Low-Glycemic-Index/Load Desserts Decrease Glycemic and Insulinemic Response in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Blood Measurements
2.4. Subjective Appetite Measurements
2.5. Statistical Analysis
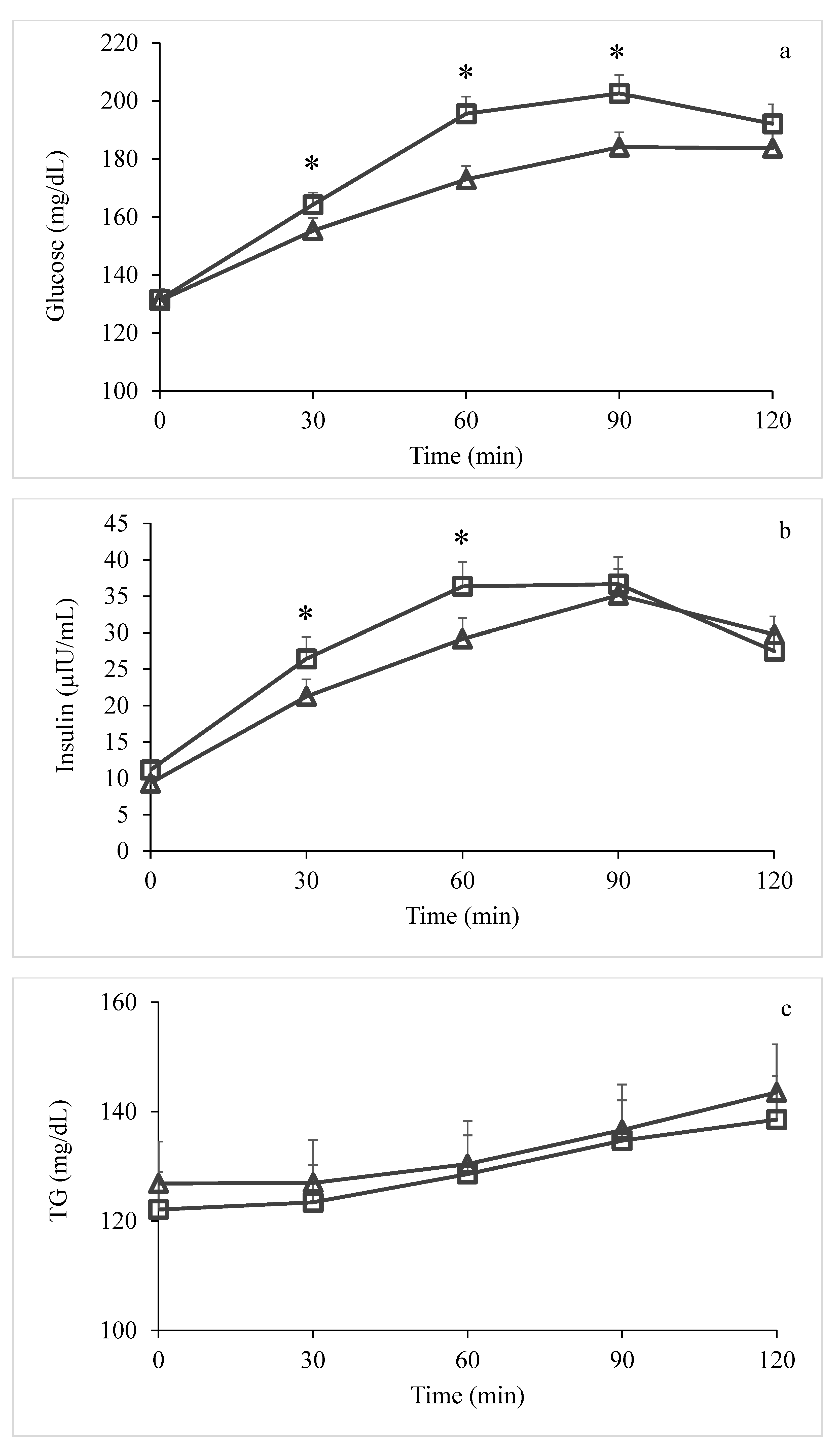
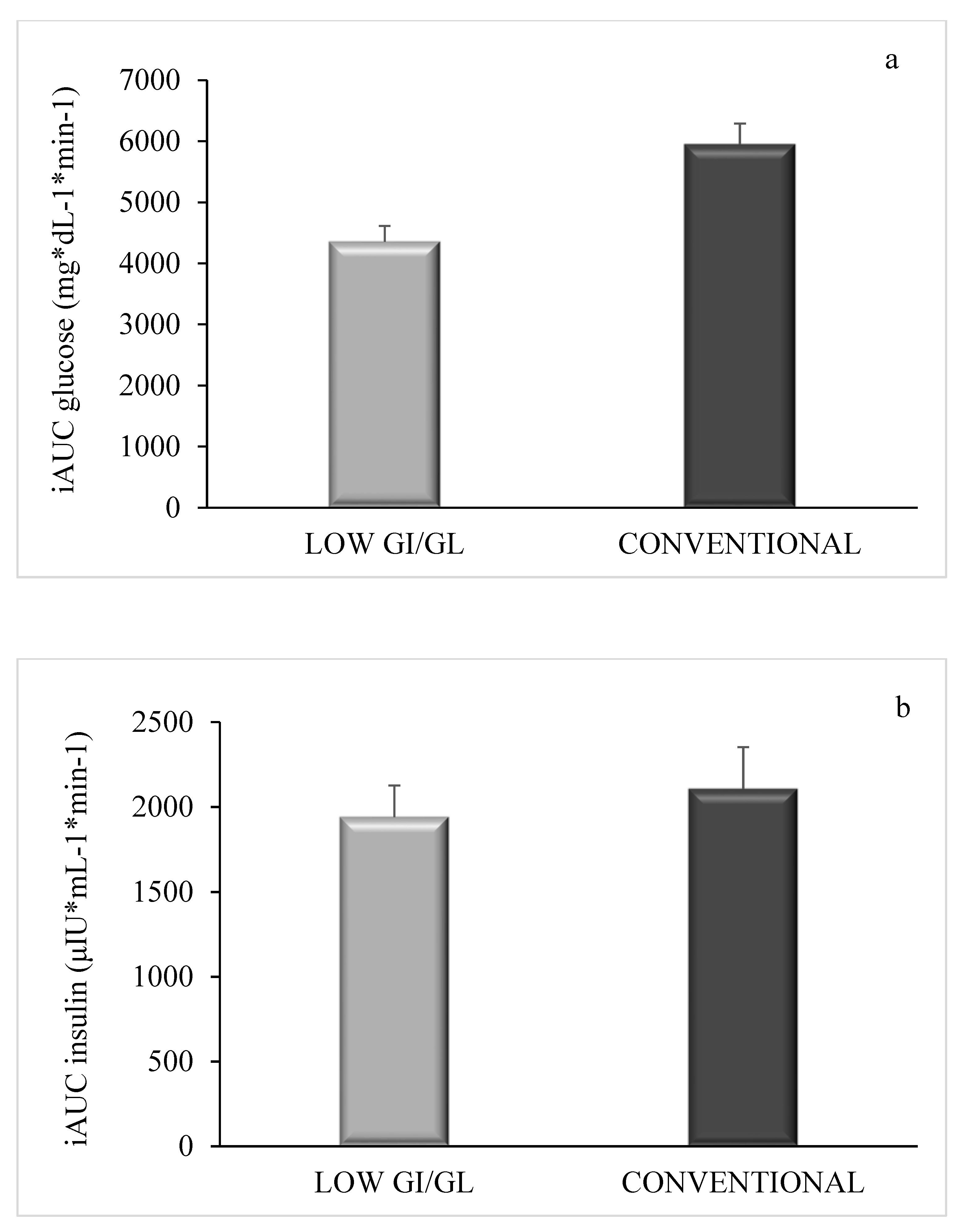
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Ceriello, A.; Davidson, J.; Hanefeld, M.; Leiter, L.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J.; International Prandial Glucose Regulation Study Group. Postprandial hyperglycaemia and cardiovascular complications of diabetes: An update. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 453–456. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2018. Diabetes Care 2019, 42 (Suppl. 1), S46–S60. [Google Scholar]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Recondo, J.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Glycemic Index and Glycemic Load Are Positively Associated with Risk of Developing Metabolic Syndrome in Middle-Aged and Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Argiana, V.; Kanellos, P.Τ.; Makrilakis, K.; Eleftheriadou, I.; Tsitsinakis, G.; Kokkinos, A.; Perrea, D.; Tentolouris, N. The effect of consumption of low-glycemic-index and low-glycemic-load desserts on anthropometric parameters and inflammatory markers in patients with type 2 diabetes mellitus. Eur. J. Nutr. 2015, 54, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Jenkins, D.J.A.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]
- Angelopoulos, T.; Kokkinos, A.; Liaskos, C.; Tentolouris, N.; Alexiadou, K.; Miras, A.D.; Mourouzis, I.; Perrea, D.; Pantos, C.; Katsilambros, N.; et al. The effect of slow spaced eating on hunger and satiety in overweight and obese patients with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care. 2014, 2, e000013. [Google Scholar] [CrossRef]
- Janket, S.J.; Benwait, J.; Isaac, P.; Ackerson, L.K.; Meurman, J.H. Oral and Systemic Effects of Xylitol Consumption. Caries Res. 2019, 53, 491–501. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Hassinger, W.; Sauer, G.; Cordes, U.; Beyer, J.; Baessler, K.H. The effects of equal caloric amounts of xylitol, sucrose and starch on insulin requirements and blood glucose levels in insulin-dependent diabetics. Diabetologia 1981, 2, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef] [PubMed]
- Trowell, H.C. Crude fiber, dietary fiber, and atherosclerosis. Atherosclerosis 1972, 16, 138e48. [Google Scholar] [CrossRef]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Mancini, M.; Stamler, J. Diet for preventing cardiovascular diseases: Light from Ancel Keys, distinguished centenarian scientist. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 52–57. [Google Scholar] [CrossRef]
- Burkitt, D.P. Relationships between diseases and their etiological significance. Am. J. Clin. Nutr. 1977, 30, 262–267. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, 1, CD006296. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Kramer, C.K.; Crispim, D.; Azevedo, M.J. A high-glycemic index, low-fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial. J. Nutr. 2015, 145, 736–741. [Google Scholar] [CrossRef]
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Östman, E.M.; Björck, I.M. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE 2012, 7, e44731. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.D.; Ismail, A.A. Glycemic responses of patients with type 2 diabetes to individual carbohydrate-rich foods and mixed meals. Ann. Nutr. Metab. 2012, 60, 27–32. [Google Scholar] [CrossRef]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Niwano, Y.; Adachi, T.; Kashimura, J.; Sakata, T.; Sasaki, H.; Sekine, K.; Yamamoto, S.; Yonekubo, A.; Kimura, S. Is glycemic index of food a feasible predictor of appetite, hunger, and satiety? J. Nutr. Sci. Vitaminol. 2009, 55, 201–207. [Google Scholar] [CrossRef] [PubMed]
| N | 51 |
|---|---|
| Gender, n Males Females | 23 28 |
| Age, year | 62 ± 1 |
| Smoking, n (%) | 10 (19.6) |
| Body weight, kg | 84.8 ± 1.9 |
| BMI, kg/m2 | 31.6 ± 0.6 |
| Waist circumference, cm | 104.4 ± 1.4 |
| Hip circumference, cm | 109.8 ± 1.1 |
| SBP, mmHg | 131.5 ± 1.9 |
| DBP, mmHg | 75.9 ± 1.1 |
| HbA1c, % | 6.4 ± 0.1 |
| Total chol., mg/dL | 171.7 ± 3.6 |
| LDL chol., mg/dL | 98.8 ± 4.4 |
| HDL chol., mg/dL | 45.3 ± 1.2 |
| TG, mg/dL | 126.8 ± 7.7 |
| SGOT, U/L | 22.2 ± 0.9 |
| SGPT, U/L | 22 ± 1.2 |
| γ-GT, U/L | 23.4 ± 1.6 |
| Nutrient | Low-GI/GL Dessert | Conventional Dessert |
|---|---|---|
| Energy (kcal/kJ) | 332/1389 | 407/1711 |
| Protein (g) | 7.0 | 6.5 |
| Carbohydrates (g) Sugars (g) | 42.5 0.3 | 59.2 33.4 |
| Polyols (g) | 14.3 | - |
| Total fat (g) Saturated (g) | 17.1 3.8 | 15.9 7.2 |
| Dietary fibers (g) | 7.9 | 0.7 |
| Sodium (g) | 0.27 | 0.26 |
| Low-GI/GL Dessert | Conventional Dessert | p-Value | |
|---|---|---|---|
| Fullness (cm) | 7.5 ± 0.3 | 6.3 ± 0.4 | 0.004 |
| Hunger (cm) | 1.6 ± 0.2 | 2.7 ± 0.4 | 0.001 |
| Additional food (cm) | 2.5 ± 0.3 | 3.6 ± 0.4 | 0.002 |
| Additional food quantity (cm) | 2.7 ± 0.3 | 3.3 ± 0.3 | 0.016 |
| Preference (cm) | 6.5 ± 0.4 | 6.9 ± 0.4 | 0.275 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argiana, V.; Kanellos, P.Τ.; Eleftheriadou, I.; Tsitsinakis, G.; Perrea, D.; Tentolouris, N.K. Low-Glycemic-Index/Load Desserts Decrease Glycemic and Insulinemic Response in Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2153. https://doi.org/10.3390/nu12072153
Argiana V, Kanellos PΤ, Eleftheriadou I, Tsitsinakis G, Perrea D, Tentolouris NK. Low-Glycemic-Index/Load Desserts Decrease Glycemic and Insulinemic Response in Patients with Type 2 Diabetes Mellitus. Nutrients. 2020; 12(7):2153. https://doi.org/10.3390/nu12072153
Chicago/Turabian StyleArgiana, Vasiliki, Panagiotis Τ. Kanellos, Ioanna Eleftheriadou, Georgios Tsitsinakis, Despoina Perrea, and Nikolaos K. Tentolouris. 2020. "Low-Glycemic-Index/Load Desserts Decrease Glycemic and Insulinemic Response in Patients with Type 2 Diabetes Mellitus" Nutrients 12, no. 7: 2153. https://doi.org/10.3390/nu12072153
APA StyleArgiana, V., Kanellos, P. Τ., Eleftheriadou, I., Tsitsinakis, G., Perrea, D., & Tentolouris, N. K. (2020). Low-Glycemic-Index/Load Desserts Decrease Glycemic and Insulinemic Response in Patients with Type 2 Diabetes Mellitus. Nutrients, 12(7), 2153. https://doi.org/10.3390/nu12072153




