Supplemental Microalgal Iron Helps Replete Blood Hemoglobin in Moderately Anemic Mice Fed a Rice-Based Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Extractions and Diets
2.2. Animals and Sample Collection
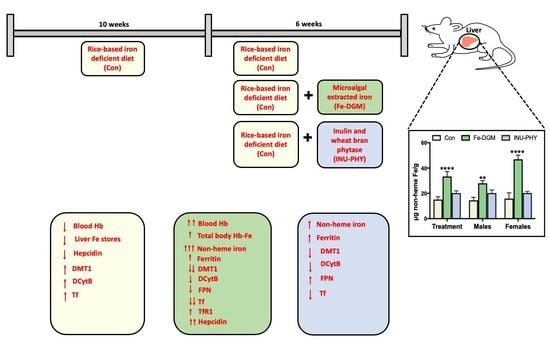
2.3. Dosage Information/Dosage Regimen
2.4. Hematology
2.5. Tissue Non-Heme Iron Concentration
2.6. Quantitative Real-Time PCR
2.7. Western Blotting
2.8. Phytate Content
2.9. Total Phenolic Content
2.10. Statistical Analysis
3. Results
3.1. Soluble Iron, Phytase, Phytate and Total Phenolic Concentrations
3.2. Growth Performance and Hematological Profile of Mice
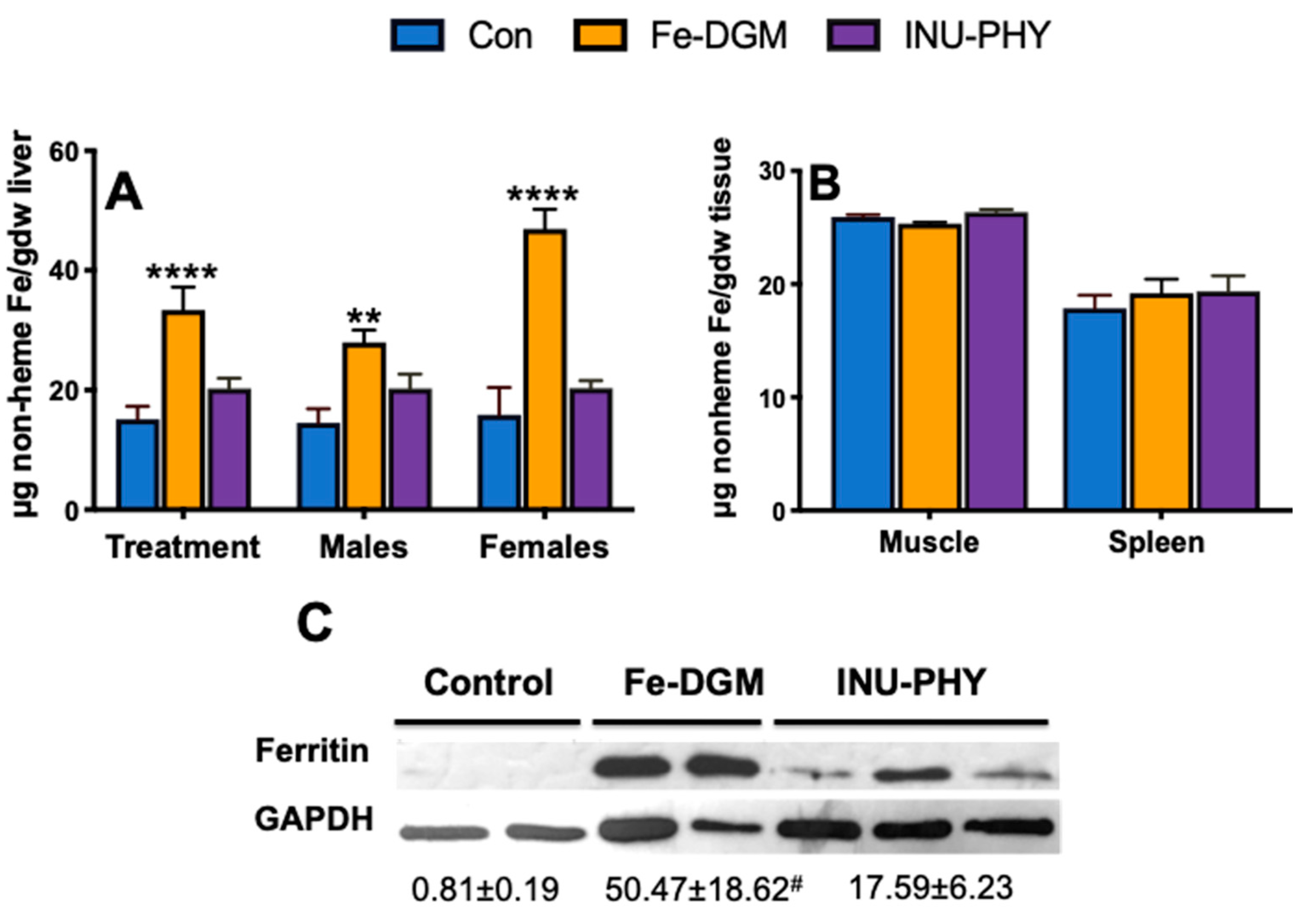
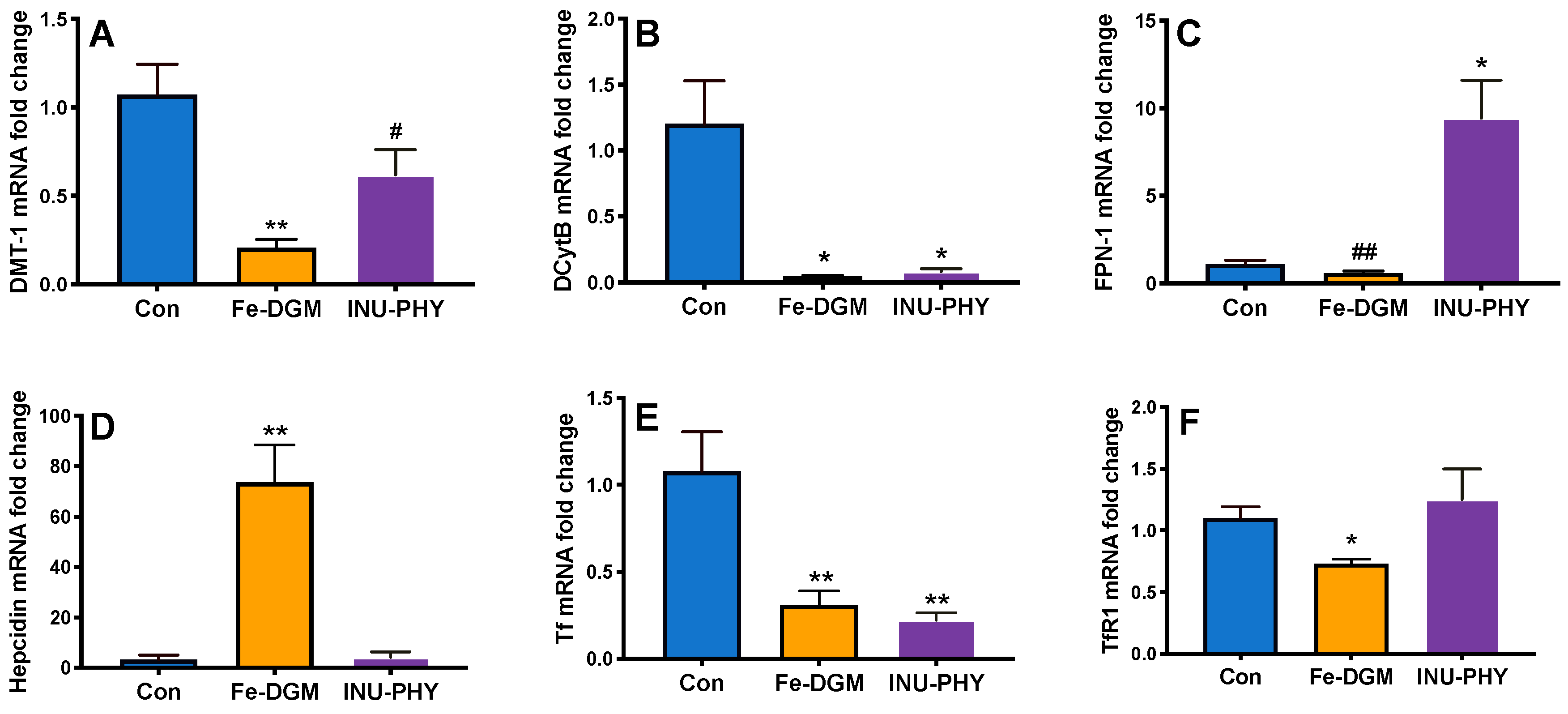
3.3. Body Iron Stores and Relative mRNA Levels of Iron Metabolism-Related Genes of Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Weaver, J.D.; Mullaney, E.; Ullah, A.H.; Azain, M.J. Phytase, a new life for an “old” enzyme. Annu. Rev. Anim. Biosci. 2013, 1, 283–309. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Hurrell, R.F. Fortification: Overcoming technical and practical barriers. J. Nutr. 2002, 132, 806–812. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Van der Westhuizen, J.; Vulpe, C.D.; Anderson, G.J.; Simpson, R.J.; McKie, A.T. Molecular and functional roles of duodenal cytochrome B (Dcytb) in iron metabolism. Blood Cells Mol. Dis. 2002, 29, 356–360. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin—A regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- García-Casal, M.N.; Pereira, A.C.; Leets, I.; Ramírez, J.; Quiroga, M.F. High iron content and bioavailability in humans from four species of marine algae. J. Nutr. 2007, 137, 2691–2695. [Google Scholar] [CrossRef] [PubMed]
- García-Casal, M.N.; Ramírez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr. 2009, 101, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.S.; Liu, Y.H. Bioavailability of iron from purple laver (Porphyra spp.) estimated in a rat hemoglobin regeneration bioassay. J. Agric. Food Chem. 2000, 48, 1734–1737. [Google Scholar] [CrossRef]
- Bocanegra, A.; Nieto, A.; Blas, B.; Sánchez-Muniz, F.J. Diets containing a high percentage of Nori or Konbu algae are well-accepted and efficiently utilised by growing rats but induce different degrees of histological changes in the liver and bowel. Food Chem. Toxicol. 2003, 41, 1473–1480. [Google Scholar] [CrossRef]
- Manor, M.L.; Kim, J.; Derksen, T.J.; Schwartz, R.L.; Roneker, C.A.; Bhatnagar, R.S.; Lei, X.G. Defatted microalgae serve as a dual dietary source of highly bioavailable iron and protein in an anemic pig model. Algal Res. 2017, 26, 409–414. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA’s source. LWT Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Tańska, M.; Konopka, I.; Ruszkowska, M. Sensory, Physico-Chemical and Water Sorption Properties of Corn Extrudates Enriched with Spirulina. Plant Foods Hum. Nutr. 2017, 72, 250–257. [Google Scholar] [CrossRef]
- Palabiyik, I.; Durmaz, Y.; Öner, B.; Toker, O.S.; Coksari, G.; Konar, N.; Tamtürk, F. Using spray-dried microalgae as a natural coloring agent in chewing gum: Effects on color, sensory, and textural properties. J. Appl. Phycol. 2018, 30, 1031–1039. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep. 1998, 47, 1–29. [Google Scholar]
- Stahl, C.H.; Han, Y.M.; Roneker, K.R.; House, W.A.; Lei, X.G. Phytase improves iron bioavailability for hemoglobin synthesis in young pigs. J. Anim. Sci. 1999, 77, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Egli, I.; Davidsson, L.; Juillerat, M.-A.; Barclay, D.; Hurrell, R. Phytic Acid Degradation in Complementary Foods Using Phytase Naturally Occurring in Whole Grain Cereals. J. Food Sci. 2003, 68, 1855–1859. [Google Scholar] [CrossRef]
- Yasuda, K.; Roneker, K.R.; Miller, D.D.; Welch, R.M.; Lei, X.G. Supplemental dietary inulin affects the bioavailability of iron in corn and soybean meal to young pigs. J. Nutr. 2006, 136, 3033–3038. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary inulin affects the expression of intestinal enterocyte iron transporters, receptors and storage protein and alters the microbiota in the pig intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef]
- Yasuda, K.; Dawson, H.D.; Wasmuth, E.V.; Roneker, C.A.; Chen, C.; Urban, J.F.; Welch, R.M.; Miller, D.D.; Lei, X.G. Supplemental dietary inulin influences expression of iron and inflammation related genes in young pigs. J. Nutr. 2009, 139, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Jolliff, J.S.; Mahan, D.C. Effect of Dietary Inulin and Phytase on Mineral Digestibility and Tissue Retention in Weanling and Growing Swine. J. Anim. Sci. 2012, 90, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Eagling, T.; Neal, A.L.; McGrath, S.P.; Fairweather-Tait, S.; Shewry, P.R.; Zhao, F.J. Distribution and Speciation of Iron and Zinc in Grain of Two Wheat Genotypes. J. Agric. Food Chem. 2014, 62, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Funahashi, S. Phytase (myoinositolhexaphosphate phosphohydrolase) from Wheat Bran. Agric. Biol. Chem. 1962, 26, 794–803. [Google Scholar] [CrossRef]
- Rodriguez, E.; Porres, J.M.; Han, Y.; Lei, X.G. Different Sensitivity of RecombinantAspergillus nigerPhytase (r-PhyA) andEscherichia colipH 2.5 Acid Phosphatase (r-AppA) to Trypsin and Pepsinin Vitro. Arch. Biochem. Biophys. 1999, 365, 262–267. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition; National Academies Press: Washington DC, USA, 1995; ISBN 978-0-309-05126-2. [Google Scholar]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food Intake, Water Intake, and Drinking Spout Side Preference of 28 Mouse Strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Monsen, E.R.; Balintfy, J.L. Calculating dietary iron bioavailability: Refinement and computerization. J. Am. Diet. Assoc. 1982, 80, 307–311. [Google Scholar] [PubMed]
- South, P.K.; Lei, X.; Miller, D.D. Meat enhances nonheme iron absorption in pigs. Nutr. Res. 2000, 20, 1749–1759. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Laparra, J.M.; Welch, R.M.; Lei, X.; Kelly, J.D.; Rutzke, M.A.; Miller, D.D. Iron and zinc bioavailabilities to pigs from red and white beans (Phaseolus vulgaris L.) are similar. J. Agric. Food Chem. 2009, 57, 3134–3140. [Google Scholar] [CrossRef]
- Johnson, P.E.; Shubert, L.E. Availability of iron to rats from spirulina, a blue-green alga. Nutr. Res. 1986, 6, 85–94. [Google Scholar] [CrossRef]
- Puyfoulhoux, G.; Rouanet, J.-M.; Besançon, P.; Baroux, B.; Baccou, J.-C.; Caporiccio, B. Iron Availability from Iron-Fortified Spirulina by an in Vitro Digestion/Caco-2 Cell Culture Model. J. Agric. Food Chem. 2001, 49, 1625–1629. [Google Scholar] [CrossRef]
- Yip, R. Iron Deficiency and Anemia. In Nutrition and Health in Developing Countries; Semba, R.D., Bloem, M.W., Eds.; Nutrition and Health; Humana Press: Totowa, NJ, USA, 2001; pp. 327–342. ISBN 978-1-59259-225-8. [Google Scholar]
- Tako, E.; Blair, M.W.; Glahn, R.P. Biofortified red mottled beans (Phaseolus vulgaris L.) in a maize and bean diet provide more bioavailable iron than standard red mottled beans: Studies in poultry (Gallus gallus) and an in vitro digestion/Caco-2 model. Nutr. J. 2011, 10, 113. [Google Scholar] [CrossRef]
- Hahn, P.; Song, Y.; Ying, G.; He, X.; Beard, J.; Dunaief, J.L. Age-dependent and gender-specific changes in mouse tissue iron by strain. Exp. Gerontol. 2009, 44, 594–600. [Google Scholar] [CrossRef]
- Morgan, E.H. Factors Affecting the Synthesis of Transferrin by Rat Tissue Slices. J. Biol. Chem. 1969, 244, 4193–4199. [Google Scholar]
- Mason, D.Y.; Taylor, C.R. Distribution of transferrin, ferritin, and lactoferrin in human tissues. J. Clin. Pathol. 1978, 31, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.A.; Cook, J.D. Mucosal iron transport by rat intestine. Blood 1980, 56, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, K.; Schümann, K.; Ehtechami, C.; Forth, W. Transferrin in isolated cells from rat duodenum and jejunum. Ann. Hematol. 1985, 51, 41–47. [Google Scholar] [CrossRef]
- Johnson, G.; Jacobs, P.; Purves, L.R. Iron binding proteins of iron-absorbing rat intestinal mucosa. J. Clin. Investig. 1983, 71, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Rehman, H.; Rosenkranz, C.; Böhm, J.; Zentek, J. Dietary Inulin Affects the Morphology but not the Sodium-Dependent Glucose and Glutamine Transport in the Jejunum of Broilers. Poult. Sci. 2007, 86, 118–122. [Google Scholar] [CrossRef]
- Zoller, H.; Weiss, G.; Theurl, I.; Koch, R.O.; Vogel, W.; Obrist, P.; Pietrangelo, A.; Montosi, G.; Haile, D.J. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 2001, 120, 1412–1419. [Google Scholar] [CrossRef]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Diez Simon, C.; Sagdic, O.; Capanoglu, E. Effect of dietary fiber (inulin) addition on phenolics and in vitro bioaccessibility of tomato sauce. Food Res. Int. 2018, 106, 129–135. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Sorbie, J.; Valberg, L.S. Iron balance in the mouse. Lab. Anim. Sci. 1974, 24, 900–904. [Google Scholar]
- Ramos, E.; Kautz, L.; Rodriguez, R.; Hansen, M.; Gabayan, V.; Ginzburg, Y.; Roth, M.P.; Nemeth, E.; Ganz, T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology 2011, 53, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A.; Valentino, P.; Daraio, F.; Porporato, P.; Gramaglia, E.; Carturan, S.; Gottardi, E.; Camaschella, C.; Roetto, A. Hepatic expression of hemochromatosis genes in two mouse strains after phlebotomy and iron overload. Haematologica 2005, 90, 1161–1167. [Google Scholar] [PubMed]
- Jacobson, L.O.; Marks, E.K.; Gaston, E.O.; Goldwasser, E. Studies on Erythropoiesis. XI. Reticulocyte response of transfusion-induced polycythemic mice to anemic plasma from nephrectomized mice to and plasma from nephrectomized rats exposed to low oxygen. Blood 1959, 14, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Criswell, K.A.; Sulkanen, A.P.; Hochbaum, A.F.; Bleavins, M.R. Effects of phenylhydrazine or phlebotomy on peripheral blood, bone marrow and erythropoietin in Wistar rats. J. Appl. Toxicol. 2000, 20, 25–34. [Google Scholar] [CrossRef]
- Das, D. Large scale algal biomass (Spirulina) production in India. In Algal Biorefinery: An Integrated Approach; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2015; pp. 151–169. ISBN 978-3-319-22812-9. [Google Scholar]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.M.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]


| Diet | Control | Fe-DGM | INU-PHY |
|---|---|---|---|
| Rice a | 41.70 | 41.70 | 40.70 |
| Sucrose | 30.00 | 30.00 | 30.00 |
| Casein | 10.45 | 10.45 | 10.45 |
| Corn Oil | 8.00 | 8.00 | 8.00 |
| Gelatin | 5.00 | 5.00 | 5.00 |
| Calcium Carbonate | 1.20 | 1.20 | 1.20 |
| Vit/Min premix b | 2.00 | 2.00 | 2.00 |
| Fe-DGM mixed with rice c | - | 1.00 | - |
| Methionine | 0.30 | 0.30 | 0.30 |
| Choline | 0.20 | 0.20 | 0.20 |
| Selenium | 0.15 | 0.15 | 0.15 |
| Inulin | - | - | 1.00 |
| Phytase mixed with rice d | - | - | 1.00 |
| Calculated values | - | - | - |
| Crude Protein, % | 16.60 | 16.00 | 16.00 |
| Crude Fat, % | 7.90 | 7.20 | 7.80 |
| Fe, mg/kg | 6.00 | 39.00 | 6.00 |
| Gene a | Forward (5′ to 3′) | Accession Number |
|---|---|---|
| Reverse (3′ to 5′) | ||
| β-actin | CACCCTGTGCTGCTCACC | NM_007393 |
| GCACGATTTCCCTCTCAG | ||
| DCytB | CATCCTCGCCATCATCTC | AF354666 |
| GGCATTGCCTCCATTTAGCTG | ||
| DMT1 | GGCTTTCTTATGAGCATTGCCTA | L33415 |
| GGAGCACCCAGAGCAGCTTA | ||
| FPN | TTGCAGGAGTCATTGCTGCTA | AF226613 |
| TGGAGTTCTGCACACCATTGAT | ||
| TfR1 | TCATGAGGGAAATCAATGATCGTA | X57349 |
| GCCCCAGAAGATATGTCGGAA | ||
| HAMP | CCTATCTCCATCAACAGAT | AF297664 |
| TGCAACAGATACCACACTG | ||
| Tf | ATACCGATGCTATGACCTTGGAT | NM_133977 |
| CAGGACTTCTTGCCTTCGAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatnagar, R.S.; Miller, D.D.; Padilla-Zakour, O.I.; Lei, X.G. Supplemental Microalgal Iron Helps Replete Blood Hemoglobin in Moderately Anemic Mice Fed a Rice-Based Diet. Nutrients 2020, 12, 2239. https://doi.org/10.3390/nu12082239
Bhatnagar RS, Miller DD, Padilla-Zakour OI, Lei XG. Supplemental Microalgal Iron Helps Replete Blood Hemoglobin in Moderately Anemic Mice Fed a Rice-Based Diet. Nutrients. 2020; 12(8):2239. https://doi.org/10.3390/nu12082239
Chicago/Turabian StyleBhatnagar, Rohil S., Dennis D. Miller, Olga I. Padilla-Zakour, and Xin Gen Lei. 2020. "Supplemental Microalgal Iron Helps Replete Blood Hemoglobin in Moderately Anemic Mice Fed a Rice-Based Diet" Nutrients 12, no. 8: 2239. https://doi.org/10.3390/nu12082239
APA StyleBhatnagar, R. S., Miller, D. D., Padilla-Zakour, O. I., & Lei, X. G. (2020). Supplemental Microalgal Iron Helps Replete Blood Hemoglobin in Moderately Anemic Mice Fed a Rice-Based Diet. Nutrients, 12(8), 2239. https://doi.org/10.3390/nu12082239