A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Design and Participants
2.2. Treatments
2.3. Cognitive and Mood Assessment
2.4. NIRS
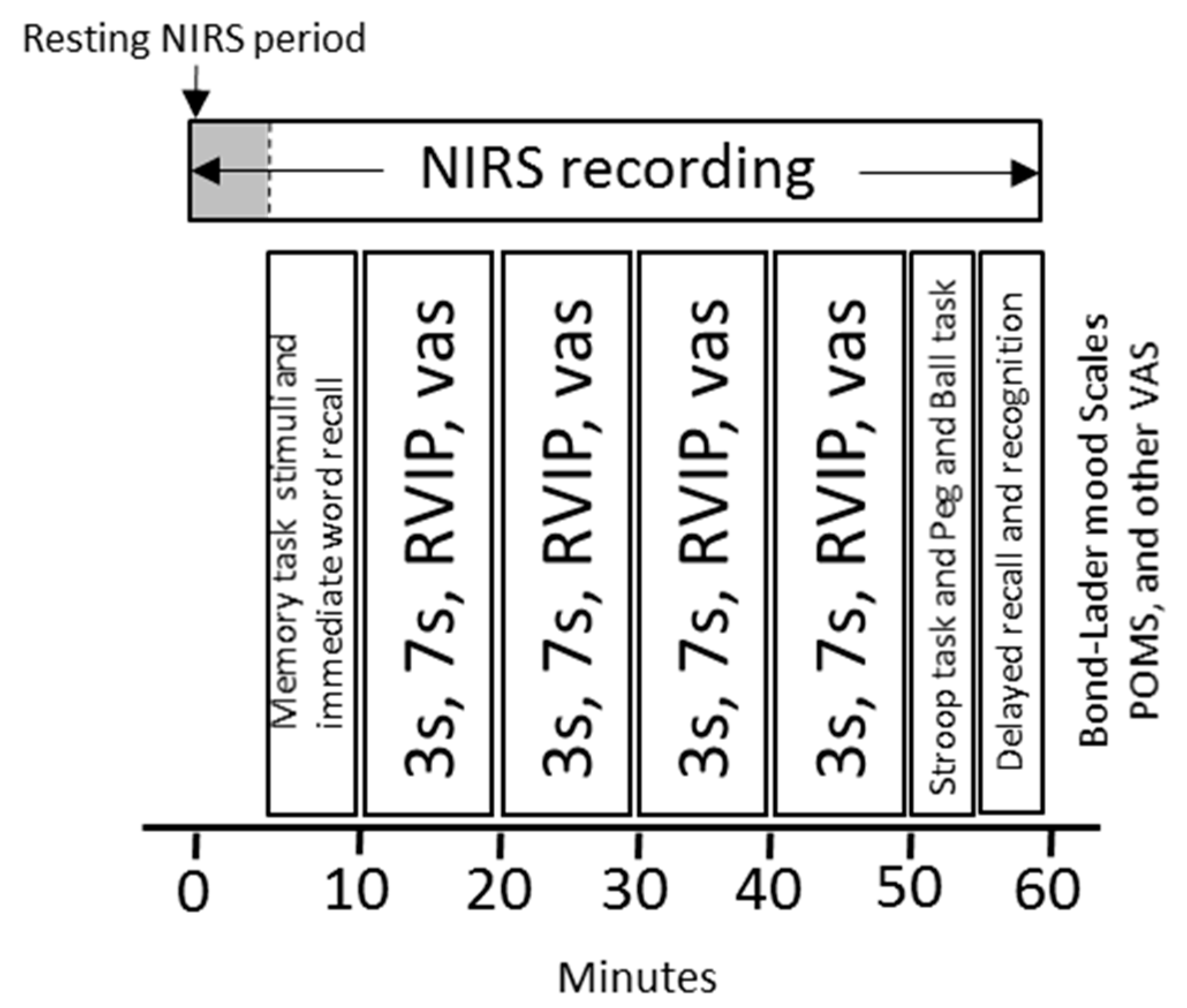
2.5. Procedure
2.6. Statistics
2.6.1. Cognitive and Mood Assessment
2.6.2. NIRS Assessment
3. Results
3.1. Baseline Scores
3.2. Cognitive and Mood Assessment
3.2.1. Cognitive Performance
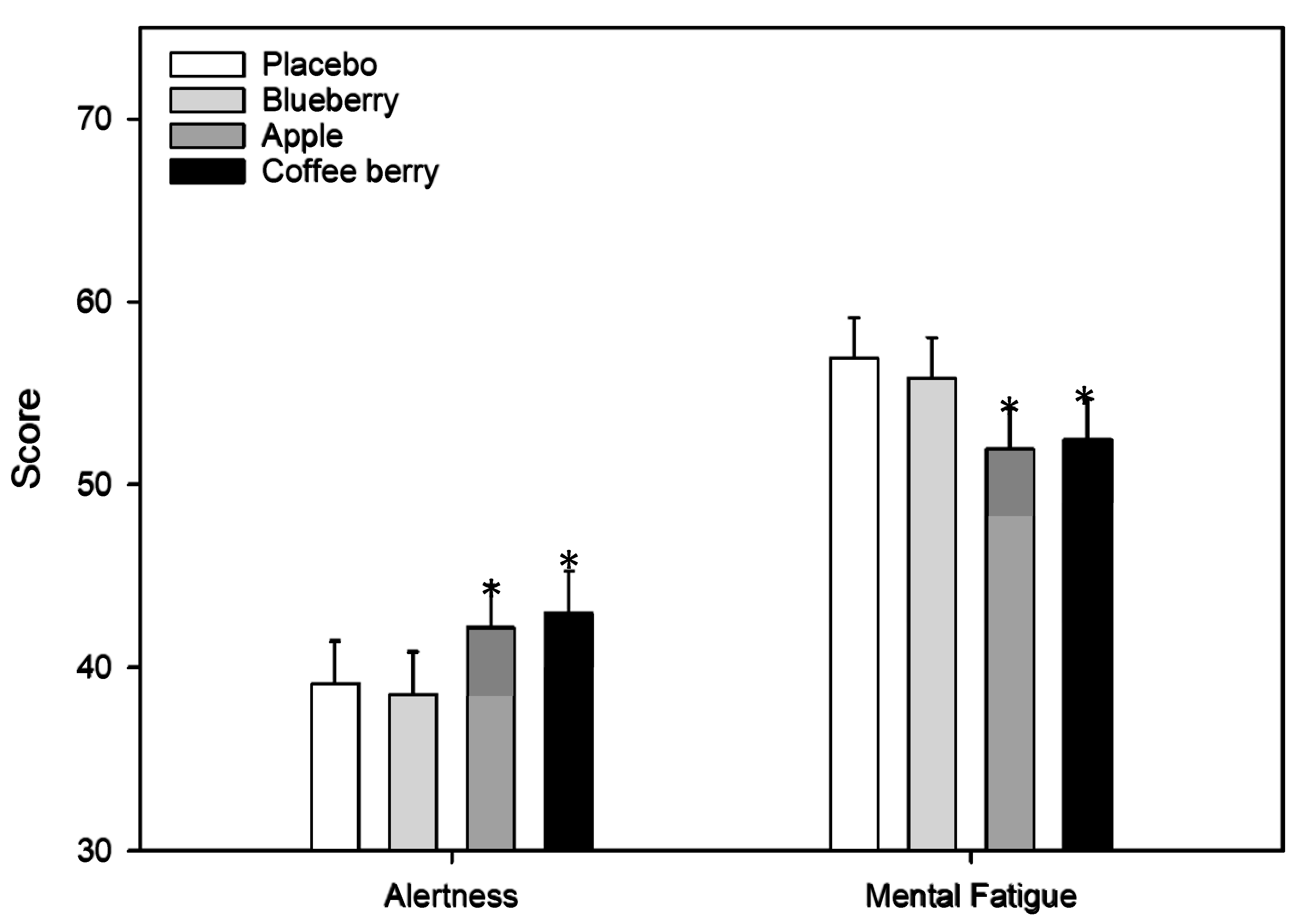
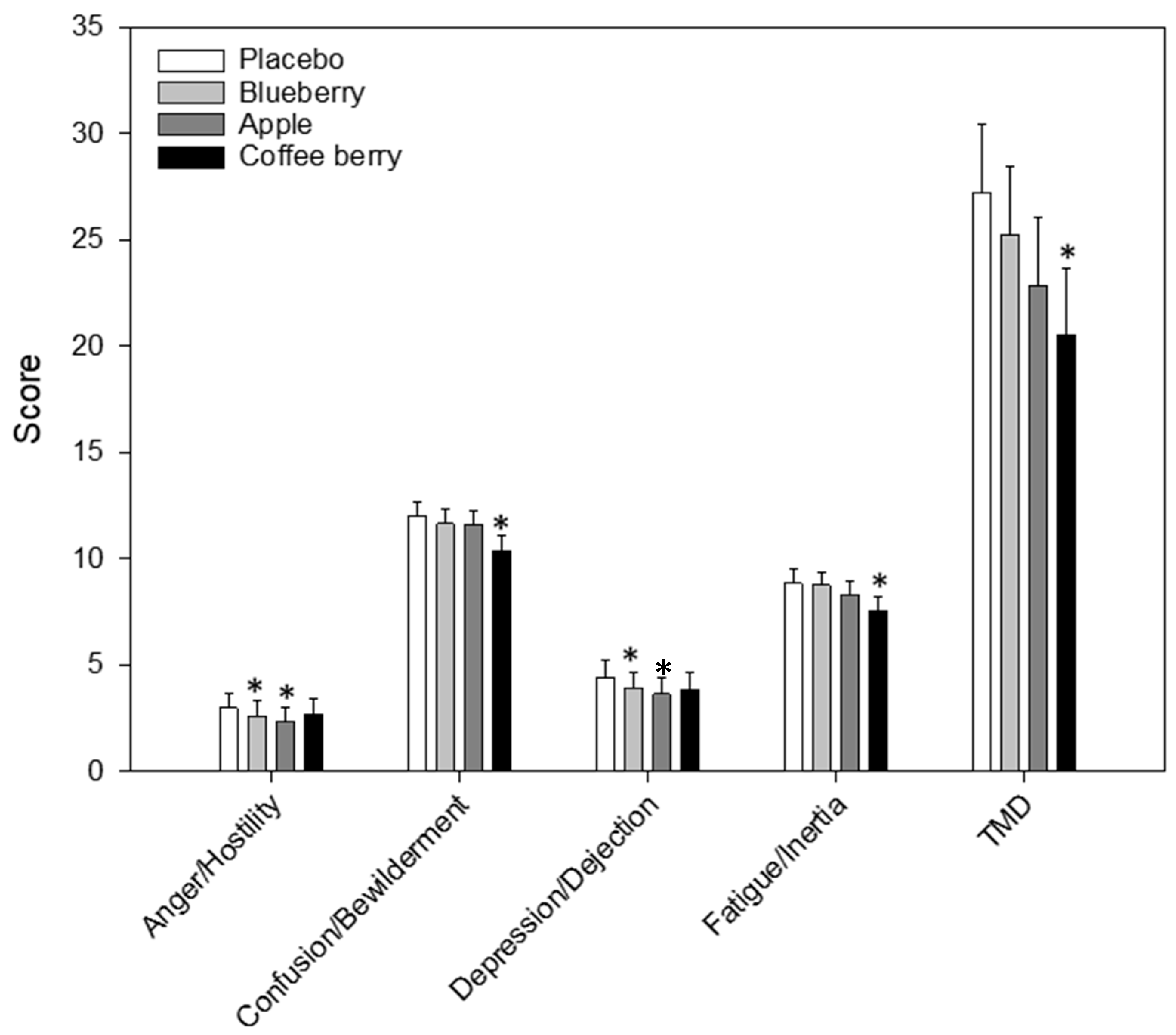
3.2.2. Mood
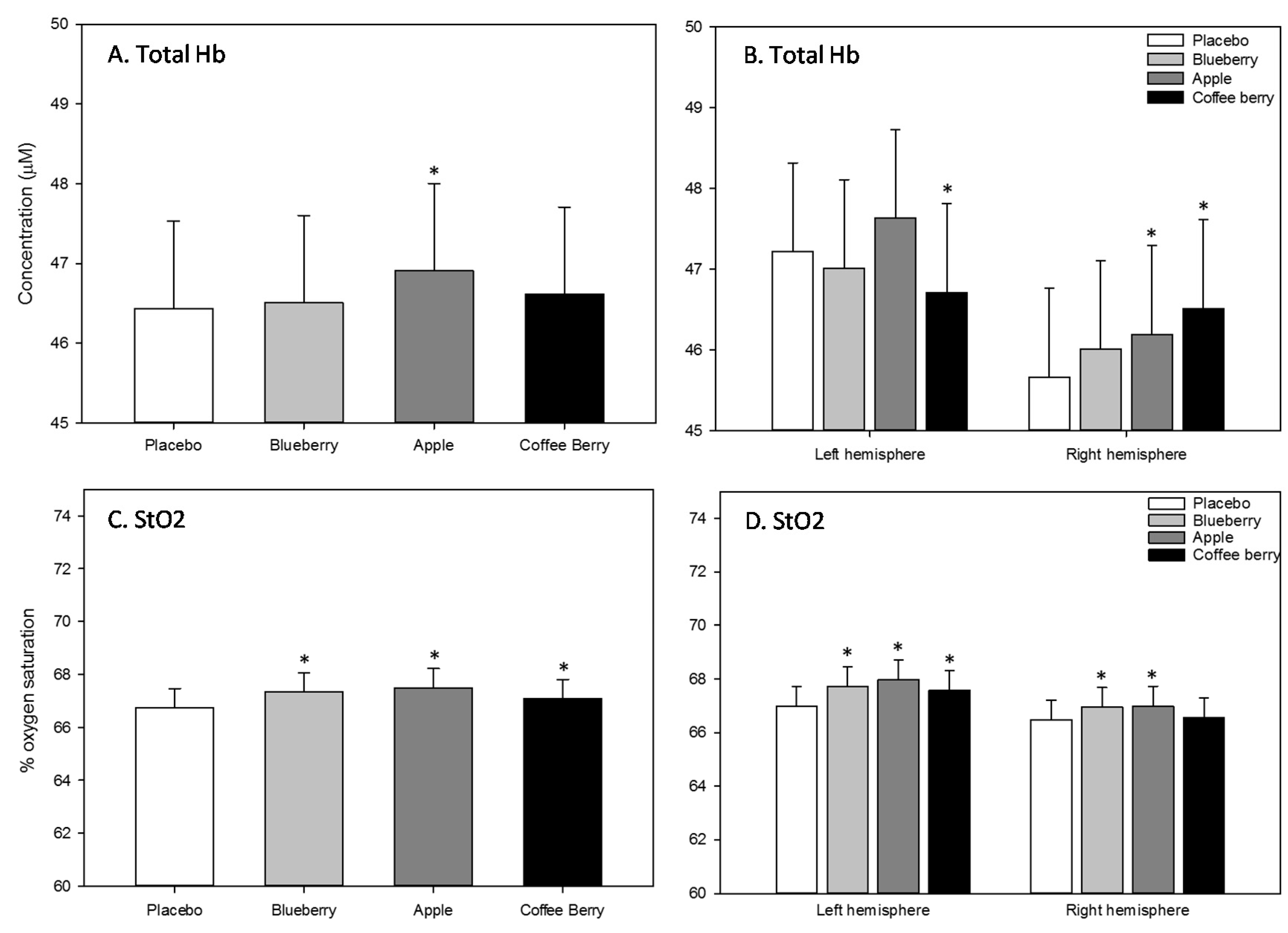
3.2.3. NIRS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NO | nitric oxide |
| CBF | cerebral blood flow |
| NIRS | near-infrared spectroscopy |
| THb | total hemoglobin |
| HbO2 | oxygenated hemoglobin |
| HHb | deoxygenated hemoglobin |
| StO2 | oxygen saturation |
| CGA | chlorogenic acid |
References
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Shrime, M.G.; Bauer, S.R.; McDonald, A.C.; Chowdhury, N.H.; Coltart, C.E.M.; Ding, E.L. Flavonoid-Rich Cocoa Consumption Affects Multiple Cardiovascular Risk Factors in a Meta-Analysis of Short-Term Studies. J. Nutr. 2011, 141, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Field, D.T.; Williams, C.M.; Butler, L.T. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 2011, 103, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010, 24, 1505–1514. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.P.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef]
- Watson, A.W.; Haskell-Ramsay, C.F.; Kennedy, D.O.; Cooney, J.M.; Trower, T.; Scheepens, A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J. Funct. Foods 2015, 17, 524–539. [Google Scholar] [CrossRef]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef]
- Furuuchi, R.; Shimizu, I.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Suda, M.; Katsuumi, G.; Wakasugi, T.; Nakao, M.; Minamino, T. Boysenberry polyphenol inhibits endothelial dysfunction and improves vascular health. PLoS ONE 2018, 13, e0202051. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4. [Google Scholar] [CrossRef]
- Hollands, W.J.; Hart, D.J.; Dainty, J.R.; Hasselwander, O.; Tiihonen, K.; Wood, R.; Kroon, P.A. Bioavailability of epicatechin and effects on nitric oxide metabolites of an apple flavanol-rich extract supplemented beverage compared to a whole apple puree: A randomized, placebo-controlled, crossover trial. Mol. Nutr. Food Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zand, J.; Lanza, F.; Garg, H.K.; Bryan, N.S. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr. Res. 2011, 31, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Haskell, C.F.; Dodd, F.L.; Wightman, E.L.; Kennedy, D.O. Behavioural effects of compounds co-consumed in dietary forms of caffeinated plants. Nutr. Res. Rev. 2013, 26, 49–70. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Haskell, C.F.; Wesnes, K.A.; Scholey, A.B. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: Comparison and interaction with Panax ginseng. Pharm. Biochem. Behav. 2004, 79, 401–411. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. Differential, dose dependent changes in cognitive performance following acute administration of a Ginkgo biloba/Panax ginseng combination to healthy young volunteers. Nutr. Neurosci. 2001, 4, 399–412. [Google Scholar] [CrossRef]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef]
- Williamson, G.; Dionisi, F.; Renouf, M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Muller, U.; Hoglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Thompson, K.G.; Blackwell, J.R.; Winyard, P.G.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Tildesley, N.T.; Ballard, C.G.; Wesnes, K.A.; Tasker, A.; Perry, E.K.; Kennedy, D.O. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacol. (Berl.) 2008, 198, 127–139. [Google Scholar] [CrossRef]
- Kennedy, D.; Scholey, A.; Wesnes, K.A. Dose-dependent enhancement of cognitive performance in young volunteers by a single doses of Ginseng. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2000, 10, 391–392. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Pace, S.; Haskell, C.; Okello, E.J.; Milne, A.; Scholey, A.B. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology 2006, 31, 845–852. [Google Scholar] [CrossRef]
- Reay, J.L.; Kennedy, D.O.; Scholey, A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J. Psychopharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef]
- Al Disi, S.S.; Anwar, M.A.; Eid, A.H. Anti-hypertensive Herbs and their Mechanisms of Action: Part I. Front. Pharm. 2015, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Savelev, S.; Okello, E.; Perry, N.S.; Wilkins, R.M.; Perry, E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharm. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Pinto, N.V.; Assreuy, A.M.; Coelho-de-Souza, A.N.; Ceccatto, V.M.; Magalhaes, P.J.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef]
- Horst, K.; Rychlik, M. Quantification of 1,8-cineole and of its metabolites in humans using stable isotope dilution assays. Mol. Nutr. Food Res. 2010, 54, 1515–1529. [Google Scholar] [CrossRef]
- Pang, H.; Wang, H.L.; Fu, L.; Su, C.Y. Pharmacokinetic studies of 20(R)-ginsenoside RG3 in human volunteers. Yao Xue Xue Bao 2001, 36, 170–173. [Google Scholar]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Jackson, P.A.; Forster, J.; Khan, J.; Grothe, T.; Perrinjaquet-Moccetti, T.; Haskell-Ramsay, C.F. Acute effects of a wild green-oat (Avena sativa) extract on cognitive function in middle-aged adults: A double-blind, placebo-controlled, within-subjects trial. Nutr. Neurosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Forster, J.; Khan, J.; Haskell-Ramsay, C.F.; Jackson, P.A. Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study. Nutrients 2017, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Dodd, F.L.; Robertson, B.C.; Okello, E.J.; Reay, J.L.; Scholey, A.B.; Haskell, C.F. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J. Psychopharmacol. 2011, 25, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Veasey, R.C.; Gonzalez, J.T.; Kennedy, D.O.; Haskell, C.F.; Stevenson, E.J. Breakfast consumption and exercise interact to affect cognitive performance and mood later in the day. A randomized controlled trial. Appetite 2013, 68, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Haskell, C.F. Cerebral blood flow and behavioural effects of caffeine in habitual and non-habitual consumers of caffeine: A near infrared spectroscopy study. Biol. Psychol. 2011, 86, 298–306. [Google Scholar] [CrossRef]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: A Near Infrared Spectroscopy pilot study. Br. J. Nutr. 2012, 107, 1093–1098. [Google Scholar] [CrossRef]
- Bond, A.; Lader, M. Use of Analog Scales in Rating Subjective Feelings. Br. J. Med. Psychol. 1974, 47, 211–218. [Google Scholar] [CrossRef]
- Heuchert, J.P.; McNair, D.M. POMS 2 Manual: Profile of Mood States; Multi-Health Systems Inc.: Toronto, ON, Canada, 2012. [Google Scholar]
- Hueber, D.M.; Franceschini, M.A.; Ma, H.Y.; Zhang, Q.; Ballesteros, J.R.; Fantini, S.; Wallace, D.; Ntziachristos, V.; Chance, B. Non-invasive and quantitative near-infrared haemoglobin spectrometry in the piglet brain during hypoxic stress, using a frequency-domain multidistance instrument. Phys. Med. Biol. 2001, 46, 41–62. [Google Scholar] [CrossRef][Green Version]
- Rogers, P.J.; Heatherley, S.V.; Mullings, E.L.; Smith, J.E. Faster but not smarter: Effects of caffeine and caffeine withdrawal on alertness and performance. Psychopharmacol. (Berl.) 2013, 226, 229–240. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef]
- Haskell, C.F.; Kennedy, D.O.; Wesnes, K.A.; Scholey, A.B. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacol. (Berl.) 2005, 179, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Christopher, G.; Sutherland, D. Acute effects of caffeine on attention: A comparison of non-consumers and withdrawn consumers. J. Psychopharmacol. 2013, 27, 77–83. [Google Scholar] [CrossRef]
- Camfield, D.A.; Silber, B.Y.; Scholey, A.B.; Nolidin, K.; Goh, A.; Stough, C. A Randomised Placebo-Controlled Trial to Differentiate the Acute Cognitive and Mood Effects of Chlorogenic Acid from Decaffeinated Coffee. PLoS ONE 2013, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Cropley, V.; Croft, R.; Silber, B.; Neale, C.; Scholey, A.; Stough, C.; Schmitt, J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacol. (Berl.) 2012, 219, 737–749. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Jackson, P.A.; Forster, J.S.; Dodd, F.L.; Bowerbank, S.L.; Kennedy, D.O. The Acute Effects of Caffeinated Black Coffee on Cognition and Mood in Healthy Young and Older Adults. Nutrients 2018, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nakayama, K.; Nakamura, M.; Sookwong, P.; Tsuduki, T.; Niino, H.; Nimura, F.; Miyazawa, T. Effects of Co-Administration of Tea Epigallocatechin-3-gallate (EGCG) and Caffeine on Absorption and Metabolism of EGCG in Humans. Biosci. Biotechnol. Biochem. 2009, 73, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Stringer, T.P.; Guerrieri, D.; Vivar, C.; van Praag, H. Plant-derived flavanol (-)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl. Psychiatry 2015, 5, e493. [Google Scholar] [CrossRef]
- Khalid, S.; Barfoot, K.L.; May, G.; Lamport, D.J.; Reynolds, S.A.; Williams, C.M. Effects of Acute Blueberry Flavonoids on Mood in Children and Young Adults. Nutrients 2017, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.B.; Rauter, A.P.; Serralheiro, M.L.M. Polyphenols as acetylcholinesterase inhibitors: Structural specificity and impact on human disease. Nutr. Aging 2012, 1, 99–111. [Google Scholar] [CrossRef]
- Jodra, P.; Domínguez, R.; Sánchez-Oliver, A.J.; Veiga-Herreros, P.; Bailey, S.J. Effect of Beetroot Juice Supplementation on Mood, Perceived Exertion, and Performance During a 30-Second Wingate Test. Int. J. Sports Physiol. Perform. 2019, 1–6. [Google Scholar] [CrossRef]
- Reay, J.L.; van Schaik, P.; Wilson, C.J. A systematic review of research investigating the physiological and psychological effects of combining Ginkgo biloba and Panax ginseng into a single treatment in humans: Implications for research design and analysis. Brain Behav. 2019, 9, e01217. [Google Scholar] [CrossRef]
- Burbaud, P.; Camus, O.; Guehl, D.; Bioulac, B.; Caillé, J.M.; Allard, M. A functional magnetic resonance imaging study of mental subtraction in human subjects. Neurosci. Lett. 1999, 273, 195–199. [Google Scholar] [CrossRef]
- Ambrosini, E.; Vallesi, A. Domain-general Stroop Performance and Hemispheric Asymmetries: A Resting-state EEG Study. J. Cogn. Neurosci. 2017, 29, 769–779. [Google Scholar] [CrossRef]
- Hoshi, Y. Functional near-infrared spectroscopy: Current status and future prospects. J. Biomed. Opt. 2007, 12. [Google Scholar] [CrossRef]
- Bertoni-Freddari, C.; Fattoretti, P.; Giorgetti, B.; Solazzi, M.; Balietti, M.; Meier-Ruge, W. Role of mitochondrial deterioration in physiological and pathological brain aging. Gerontology 2004, 50, 187–192. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
| Placebo | Blueberry | Apple | Coffee Berry |
|---|---|---|---|
| Base 1 | Base 1 | Base 1 | Base 1 |
| Blueberry extract 2 | Apple extract 6 | Coffee berry extract 7 | |
| Beetroot extract 3 | Beetroot extract 3 | Beetroot extract 3 | |
| Ginseng extract 4 | Ginseng extract 4 | Ginseng extract 4 | |
| Sage extract 5 | Sage extract 5 | Sage extract 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, P.A.; Wightman, E.L.; Veasey, R.; Forster, J.; Khan, J.; Saunders, C.; Mitchell, S.; Haskell-Ramsay, C.F.; Kennedy, D.O. A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans. Nutrients 2020, 12, 2254. https://doi.org/10.3390/nu12082254
Jackson PA, Wightman EL, Veasey R, Forster J, Khan J, Saunders C, Mitchell S, Haskell-Ramsay CF, Kennedy DO. A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans. Nutrients. 2020; 12(8):2254. https://doi.org/10.3390/nu12082254
Chicago/Turabian StyleJackson, Philippa A., Emma L. Wightman, Rachel Veasey, Joanne Forster, Julie Khan, Caroline Saunders, Siobhan Mitchell, Crystal F. Haskell-Ramsay, and David O. Kennedy. 2020. "A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans" Nutrients 12, no. 8: 2254. https://doi.org/10.3390/nu12082254
APA StyleJackson, P. A., Wightman, E. L., Veasey, R., Forster, J., Khan, J., Saunders, C., Mitchell, S., Haskell-Ramsay, C. F., & Kennedy, D. O. (2020). A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans. Nutrients, 12(8), 2254. https://doi.org/10.3390/nu12082254





