Impact of Treatment with RUTF on Plasma Lipid Profiles of Severely Malnourished Pakistani Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metabolomics Measurements
2.1.1. Total Glycerophospholipid Fatty Acid Analysis
2.1.2. Analysis of Individual Phospholipid Species and Acyl Carnitines
2.2. Quality Control (QC) and Preprocessing
2.3. Statistical Analysis
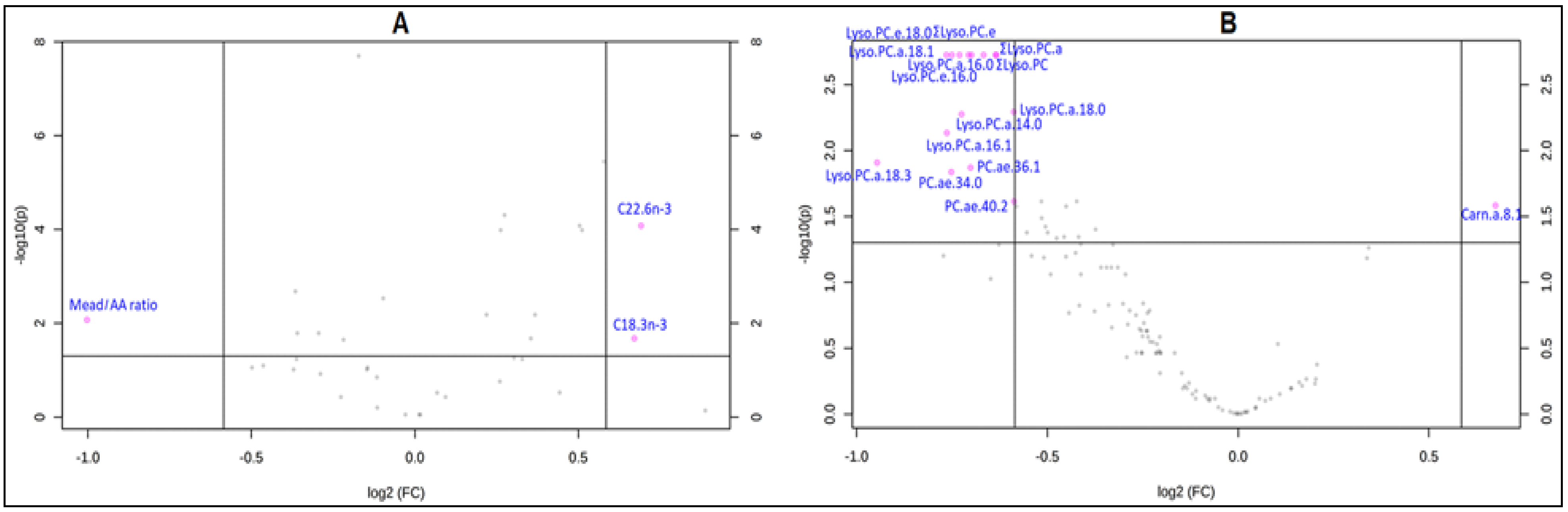
3. Results
3.1. Characteristics of the Study Population
3.2. Changes in Lipid Metabolites after Treatment with Ready-to-Use Therapeutic Foods (RUTF)
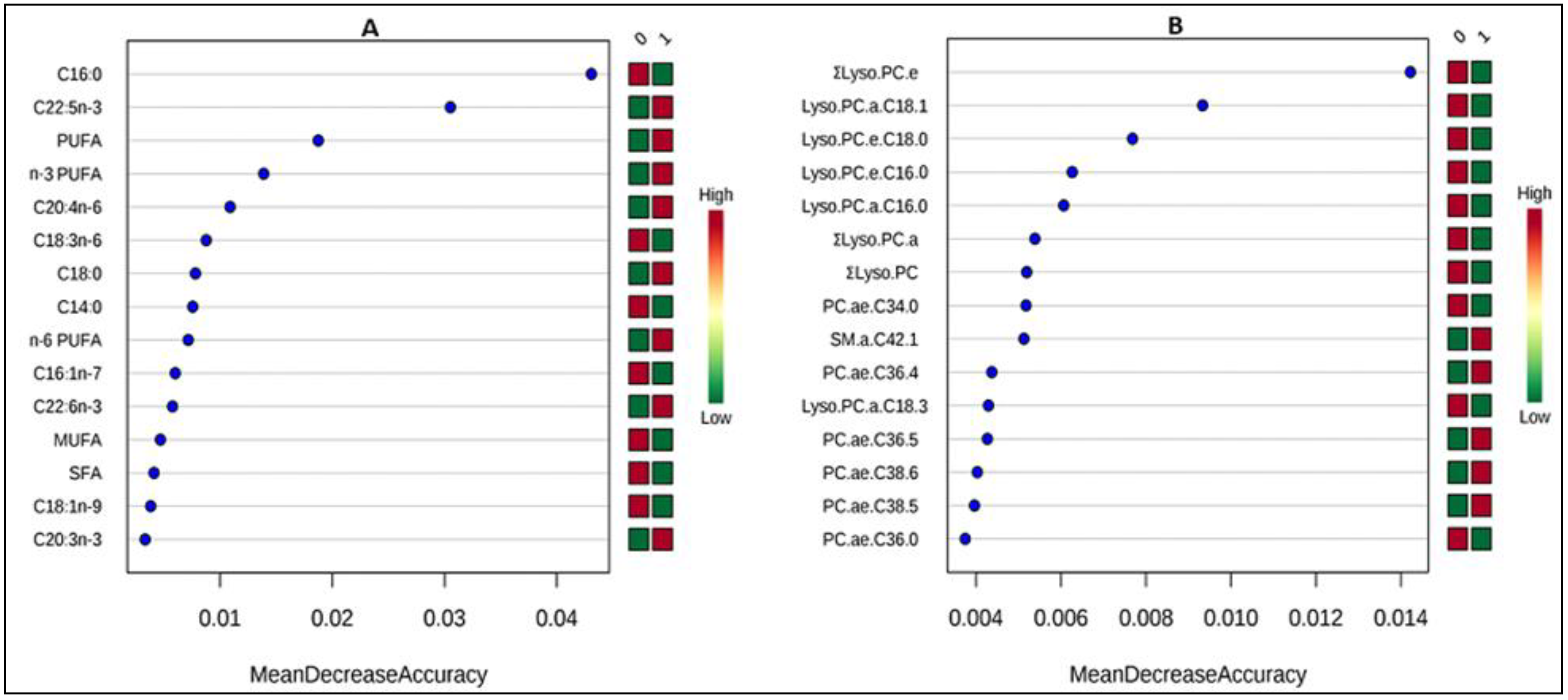
3.3. Correlates of Change in Metabolite Concentrations after Treatment
4. Discussion
4.1. Changes in Lipid Metabolites after Treatment with RUTF
4.2. Correlates of Change in Metabolite Concentrations after Treatment
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Islam, M.M.; Alam, M.; Tariquzaman, M.; Kabir, M.A.; Pervin, R.; Begum, M.; Khan, M.M.H. Predictors of the number of under-five malnourished children in Bangladesh: Application of the generalized poisson regression model. BMC Public Health 2013, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebre, A.; Surender Reddy, P.; Mulugeta, A.; Sedik, Y.; Kahssay, M. Prevalence of Malnutrition and Associated Factors among Under-Five Children in Pastoral Communities of Afar Regional State, Northeast Ethiopia: A Community-Based Cross-Sectional Study. J. Nutr. Metab. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization, United Nations Children’s Fund. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. A Joint Statement. 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/44129/9789241598163_eng.pdf?ua=1 (accessed on 17 May 2020).
- Bandsma, R.H.J.; Mendel, M.; Spoelstra, M.N.; Reijngoud, D.J.; Boer, T.; Stellaard, F.; Brabin, B.; Schellekens, R.; Senga, E.; Heikens, G.T. Mechanisms behind decreased endogenous glucose production in malnourished children. Pediatr. Res. 2010, 68, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahoor, F.; Badaloo, A.; Reid, M.; Forrester, T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am. J. Clin. Nutr. 2005, 82, 792–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manary, M.J.; Hart, C.; Whyte, M.P. Severe hypophosphatemia in children with kwashiorkor is associated with increased mortality. J. Pediatr. 1998, 133, 789–791. [Google Scholar] [CrossRef]
- Spoelstra, M.N.; Mari, A.; Mendel, M.; Senga, E.; Van Rheenen, P.; Van Dijk, T.H.; Reijngoud, D.J.; Zegers, R.G.; Heikens, G.T.; Bandsma, R.H. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic b-cell dysfunction. Metabolism 2012, 61, 1224–1230. [Google Scholar] [CrossRef]
- UN Development Programme—Inter-Agency Procurement Support Office. Unicef Ready-to-Use Therapeutic Food for Children with Severe Acute Malnutrition. In Emergency Relief Items—Compendium of Generic Specifications; Unicef Position Paper; UNDP: Copenhagen, Denmark, 2013; Volume 1. [Google Scholar]
- Prudhon, C.; Briend, A.; Prinzo, Z.W.; Daelmans, B.M.E.G.; Mason, J.B. WHO, UNICEF, and SCN Informal consultation on community-based management of severe malnutrition in children. SCN Nutr. Policy Paper 2006, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Nutriset. Plumpy’Nut® Is a Ready-to-Use Therapeutic Food (RUTF). 2018. Available online: https://www.nutriset.fr/products/en/plumpy-nut (accessed on 15 January 2020).
- Di Giovanni, V.; Bourdon, C.; Wang, D.X.; Seshadri, S.; Senga, E.; Versloot, C.J.; Voskuijl, W.; Semba, R.D.; Trehan, I.; Moaddel, R.; et al. Metabolomic Changes in Serum of Children with Different Clinical Diagnoses of Malnutrition. J. Nutr. 2016, 146, 2436–2444. [Google Scholar] [CrossRef] [Green Version]
- Bartz, S.; Mody, A.; Hornik, C.; Bain, J.; Muehlbauer, M.; Kiyimba, T.; Kiboneka, E.; Stevens, R.; Bartlett, J.; St Peter, J.V.; et al. Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J. Clin. Endocrinol. Metab. 2014, 99, 2128–2137. [Google Scholar] [CrossRef] [Green Version]
- Antonny, B.; Vanni, S.; Shindou, H.; Ferreira, T. From zero to six double bonds: Phospholipid unsaturation and organelle function. Trends Cell Biol. 2015, 25, 427–436. [Google Scholar] [CrossRef]
- Bacle, A.; Kadri, L.; Khoury, S.; Ferru-Clément, R.; Faivre, J.F.; Cognard, C.; Bescond, J.; Krzesiak, A.; Contzler, H.; Delpech, N.; et al. A comprehensive study of phospholipid fatty acid rearrangements in metabolic syndrome: Correlations with organ dysfunction. Dis. Models Mech. 2020, 13, dmm043927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoonees, A.; Lombard, M.J.; Musekiwa, A.; Nel, E.; Volmink, J. Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst. Rev. 2019, 5, CD009000. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Ali, R.; Khasira, M.A.; Odera, D.; West, A.L.; Koster, G.; Akomo, P.; Talbert, A.W.; Goss, V.M.; Ngari, M.; et al. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: A randomized controlled trial. BMC Med. 2015, 13, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization, AED, USAID, FANTA, University of California Davis, IFPRI, UNICEF. Indicators for Assessing Infant and Young Child Feeding Indicators. Part II; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Glaser, C.; Demmelmair, H.; Sausenthaler, S.; Herbarth, O.; Heinrich, J.; Koletzko, B. Fatty acid composition of serum glycerophospholipids in children. J. Pediatr. 2010, 157, 826–831. [Google Scholar] [CrossRef]
- Uhl, O.; Fleddermann, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Phospholipid Species in Newborn and 4 Month Old Infants after Consumption of Different Formulas or Breast Milk. PLoS ONE 2016, 11, e0162040. [Google Scholar] [CrossRef]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: Results from the PREOBE cohort study. Acta Diabetol. 2019, 56, 421. [Google Scholar] [CrossRef]
- Siguel, E.N.; Chee, K.M.; Gong, J.X.; Schaefer, E.J. Criteria for essential fatty acid deficiency in plasma as assessed by capillary column gas-liquid chromatography. Clin. Chem. 1987, 33, 1869–1873. [Google Scholar] [CrossRef]
- Holman, R.T. The Ratio of Trienoic: Tetraenoic Acids in Tissue Lipids as a Measure of Essential Fatty Acid Requirement. J. Nutr. 1960, 70, 405–410. [Google Scholar] [PubMed]
- Gurr, M.I.; Harwood, J.L.; Frayn, K.N. (Eds.) Fatty acid structure and metabolism. In Lipid Biochemistry, 5th ed.; Blackwell Science: Oxford, UK, 2002; pp. 13–92. [Google Scholar]
- Leichsenring, M.; Ahmed, H.M.; Laryea, M.D.; Welchering, T.; El Amin, E.O.; El Karib, A.O.; Bremer, H.J. Polyunsaturated and essential fatty acids in malnourished children. Nutr. Res. 1992, 12, 595–603. [Google Scholar]
- Babirekere-Iriso, E.; Mortensen, C.G.; Mupere, E.; Rytter, M.J.; Namusoke, H.; Michaelsen, K.F.; Briend, A.; Stark, K.D.; Friis, H.; Lauritzen, L. Changes in whole-blood PUFA and their predictors during recovery from severe acute malnutrition. Br. J. Nutr. 2016, 115, 1730–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaméogo, C.W.; Cichon, B.; Fabiansen, C.; Rytter, M.J.H.; Faurholt-Jepsen, D.; Stark, K.D.; Briend, A.; Shepherd, S.; Traoré, A.S.; Christensen, V.B.; et al. Correlates of whole-blood polyunsaturated fatty acids among young children with moderate acute malnutrition. Nutr. J. 2017, 16, 44. [Google Scholar]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 27, 4313–4314. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Koletzko, B.; Abiodun, P.O.; Larya, M.D.; Bremer, H.J. Fatty acid composition of plasma in Nigerian children with protein-energy malnutrition. Eur. J. Pediatr. 1986, 145, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.N.; Dijkstra, J.M.; Schnater, T.A.; Seerat, E.; Muskiet, F.A.; Boersma, E.R. Effects of malnutrition on the fatty acid composition and plasma vitamin E levels of Pakistani children. Acta Paediatr. 1997, 86, 690–695. [Google Scholar] [CrossRef]
- Action Against Hunger-USA. USA Nutrition Causal Analysis, Thatta and Dadu District, Sindh Province, Pakistan; Action Against Hunger: New York, NY, USA, 2012. [Google Scholar]
- World Bank 2016. Revisiting the Poverty Debate in Pakistan: Forensics and the Way Forward; World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Mølgaard, C.; Girma, T.; Friis, H. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, L.; Cervantes, E.; Ortiz, R. Malnutrition and gastrointestinal and respiratory infections in children: A public health problem. Int. J. Environ. Res. Publ. Health 2011, 8, 1174–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomkins, A.; Watson, F. Malnutrition and Infection—A Review—Nutrition Policy Discussion Paper No. 5. United Nations—Administrative Commitee on Coordination—Subcommitee on Nutrition. Discuss. Paper 1989, 5, 3–4. [Google Scholar]
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 21, 21–44. [Google Scholar] [CrossRef]
- Isanaka, S.; Roederer, T.; Djibo, A.; Luquero, F.J.; Nombela, N.; Guerin, P.J.; Grais, R.F. Reducing wasting in young children with preventive supplementation: A cohort study in Niger. Pediatrics 2010, 126, e442–e450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, J.C.; Liu, L.; Zeilani, M.; Ickes, S.; Trehan, I.; Maleta, K.; Craig, C.; Thakwalakwa, C.; Singh, L.; Brenna, J.T.; et al. High oleic ready to-use therapeutic food maintains docosahexaenoic acid status in severe malnutrition: A randomized, blinded trial. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme Codex Committee on Nutrition and Foods for Special Dietary Uses. In Proceedings of the Proposed Drsft Guidelines for Ready-to-Use Therapeutic Foods, Thirty-ninth Session, Berlin, Germany, 4–8 December 2017. [Google Scholar]
- World Health Organization, United Nations Children’s Fund. Community-Based Management of Severe Acute Malnutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Olofin, I.; McDonald, C.M.; Ezzati, M.; Flaxman, S.; Black, R.E.; Fawzi, W.W.; Caulfield, L.E.; Danaei, G. Nutrition impact model study (anthropometry cohort pooling) associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: A pooled analysis of ten prospective studies. PLoS ONE 2013, 8, e64636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 30–103. [Google Scholar] [CrossRef]
- Brenna, J.T.; Akomo, P.; Bahwere, P.; Berkley, J.A.; Calder, P.C.; Jones, K.D.; Liu, L.; Manary, M.; Trehan, I.; Briend, A. Balancing omega-6 and omega-3 fatty acids in ready-to-use therapeutic foods (RUTF). BMC Med. 2015, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Decsi, T.; Zaknun, D.; Zaknun, J.; Sperl, W.; Koletzko, B. Long-chain polyunsaturated fatty acids in children with severe protein-energy malnutrition with and without human immunodeficiency virus-1 infection. Am. J. Clin. Nutr. 1995, 62, 1283–1288. [Google Scholar] [CrossRef]
- Vajreswari, A.; Narayanareddy, K.; Srinivasa Rao, P. Fatty acid composition of erythrocyte membrane lipid obtained from children suffering from kwashiorkor and marasmus. Metabolism 1990, 39, 779–782. [Google Scholar] [CrossRef]
- Marangoni, F.; Angeli, M.; Colli, S.; Eligini, S.; Tremoli, E.; Sirtori, C.R.; Galli, C. Changes of N-3 and N-6 fatty-acids in plasma and circulating cells of normal subjects, after prolonged administration of 20/5 (EPA) and 22/6 (DHA) ethyl-esters and prolonged washout. Biochim. Biophys. Acta Lipids Lipid Metab. 1994, 1210, 55–62. [Google Scholar] [CrossRef]
- Badaloo, A.V.; Forrester, T.; Reid, M.; Jahoor, F. Lipid kinetic differences between children with kwashiorkor and those with marasmus. Am. J. Clin. Nutr. 2006, 83, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.O. Serum triglycerides and fatty acids in kwashiorkor. Am. J. Clin. Nutr. 1971, 24, 1212–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J.A.; Margolis, J.A.; Bujdoso-Wolff, K.; Matusick, E.; MacLean, W.C., Jr. Plasma and red blood cell fatty acid composition in children with protein-calorie malnutrition. Ped. Res. 1984, 18, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-Dependent Metabolite Distribution, Clustering, and Protein Extraction for Serum Profiling with Mass Spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Sekas, G.; Patton, G.M.; Lincoln, E.C.; Robins, S.J. Origin of plasma lysophosphatidylcholine: Evidence for direct hepatic secretion in the rat. J. Lab. Clin. Med. 1985, 105, 190–194. [Google Scholar]
- Smit, E.N. Essential Fatty Acid Deficiency in Malnourished Children. Erythrocyte and Breastmilk Fatty Acid Compositions in Different Populations (Thesis). Friesland Nutrition Research and the Stichting Wetenschappelijk Onderzoek Zuigelingenvoeding, 2002. Available online: https://www.rug.nl/research/portal/files/14522373/thesis.pdf (accessed on 11 October 2019).
- Franco, V.H.M.; Hotta, J.K.S.; Jorge, S.M.; dos Santos, J.-E. Plasma fatty acids in children with grade III protein energy malnutrition in its different clinical forms: Marasmus, marasmic kwashiorkor and kwashiorkor. J. Trop. Pediatr. 1999, 45, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, M.C.; De Tomas, M.E.; Mercuri, O.; Fernández, A.; de Serres, C.T. Interrelationship between protein-energy malnutrition and essential fatty acid deficiency in nursing infants. Am. J. Clin. 1991, 53, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, F.; Uzunsel, S.; Atalay, A. Plasma free carnitine levels in children with malnutrition. Turk. J. Pediatr. 1994, 36, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Doherty, J.F.; Golden, M.H.; Brooks, S.E. Peroxisomes and the fatty liver of malnutrition: An hypothesis. Am. J. Clin. Nutr. 1991, 54, 674–677. [Google Scholar] [CrossRef]
- Fransen, M.; Lismont, C.; Walton, P. The Peroxisome-Mitochondria Connection: How and Why? Int. J. Mol. Sci. 2017, 18, 1126. [Google Scholar] [CrossRef]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2016, 69, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, E.N.; Martini, I.A.; Mulder, H.; Boersma, E.R.; Muskiet, F.A. Estimated biological variation of the mature human milk fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids. 2002, 66, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Bernt, K.M.; Walker, W.A. Human milk as a carrier of biochemical messages. Acta Paediatr. 1999, 430, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Fats and Fatty Acids in Human Nutrition. In Report of a joint expert consultation; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1993. [Google Scholar]
- Rocquelin, G.; Tapsoba, S.; Dop, M.; Mbemba, F.; Traissac, P.; Martin-Prével, Y. Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers’ nutritional status: Impact on infants’ EFA supply. Eur. J. Clin. Nutr. 1998, 52, 164–171. [Google Scholar] [CrossRef]
- Decsi, T.; Koletzko, B. Polyunsaturated fatty acids in infant nutrition. Acta Paediatr. 1994, 395, 31–37. [Google Scholar] [CrossRef]
- Huisman, M.; van Beusekom, C.M.; Lanting, C.I.; Nijeboer, H.J.; Muskiet, F.A.J.; Boersma, E.R. Triglycerides, fatty acids, sterols, mono and disaccharides and sugar alcohols in human milk and current types of infant formula milk. Eur. J. Clin. Nutr. 1996, 50, 255–260. [Google Scholar]
- Andersen, S.B.; Hellgren, L.I.; Larsen, M.K.; Verder, H.; Lauritzen, L. Long-Chain Polyunsaturated Fatty Acids in Breast-Milk and Erythrocytes and Neurodevelopmental Outcomes in Danish Late-Preterm Infants. J. Pregnancy. Child Health 2015, 2, 160. [Google Scholar] [CrossRef] [Green Version]
- Mellies, M.J.; Ishikawa, T.T.; Gartside, P.S.; Burton, K.; MacGee, J.; Allen, K.; Steiner, P.M.; Brady, D.; Glueck, C.J. Effects of varying maternal dietary fatty acids in lactating women and their infants. Am. J. Clin. Nutr. 1979, 32, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Pugo-Gunsam, P.; Guesnet, P.; Subratty, A.H.; Rajcoomar, D.A.; Maurage, C.; Couet, C. Fatty acid composition of white adipose tissue and breast milk of Mauritian and French mothers and erythrocyte phospholipids of their full-term breast-fed infants. Br. J. Nutr. 1999, 82, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.L.; Maude, M.; Anderson, R.E.; Heird, W.C. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am. J. Clin. Nutr. 2000, 71, 292S–299S. [Google Scholar] [CrossRef] [Green Version]
- Much, D.; Brunner, S.; Vollhardt, C.; Schmid, D.; Sedlmeier, E.M.; Brüderl, M.; Heimberg, E.; Bartke, N.; Boehm, G.; Bader, B.L.; et al. Breast milk fatty acid profile in relation to infant growth and body composition: Results from the INFAT study. Pediatr. Res. 2013, 74, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Health, Government of Pakistan, Nutrition Division. National Nutrition Survey 1985–1987; PanGraphics: Islamabad, Pakistan, 1988.
- Holman, R.T.; Smythe, L.; Johnson, S. Effect of sex and age on fatty acid composition of human serum lipids. Am. J. Clin. Nutr. 1979, 32, 2390–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decsi, T.; Kennedy, K. Sex-specific differences in essential fatty acids metabolism. Am. J. Clin. Nutr. 2011, 94, 1914S–1919S. [Google Scholar] [CrossRef] [PubMed]
- Mashavave, G.; Kuona, P.; Tinago, W.; Stray-Pedersen, B.; Munjoma, M.; Musarurwa, C. Dried blood spot omega-3 and omega-6 long chain polyunsaturated fatty acid levels in 7–9 year old Zimbabwean children: A cross sectional study. BMC Clin. Pathol. 2016, 16, 14. [Google Scholar] [CrossRef] [Green Version]
| Fatty Acid | % |
|---|---|
| C14:0 | 0.75 |
| C15:0 | 0.05 |
| C16:0 | 28.82 |
| C16:1n-7 | 0.16 |
| C18:0 | 3.61 |
| C18:1n-9 | 48.57 |
| C18:1n-9t | 1.03 |
| C18:2n-6 | 12.62 |
| C18:3n-6 | 0.06 |
| C18:3n-3 | 1.11 |
| C20:0 | 0.55 |
| C20:1n-9 | 1.03 |
| C20:2n-6 | 0.03 |
| C20:3n-6 | 0.00 |
| C20:4n-6 | 0.98 |
| C20:4n-3 | 0.09 |
| C20:5n-3 | 0.04 |
| C24:0 | 0.45 |
| C24:1n-9 | 0.06 |
| C22:5n-3 | 0.00 |
| C22:6n-3 | 0.00 |
| Σn-6 PUFA | 13.68 |
| Σn-3 PUFA | 1.24 |
| n-6: n-3 PUFA | 11.05 |
| n-6 (% energy) | 7.25 |
| n-3 (% energy) | 0.66 |
| Nutritional Value: | g/92 g |
| Carbohydrates | 45 |
| Proteins | 12.8 |
| Fat | 30.3 |
| Energy (kJ/kcal) | 2100/500 |
| Characteristics | At Baseline | After Treatment | p-Value |
|---|---|---|---|
| Sex | Males (22; 54%), Females (19; 46%) | ||
| Breastfeeding status (y/n) | 14 (34%) | 14 (34%) | ns |
| Hemoglobin (Hb; (g/dL)) | 8.35 ± 2.13 [6] | 9.71 ± 1.52 [7] | ** |
| Length (cm) | 77.14 ± 8.09 | 78.94 ± 7.90 [4] | *** |
| Weight (kg) | 7.36 ± 1.45 | 8.78 ± 1.67 [4] | *** |
| Weight for height z-scores (WHZ) | −3.56 ± 0.56 | −1.91 ± 0.76 [7] | *** |
| Co-morbidity (y/n) | 23 (56%) | 18 (53%) [7] | ns |
| Diarrhea (y/n) | 14 (34%) | 14 (41%) [7] | ns |
| Repeated episodes of cough/flu/sore throat (y/n) | 19 (46%) | 11(32%) [7] | ns |
| Pneumonia (y/n) | 1 (2.4%) | na | |
| Measles (y/n) | 0(0%) | na | |
| Fever | na | 12 (35%) [7] | |
| Vomiting | na | 3 (8.8%) [7] | |
| Food Groups | Dietary Components | At Baseline (%) | After Treatment (%) | p-Value |
|---|---|---|---|---|
| 1 | Grains, roots and tubers | 100 | 100 (7) | ns |
| 2 | Legumes and Nuts | 12.2 | 20.6 (7) | ns |
| 3 | Dairy Products (milk, yogurt, cheese) | 51.2 | 55.9 (7) | ns |
| 4 | Flesh foods (meat, fish, poultry, and liver/organ meats) | 4.9 | 11.8 (7) | ns |
| 5 | Eggs | 2.4 | 5.9 (7) | ns |
| 6 | Vitamin A rich fruits and Vegetables | 12.2 | 14.7 (7) | ns |
| 7 | Other fruits and Vegetables | 14.6 | 11.8 (7) | ns |
| 8 | Breastmilk consumption | 5.4 ± 1.6 | 5.2 ± 1.1 (7) | ns |
| Metabolites | Baseline | After Treatment with RUTF | p-Value (after False Discovery Rate (FDR) Correction)) |
|---|---|---|---|
| A. Fatty acids | |||
| Saturated fatty acids: | |||
| C16:0 | 30.97 ± 1.96 | 27.49 ± 2.46 | <0.001 *** |
| C17:0 | 0.56 ± 0.15 | 0.48 ± 0.13 | 0.01 * |
| C18:0 | 15.93[2.34] | 17.73 [3.83] | 0.02 * |
| ΣSFA | 48.91 ± 3.81 | 45.74 ± 4.34 | 0.001 ** |
| Monounsaturated fatty acids: | |||
| C16:1n-7 | 1.22[0.83] | 0.90[0.39] | 0.005 ** |
| Polyunsaturated fatty acids: | |||
| n-6: | |||
| C18:2n-6 | 16.40 ± 3.70 | 19.09 ± 3.85 | 0.002 ** |
| C20:4n-6 | 4.19[2.07] | 5.97[2.12] | <0.001 *** |
| C22:4n-6 | 0.38 ± 0.14 | 0.49 ± 0.17 | 0.002 ** |
| C22:5n-6 | 0.33 ± 0.16 | 0.42 ± 0.15 | 0.009 ** |
| Σn-6 PUFA | 24.35 ± 4.61 | 29.21 ± 5.04 | <0.001 *** |
| n-3: | |||
| C18:3n-3 | 0.15[0.11] | 0.19[0.16] | 0.01 * |
| C22:5n-3 | 0.42 ± 0.17 | 0.59 ± 0.18 | <0.001 *** |
| C22:6n-3 | 0.71 ± 0.37 | 1.15 ± 0.47 | <0.001 *** |
| Σn3 PUFA | 1.60 ± 0.58 | 2.38 ± 0.67 | <0.001 *** |
| ΣPUFA | 26.42 ± 4.82 | 31.95 ± 5.28 | <0.001 *** |
| Ratios: | |||
| n-6/n-3 -PUFA | 16.79 ± 5.65 | 13.03 ± 3.36 | <0.001 *** |
| Mead acid/AA | 0.07[0.09] | 0.03[0.05] | 0.002 ** |
| C22:5n-6/DHA | 0.49[0.27] | 0.40[0.15] | 0.006 ** |
| AA/LA | 0.28 ± 0.10 | 0.34 ± 0.12 | 0.020 ** |
| B. Polar lipid species | |||
| Lysophosphatidylcholines: | |||
| Lyso.PC.e.C16.0 | 1.04 ± 0.40 | 0.64 ± 0.45 | <0.001 *** |
| Lyso.PC.e.C18.0 | 3.38 ± 1.58 | 1.99 ± 1.45 | <0.001 *** |
| Lyso.PC.e.C18.1 | 0.35 ± 0.14 | 0.25 ± 0.18 | 0.003 ** |
| ΣLyso.PC.e | 4.77 ± 1.97 | 2.87 ± 2.02 | <0.001 *** |
| Lyso.PC.a.C18.3 | 0.09[0.06] | 0.04[0.06] | 0.005 ** |
| Lyso.PC.a.C14.0 | 0.32 ± 0.18 | 0.19 ± 0.13 | <0.001 *** |
| Lyso.PC.a.C16.0 | 97.73 ± 35.09 | 61.55 ± 45.53 | <0.001 *** |
| Lyso.PC.a.C16.1 | 0.44 ± 0.24 | 0.26 ± 0.23 | <0.001 *** |
| Lyso.PC.a.C18.0 | 75.25 ± 30.13 | 50.06 ± 31.39 | 0.009 ** |
| Lyso.PC.a.C18.1 | 10.87 ± 4.65 | 6.46 ± 5.03 | <0.001 *** |
| Lyso.PC.a.C20.3 | 0.41[0.29] | 0.30 ± 0.25 | 0.046 * |
| ΣLyso.PC.a | 187.72 ± 66.70 | 121.02 ± 81.21 | <0.001 *** |
| ΣLyso.PC | 192.48 ± 68.15 | 123.89 ± 83.11 | <0.001 *** |
| Phosphatidylcholines: | |||
| PC.aa.C30.0 | 1.41[0.73] | 1.06[1.19] | 0.046 * |
| PC.aa.C32.0 | 9.21[2.93] | 7.97[4.52] | 0.03 * |
| PC.aa.C34.1 | 103.78 ± 52.01 | 74.63 ± 49.20 | 0.01 * |
| PC.aa.C36.1 | 28.69 ± 13.61 | 20.08 ± 13.86 | 0.006 ** |
| PC.ae.C30.0 | 0.13[0.10] | 0.08[0.15] | 0.03 * |
| PC.ae.C34.0 | 0.99[0.35] | 0.63[0.56] | 0.003 ** |
| PC.ae.C36.0 | 0.29 ± 0.13 | 0.18 ± 0.12 | <0.001 *** |
| PC.ae.C36.1 | 2.14[1.07] | 1.43[1.20] | 0.006 ** |
| PC.ae.C38.3 | 2.64 ± 1.22 | 1.92 ± 1.23 | 0.01 * |
| PC.ae.C40.2 | 0.77 ± 0.44 | 0.51 ± 0.32 | 0.003 ** |
| Sphingomyelins: | |||
| SM.a.C30.1 | 0.32 [0.23] | [0.17]0.18 | 0.01 |
| SM.a.C31.1 | 0.23 ± 0.13 | 0.15 ± 0.10 | 0.005 ** |
| SM.a.C32.2 | 0.65 ± 0.27 | 0.47 ± 0.27 | 0.004 ** |
| SM.a.C33.2 | 0.17 ± 0.09 | 0.12 ± 0.07 | 0.007 ** |
| SM.a.C35.1 | 2.80 ± 1.00 | 2.09 ± 1.13 | 0.003 ** |
| SM.a.C35.2 | 0.36 ± 0.17 | 0.27 ± 0.15 | 0.01 * |
| SM.a.C36.1 | 17.46 ± 5.64 | 13.48 ± 7.42 | 0.008 ** |
| Acyl carnitines: | |||
| Carn.a.C8.1 | 0.06[0.05] | 0.09[0.09] | 0.01 * |
| Metabolite | Regression Coefficient (β) | p-Value | p-Value (after FDR Correction) |
|---|---|---|---|
| A. Total Glycerophospholipid Fatty Acids | |||
| Saturated fatty acids: | |||
| C14:0 | −0.06 | 0.002 | 0.013 * |
| C18:0 | 0.07 | 0.001 | 0.010 * |
| ΣSFA | 0.07 | 0.001 | 0.010 * |
| Monounsaturated fatty acids: | |||
| C15:1 | −0.08 | 0.001 | 0.010 * |
| C16:1n-7 | −0.07 | 0.010 | 0.045 * |
| C18:1n-9 | −0.08 | 0.001 | 0.010 * |
| C22:1t | −0.07 | 0.011 | 0.045 * |
| ΣMUFA | −0.08 | 0.001 | 0.010 * |
| Polyunsaturated fatty acids: | |||
| C20:3n.6 | 0.06 | 0.007 | 0.037 * |
| B. Polar lipid species | |||
| Lysophosphatidylcholines: | |||
| lyso.PC.a.C16.0 | 0.06 | 0.014 | 0.030 * |
| lyso.PC.a.C18.0 | 0.05 | 0.012 | 0.027 * |
| lyso.PC.a.C18.1 | 0.06 | 0.016 | 0.034 * |
| lyso.PC.a.C18.2 | 0.08 | 0.005 | 0.024 * |
| lyso.PC.a.C20.3 | 0.08 | 0.001 | 0.015 * |
| lyso.PC.a.C20.5 | 0.06 | 0.010 | 0.026 * |
| ΣLyso.PC.a | 0.06 | 0.008 | 0.026 * |
| ΣLyso.PC | 0.06 | 0.009 | 0.026 * |
| Phosphatidylcholines: | |||
| PC.aa.C32.0 | 0.04 | 0.011 | 0.026 * |
| PC.aa.C34.1 | 0.06 | 0.010 | 0.026 * |
| PC.aa.C34.2 | 0.06 | 0.008 | 0.026 * |
| PC.aa.C34.3 | 0.05 | 0.008 | 0.026 * |
| PC.aa.C36.1 | 0.05 | 0.020 | 0.041 * |
| PC.aa.C36.2 | 0.07 | 0.004 | 0.024 * |
| PC.aa.C36.3 | 0.07 | 0.001 | 0.015 * |
| PC.aa.C36.4 | 0.06 | 0.009 | 0.026 * |
| PC.aa.C36.5 | 0.07 | 0.003 | 0.021 * |
| PC.aa.C38.2 | 0.07 | 0.002 | 0.016 * |
| PC.aa.C38.3 | 0.07 | 0.001 | 0.015 * |
| PC.aa.C38.4 | 0.07 | 0.004 | 0.024 * |
| PC.aa.C38.5 | 0.08 | 0.001 | 0.015 * |
| PC.aa.C38.6 | 0.06 | 0.010 | 0.026 * |
| PC.aa.C40.4 | 0.08 | 0.001 | 0.015 * |
| PC.aa.C40.5 | 0.08 | <0.001 | 0.015 * |
| PC.aa.C40.6 | 0.06 | 0.005 | 0.024 * |
| PC.aa.C44.12 | 0.07 | 0.002 | 0.018 * |
| ΣPC.aa | 0.07 | 0.003 | 0.021 * |
| PC.ae.C32.0 | 0.04 | 0.010 | 0.026 * |
| PC.ae.C32.1 | 0.05 | 0.005 | 0.024 * |
| PC.ae.C32.2 | 0.05 | 0.014 | 0.030 * |
| PC.ae.C34.1 | 0.04 | 0.008 | 0.026 * |
| PC.ae.C34.2 | 0.07 | <0.001 | 0.015 * |
| PC.ae.C34.3 | 0.08 | 0.001 | 0.015 * |
| PC.ae.C34.4 | 0.07 | <0.001 | 0.015 * |
| PC.ae.C36.1 | 0.03 | 0.026 | 0.049 |
| PC.ae.C36.2 | 0.05 | 0.010 | 0.026 * |
| PC.ae.C36.3 | 0.07 | 0.001 | 0.015 * |
| PC.ae.C36.4 | 0.06 | 0.010 | 0.026 * |
| PC.ae.C36.5 | 0.06 | 0.012 | 0.027 * |
| PC.ae.C36.6 | 0.05 | 0.023 | 0.044 * |
| PC.ae.C38.3 | 0.06 | 0.005 | 0.024 * |
| PC.ae.C38.4 | 0.06 | 0.010 | 0.026 * |
| PC.ae.C38.5 | 0.06 | 0.014 | 0.030 * |
| PC.ae.C38.6 | 0.06 | 0.011 | 0.026 * |
| PC.ae.C40.0 | 0.07 | 0.001 | 0.015 * |
| PC.ae.C40.1 | 0.06 | 0.014 | 0.030 * |
| PC.ae.C40.2 | 0.05 | 0.005 | 0.025 * |
| PC.ae.C40.4 | 0.07 | 0.007 | 0.026 * |
| PC.ae.C40.5 | 0.08 | 0.002 | 0.016 * |
| PC.ae.C42.6 | 0.07 | 0.008 | 0.026 * |
| ΣPC.ae | 0.07 | 0.003 | 0.021 * |
| ΣPC | 0.07 | 0.003 | 0.021 * |
| Sphingomyelins: | |||
| SM.a.C34.1 | 0.05 | 0.023 | 0.044 * |
| SM.a.C34.2 | 0.07 | 0.006 | 0.025 * |
| SM.a.C36.2 | 0.06 | 0.011 | 0.026 * |
| SM.a.C36.3 | 0.06 | 0.021 | 0.042 * |
| SM.a.C38.1 | 0.06 | 0.010 | 0.026 * |
| SM.a.C38.2 | 0.06 | 0.009 | 0.026 * |
| SM.a.C40.2 | 0.07 | 0.007 | 0.026 * |
| SM.a.C42.3 | 0.06 | 0.009 | 0.026 * |
| SM.a.C42.4 | 0.08 | 0.001 | 0.015 * |
| SM.a.C42.2 | 0.06 | 0.021 | 0.042 * |
| ΣSM | 0.06 | 0.021 | 0.042 * |
| Metabolite | Regression Coefficient (β) | p-Value | p-Value (after FDR Correction) |
|---|---|---|---|
| A. Total Glycerophospholipid Fatty Acids | |||
| Saturated fatty acids: | |||
| C18:0 | 1.00 | 0.002 | 0.028 * |
| SFA | 0.92 | 0.004 | 0.035 * |
| Monounsaturated fatty acids: | |||
| C18.1n-9 | −1.24 | 0.001 | 0.028 * |
| MUFA | −1.22 | 0.002 | 0.028 * |
| C18:3n−3 | −1.19 | 0.006 | 0.045 * |
| B. Polar lipid species | |||
| Lysophosphatidylcholines: | |||
| lyso.PC.a.C18.0 | 0.66 | 0.017 | 0.043 * |
| Phosphatidylcholines: | |||
| PC.aa.C30.0 | 0.80 | 0.003 | 0.012 * |
| PC.aa.C32.0 | 0.68 | 0.006 | 0.018 * |
| PC.aa.C32.2 | 0.71 | 0.011 | 0.028 * |
| PC.aa.C34.2 | 1.13 | 0.001 | 0.006 ** |
| PC.aa.C34.4 | 0.92 | 0.007 | 0.020 * |
| PC.aa.C34.5 | 0.95 | 0.014 | 0.035 * |
| PC.aa.C36.2 | 1.10 | 0.001 | 0.007 ** |
| PC.aa.C36.3 | 1.15 | 0.001 | 0.006 ** |
| PC.aa.C36.4 | 1.30 | 0.000 | 0.006 ** |
| PC.aa.C36.5 | 1.05 | 0.001 | 0.007 ** |
| PC.aa.C38.2 | 0.89 | 0.007 | 0.020 * |
| PC.aa.C38.3 | 1.08 | 0.001 | 0.006 ** |
| PC.aa.C38.4 | 1.26 | 0.001 | 0.006 ** |
| PC.aa.C38.5 | 1.21 | 0.000 | 0.006 ** |
| PC.aa.C38.6 | 1.19 | 0.001 | 0.006 ** |
| PC.aa.C40.4 | 1.21 | 0.000 | 0.006 ** |
| PC.aa.C40.5 | 1.24 | 0.000 | 0.006 ** |
| PC.aa.C40.6 | 1.09 | 0.001 | 0.006 ** |
| PC.aa.C44.12 | 0.93 | 0.005 | 0.018 * |
| ΣPC.aa | 1.11 | 0.001 | 0.006 ** |
| PC.ae.C32.0 | 0.53 | 0.022 | 0.050 |
| PC.ae.C32.1 | 0.75 | 0.005 | 0.017 * |
| PC.ae.C32.2 | 0.76 | 0.018 | 0.043 * |
| PC.ae.C34.2 | 0.85 | 0.007 | 0.020 * |
| PC.ae.C34.3 | 0.91 | 0.010 | 0.027 * |
| PC.ae.C34.4 | 0.99 | 0.000 | 0.006 ** |
| PC.ae.C36.2 | 0.66 | 0.021 | 0.049 * |
| PC.ae.C36.3 | 0.83 | 0.013 | 0.034 * |
| PC.ae.C36.4 | 1.07 | 0.003 | 0.012 * |
| PC.ae.C36.5 | 1.11 | 0.003 | 0.011 * |
| PC.ae.C36.6 | 1.16 | 0.000 | 0.006 ** |
| PC.ae.C38.0 | 1.02 | 0.004 | 0.015 * |
| PC.ae.C38.3 | 1.00 | 0.001 | 0.007 ** |
| PC.ae.C38.4 | 1.09 | 0.003 | 0.012 * |
| PC.ae.C38.5 | 1.15 | 0.001 | 0.007 ** |
| PC.ae.C38.6 | 1.19 | 0.001 | 0.007 ** |
| PC.ae.C40.0 | 1.16 | 0.000 | 0.006 ** |
| PC.ae.C40.2 | 0.64 | 0.012 | 0.032 * |
| PC.ae.C40.4 | 1.29 | 0.001 | 0.007 ** |
| PC.ae.C40.5 | 1.33 | 0.000 | 0.006 ** |
| PC.ae.C40.6 | 1.10 | 0.004 | 0.015 * |
| PC.ae.C42.6 | 1.11 | 0.002 | 0.008 ** |
| ΣPC.ae | 1.04 | 0.001 | 0.007 ** |
| ΣPC | 1.11 | 0.001 | 0.006 ** |
| Sphingomyelins: | |||
| SM.a.C32.1 | 0.93 | 0.009 | 0.025 * |
| SM.a.C32.2 | 0.86 | 0.006 | 0.018 * |
| SM.a.C38.1 | 1.00 | 0.005 | 0.017 * |
| SM.a.C38.2 | 0.99 | 0.005 | 0.017 * |
| SM.a.C40.2 | 0.83 | 0.022 | 0.049 * |
| SM.a.C42.3 | 0.77 | 0.022 | 0.049 * |
| SM.a.C42.4 | 1.14 | 0.001 | 0.006 ** |
| Carn.a.C14.0 | 1.08 | 0.004 | 0.014 * |
| Metabolite | Regression Coefficient (β) | p-Value | p-Value (after FDR Correction) |
|---|---|---|---|
| A. Fatty acids | |||
| Saturated fatty acids: | |||
| C17:0 | −0.22 | 0.001 | 0.009 ** |
| Polyunsaturated fatty acids: | |||
| C18:2tt | −0.27 | <0.001 | 0.009 ** |
| B. Phospholipid species | |||
| Phosphatidylcholines: | |||
| PC.aa.C30.0 | −0.18 | 0.001 | 0.010 * |
| PC.aa.C32.0 | −0.12 | 0.017 | 0.034 * |
| PC.aa.C32.1 | −0.12 | 0.007 | 0.022 * |
| PC.aa.C32.2 | −0.21 | 0.000 | 0.010 * |
| PC.aa.C32.3 | −0.18 | 0.001 | 0.010 * |
| PC.aa.C34.3 | −0.15 | 0.005 | 0.022 * |
| PC.aa.C34.4 | −0.22 | 0.001 | 0.010 * |
| PC.aa.C36.1 | −0.15 | 0.012 | 0.027 * |
| PC.aa.C36.3 | −0.18 | 0.006 | 0.022 * |
| PC.aa.C36.4 | −0.21 | 0.002 | 0.013 * |
| PC.aa.C36.5 | −0.21 | 0.001 | 0.010 * |
| PC.aa.C38.3 | −0.20 | 0.001 | 0.010 * |
| PC.aa.C38.4 | −0.22 | 0.002 | 0.011 * |
| PC.aa.C38.5 | −0.22 | 0.001 | 0.010 * |
| PC.aa.C38.6 | −0.20 | 0.004 | 0.017 * |
| PC.aa.C40.4 | −0.22 | 0.001 | 0.010 * |
| PC.aa.C40.5 | −0.27 | 0.000 | 0.004 ** |
| PC.aa.C40.6 | −0.20 | 0.001 | 0.010 * |
| PC.aa.C44.12 | −0.18 | 0.007 | 0.022 * |
| ΣPC.aa | −0.17 | 0.008 | 0.023 * |
| PC.ae.C30.0 | −0.16 | 0.003 | 0.017 * |
| PC.ae.C32.0 | −0.12 | 0.008 | 0.022 * |
| PC.ae.C32.1 | −0.14 | 0.009 | 0.024 * |
| PC.ae.C34.0 | −0.14 | 0.005 | 0.020 * |
| PC.ae.C34.1 | −0.13 | 0.007 | 0.022 * |
| PC.ae.C36.1 | −0.14 | 0.002 | 0.010 * |
| PC.ae.C36.2 | −0.14 | 0.014 | 0.029 * |
| PC.ae.C36.3 | −0.17 | 0.010 | 0.025 * |
| PC.ae.C36.4 | −0.18 | 0.011 | 0.027 * |
| PC.ae.C36.5 | −0.18 | 0.012 | 0.027 * |
| PC.ae.C38.3 | −0.18 | 0.002 | 0.012 * |
| PC.ae.C38.4 | −0.21 | 0.003 | 0.017 * |
| PC.ae.C38.5 | −0.21 | 0.004 | 0.018 * |
| PC.ae.C38.6 | −0.20 | 0.006 | 0.022 * |
| PC.ae.C40.0 | −0.27 | 0.000 | 0.005 ** |
| PC.ae.C40.1 | −0.15 | 0.017 | 0.034 * |
| PC.ae.C40.2 | −0.13 | 0.009 | 0.024 * |
| PC.ae.C40.4 | −0.20 | 0.010 | 0.025 * |
| PC.ae.C40.5 | −0.21 | 0.003 | 0.017 * |
| PC.ae.C40.6 | −0.19 | 0.011 | 0.026 * |
| PC.ae.C42.6 | −0.24 | 0.001 | 0.010 * |
| ΣPC.ae | −0.19 | 0.004 | 0.017 * |
| ΣPC | −0.18 | 0.007 | 0.022 * |
| SM.a.C30.1 | −0.13 | 0.007 | 0.022 * |
| SM.a.C31.1 | −0.15 | 0.001 | 0.010 * |
| SM.a.C32.1 | −0.17 | 0.007 | 0.022 * |
| SM.a.C32.2 | −0.18 | 0.001 | 0.010 * |
| SM.a.C33.1 | −0.13 | 0.024 | 0.045 * |
| SM.a.C33.2 | −0.12 | 0.008 | 0.023 * |
| SM.a.C35.1 | −0.17 | 0.006 | 0.022 * |
| SM.a.C35.2 | −0.17 | 0.001 | 0.010 * |
| SM.a.C36.1 | −0.18 | 0.012 | 0.027 * |
| SM.a.C36.2 | −0.20 | 0.006 | 0.022 * |
| SM.a.C38.1 | −0.19 | 0.009 | 0.023 * |
| SM.a.C38.2 | −0.18 | 0.019 | 0.037 * |
| SM.a.C39.1 | −0.15 | 0.017 | 0.034 * |
| SM.a.C41.2 | −0.18 | 0.012 | 0.027 * |
| SM.a.C42.2 | −0.18 | 0.014 | 0.029 * |
| SM.a.C42.3 | −0.22 | 0.002 | 0.010 * |
| SM.a.C42.4 | −0.24 | 0.000 | 0.010 * |
| SM.a.C42.6 | −0.18 | 0.010 | 0.025 * |
| Acyl carnitines: | |||
| Carn.a.C9.0 | −0.22 | 0.007 | 0.022 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokry, E.; Sadiq, K.; Soofi, S.; Habib, A.; Bhutto, N.; Rizvi, A.; Ahmad, I.; Demmelmair, H.; Uhl, O.; Bhutta, Z.A.; et al. Impact of Treatment with RUTF on Plasma Lipid Profiles of Severely Malnourished Pakistani Children. Nutrients 2020, 12, 2163. https://doi.org/10.3390/nu12072163
Shokry E, Sadiq K, Soofi S, Habib A, Bhutto N, Rizvi A, Ahmad I, Demmelmair H, Uhl O, Bhutta ZA, et al. Impact of Treatment with RUTF on Plasma Lipid Profiles of Severely Malnourished Pakistani Children. Nutrients. 2020; 12(7):2163. https://doi.org/10.3390/nu12072163
Chicago/Turabian StyleShokry, Engy, Kamran Sadiq, Sajid Soofi, Atif Habib, Naveed Bhutto, Arjumand Rizvi, Imran Ahmad, Hans Demmelmair, Olaf Uhl, Zulfiqar A. Bhutta, and et al. 2020. "Impact of Treatment with RUTF on Plasma Lipid Profiles of Severely Malnourished Pakistani Children" Nutrients 12, no. 7: 2163. https://doi.org/10.3390/nu12072163
APA StyleShokry, E., Sadiq, K., Soofi, S., Habib, A., Bhutto, N., Rizvi, A., Ahmad, I., Demmelmair, H., Uhl, O., Bhutta, Z. A., & Koletzko, B. (2020). Impact of Treatment with RUTF on Plasma Lipid Profiles of Severely Malnourished Pakistani Children. Nutrients, 12(7), 2163. https://doi.org/10.3390/nu12072163





