38. [Google Scholar] [CrossRef]

| Time Point of Assessment and Assessment Parameters | |||||
|---|---|---|---|---|---|
| T1 (Baseline: 1 Week before RF) | T2 (Mid of RF) | T3 (Last Days of RF) | T4 (1 Week after RF) | T5 (1 Month after RF) | |
| Morning 07:00–10:00) | BC, FSS, ESS, HADS, BDI-II | BC, FSS, ESS, HADS, BDI-II | BC, FSS, ESS, HADS, BDI-II | BC, FSS, ESS, HADS, BDI-II | BC, FSS, ESS, HADS, BDI-II |
| Afternoon (13:00–15:00) | FSS, ESS | FSS, ESS | FSS, ESS | FSS, ESS | FSS, ESS |
| Evening (19:00–20:00) | FSS, ESS | FSS, ESS | FSS, ESS | FSS, ESS | FSS, ESS |
| All Participants N = 34 | Male N = 19 | Female N = 15 | p (Male vs. Female) | |
|---|---|---|---|---|
| Age | 25.1 ± 0.8 | 24.8 ± 1.0 | 25.5 ± 1.2 | 0.65 |
| Caucasian/Other | 23/11 | 14/5 | 9/6 | 0.48 |
| Body Composition | ||||
| BW (kg) | 72.0 ± 2.3 | 77.31 ± 3.1 | 65.3 ± 2.3 | <0.01 |
| BMI (kg/m2) | 24.8 ± 0.6 | 25.10 ± 0.9 | 24.4 ± 0.8 | 0.56 |
| SMM (kg) | 30.0 ± 1.2 | 34.60 ± 1.2 | 24.2 ± 0.8 | <0.001 |
| BFM (kg) | 18.4 ± 1.2 | 16.2 ± 1.7 | 21.1 ± 1.5 | <0.05 |
| FFM (kg) | 53.6 ± 1.9 | 61.1 ± 2.0 | 44.2 ± 1.4 | <0.001 |
| BFP (%) | 25.4 ± 1.5 | 20.2 ± 1.5 | 31.9 ± 1.6 | <0.001 |
| BWM (kg) | 39.3 ± 1.4 | 44.8 ± 1.4 | 32.4 ± 1.0 | <0.001 |
| BMR (J/(h kg)) | 1528.5 ± 41.4 | 1686.0 ± 85.3 | 1324.7 ± 30.3 | <0.001 |
| Mood, Fatigue, Sleepiness | ||||
| Anxiety (HADS-A) | 6.2 ± 0.6 | 6.6 ± 0.6 | 5.9 ± 1.1 | 0.57 |
| Depression (HADS-D) | 4.4 ± 0.6 | 4.8 ± 0.7 | 4.0 ± 0.8 | 0.49 |
| Depression (BDI-II) | 10.9 ± 1.3 | 9.5 ± 1.3 | 12.7 ± 2.3 | 0.21 |
| Fatigue severity scale (Median (IQR)) | 3.1 (2.1–4.5) | 3.0 (2.4–4.4) | 3.3 (2.1–4.8) | 0.73 |
| Epworth sleepiness scale (Median (IQR)) | 7.0 (5.0–10.0) | 7.0 (5.0–10.0) | 7.4 (5.0–10.0) | 0.89 |
| Time Point | p | ||||||
|---|---|---|---|---|---|---|---|
| T1 (n = 34) | T2 (n = 34) | T3 (n = 34) | T4 (n = 34) | T5 (n = 34) | |||
| Fatigue severity scale; median (IQR) | |||||||
| Morning | 3.1 (2.1–4.5) | 2.6 (2.2–3.9) | 2.67 (1.4–3.9) | 2.1 (1.2–3.9) | 3.0 (1.2–4.2) | <0.05 | <0.001 § |
| Afternoon | 3.2 (1.9–4.4) | 2.6 (1.8–3.7) | 3.15 (1.6–4.7) | 2.4 (1.2–3.8) | 2.1 (1.2–4.0) | 0.16 | |
| Evening | 3.4 (2.1–4.6) | 2.7 (1.7–4.4) | 3.28 (1.5–4.6) | 2.5 (1.3–4.1) | 2.2 (1.2–3.6) | <0.01 | |
| Sleepiness (ESS score); median (IQR) | |||||||
| Morning | 7.0 (5.0–10.0) | 7.0 (3.75–10.00) | 6.0 (2.0–9.0) | 6.0 (1.8–9.2) | 6.8 (2.0–10.2) | 0.37 | 0.66 § |
| Afternoon | 7.5 (2.8–10.0) | 6.00 (1.8–9.2) | 7.50 (3.0–11.2) | 8.0 (2.0–11.0) | 6.8 (2.0–10.2) | 0.44 | |
| Evening | 9.0 (5.0–11.0) | 7.0 (3.8–10.0) | 9.00 (3.0–13.2) | 8.5 (4.5–11.2) | 7.3 (3.8–11.2) | 0.87 | |
| ES | ||||
|---|---|---|---|---|
| All Participants | Male | Female | ||
| FSS | Morning | 0.07 | 0.08 | 0.08 |
| Afternoon | 0.01 | 0.03 | 0.26 | |
| Evening | 0.04 | 0.00 | 0.23 | |
| ESS | Morning | 0.07 | 0.08 | 0.01 |
| Afternoon | 0.02 | 0.00 | 0.10 | |
| Evening | 0.00 | 0.00 | 0.05 | |
| ES Cohen’s (d) | |||
|---|---|---|---|
| All Participants | Male | Female | |
| HADS-A | 0.54 | 0.80 | 0.31 |
| HADS-D | 0.29 | 0.18 | 0.43 |
| BDI-II | 0.54 | 0.58 | 0.54 |
| ES Cohen’s (d) | |
|---|---|
| BW | 0.08 |
| BMI | 0.14 |
| SMM | 0.11 |
| BFM | 0.02 |
| FFM | 0.10 |
| BFP | 0.07 |
| BWM | 0.10 |
| BMR | 0.10 |
| ES Cohen’s d | ||
|---|---|---|
| Male | Female | |
| BW | 0.06 | 0.14 |
| BMI | 0.13 | 0.16 |
| SMM | 0.12 | 0.30 |
| BFM | 0.00 | 0.04 |
| FFM | 0.10 | 0.27 |
| BFP | 0.05 | 0.15 |
| BWM | 0.10 | 0.27 |
| BMR | 0.10 | 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, B.; Riat, A.; Ghashang, S.K.; Eljurnazi, L.; Gutenbrunner, C. A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females—Effect on Fatigue, Sleepiness, Mood and Body Composition. Nutrients 2020, 12, 2281. https://doi.org/10.3390/nu12082281
Nugraha B, Riat A, Ghashang SK, Eljurnazi L, Gutenbrunner C. A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females—Effect on Fatigue, Sleepiness, Mood and Body Composition. Nutrients. 2020; 12(8):2281. https://doi.org/10.3390/nu12082281
Chicago/Turabian StyleNugraha, Boya, Amin Riat, Samaneh Khoshandam Ghashang, Luqman Eljurnazi, and Christoph Gutenbrunner. 2020. "A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females—Effect on Fatigue, Sleepiness, Mood and Body Composition" Nutrients 12, no. 8: 2281. https://doi.org/10.3390/nu12082281
APA StyleNugraha, B., Riat, A., Ghashang, S. K., Eljurnazi, L., & Gutenbrunner, C. (2020). A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females—Effect on Fatigue, Sleepiness, Mood and Body Composition. Nutrients, 12(8), 2281. https://doi.org/10.3390/nu12082281





