Effects of 120 vs. 60 and 90 g/h Carbohydrate Intake during a Trail Marathon on Neuromuscular Function and High Intensity Run Capacity Recovery
Abstract
1. Introduction
2. Materials and Methods
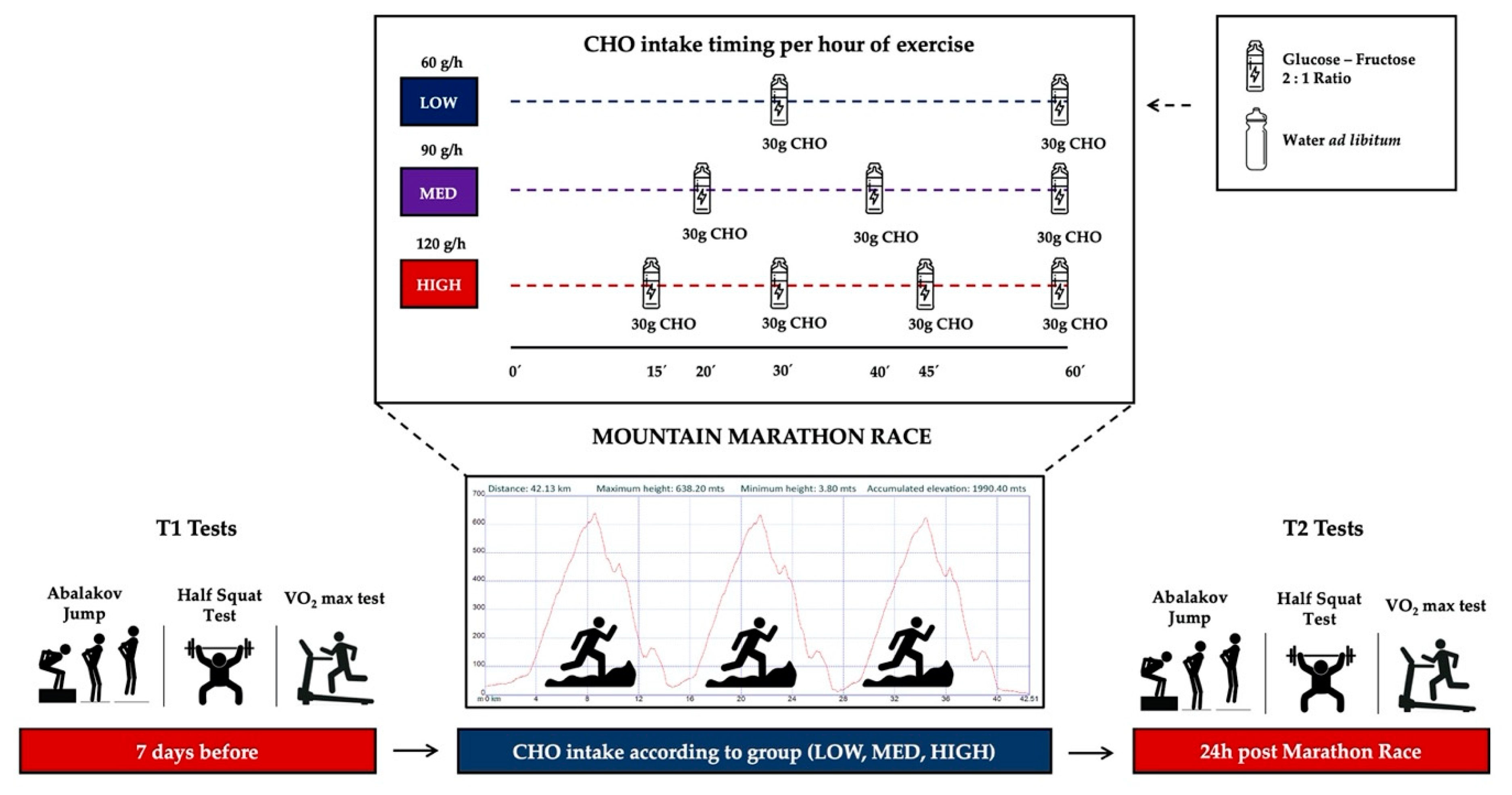
2.1. Participants and Experimental Protocol
2.2. Dietary Assessment
2.3. Athletic Performance Test
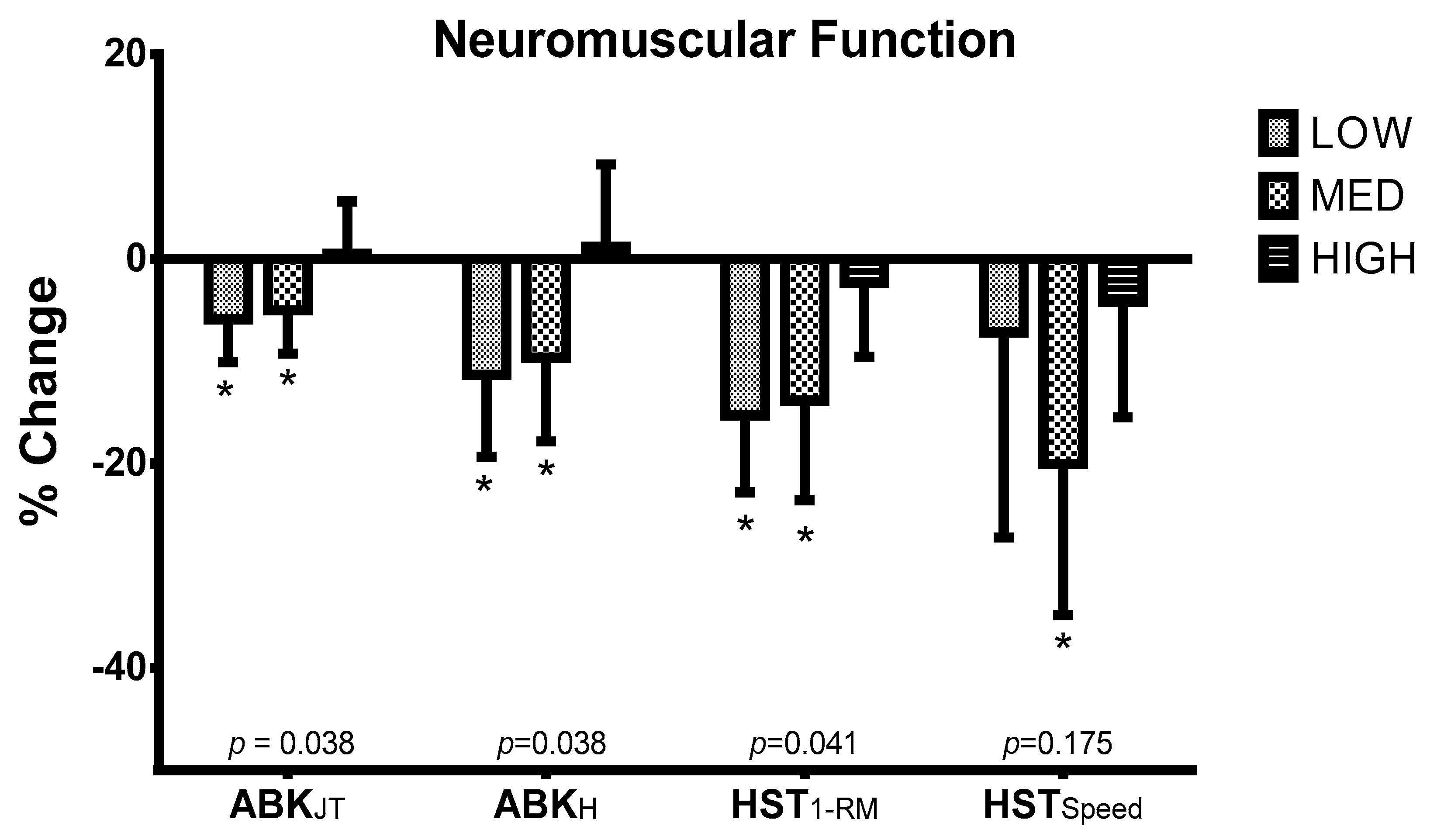
2.3.1. Neuromuscular Function
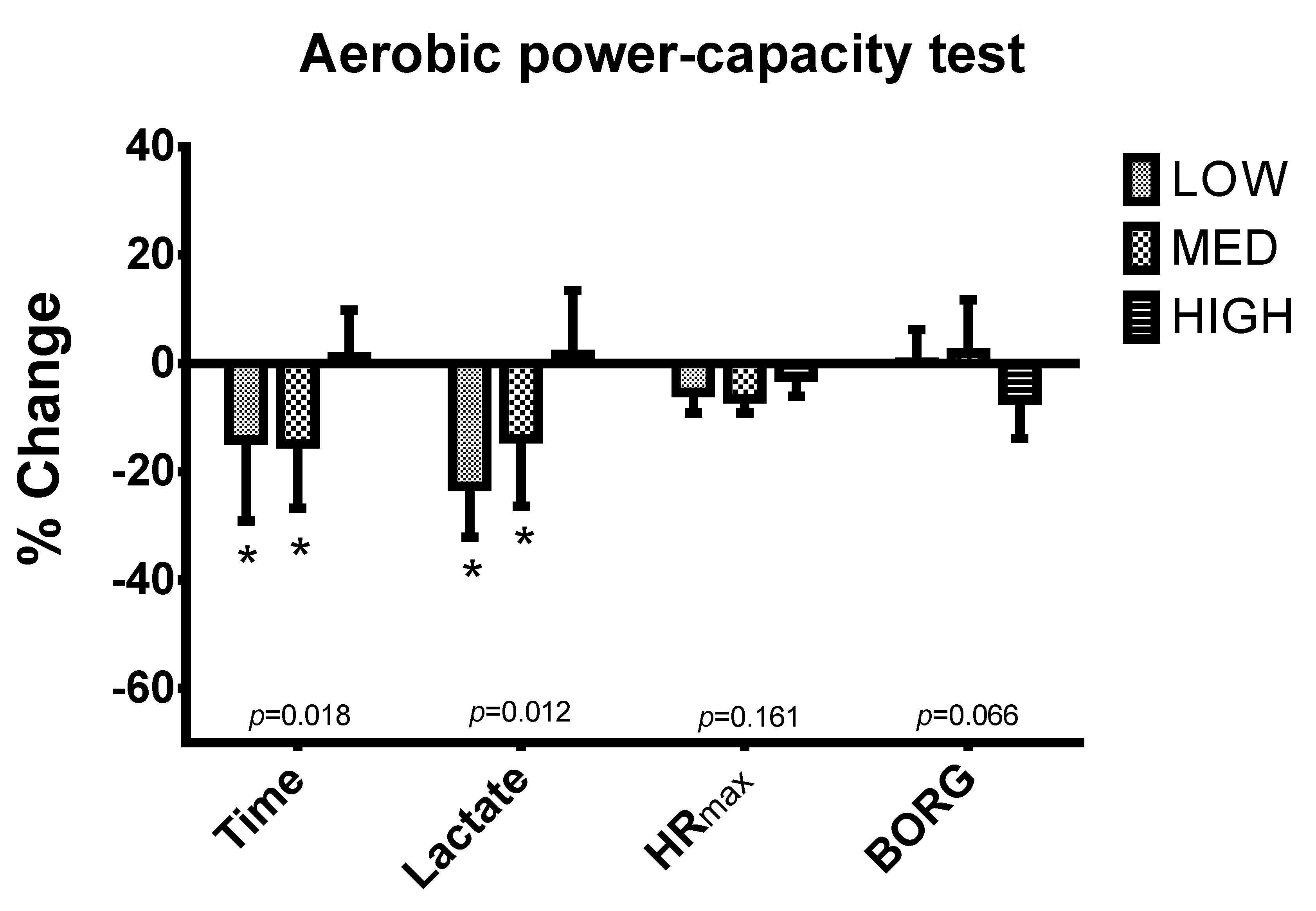
2.3.2. High Intensity Run Capacity
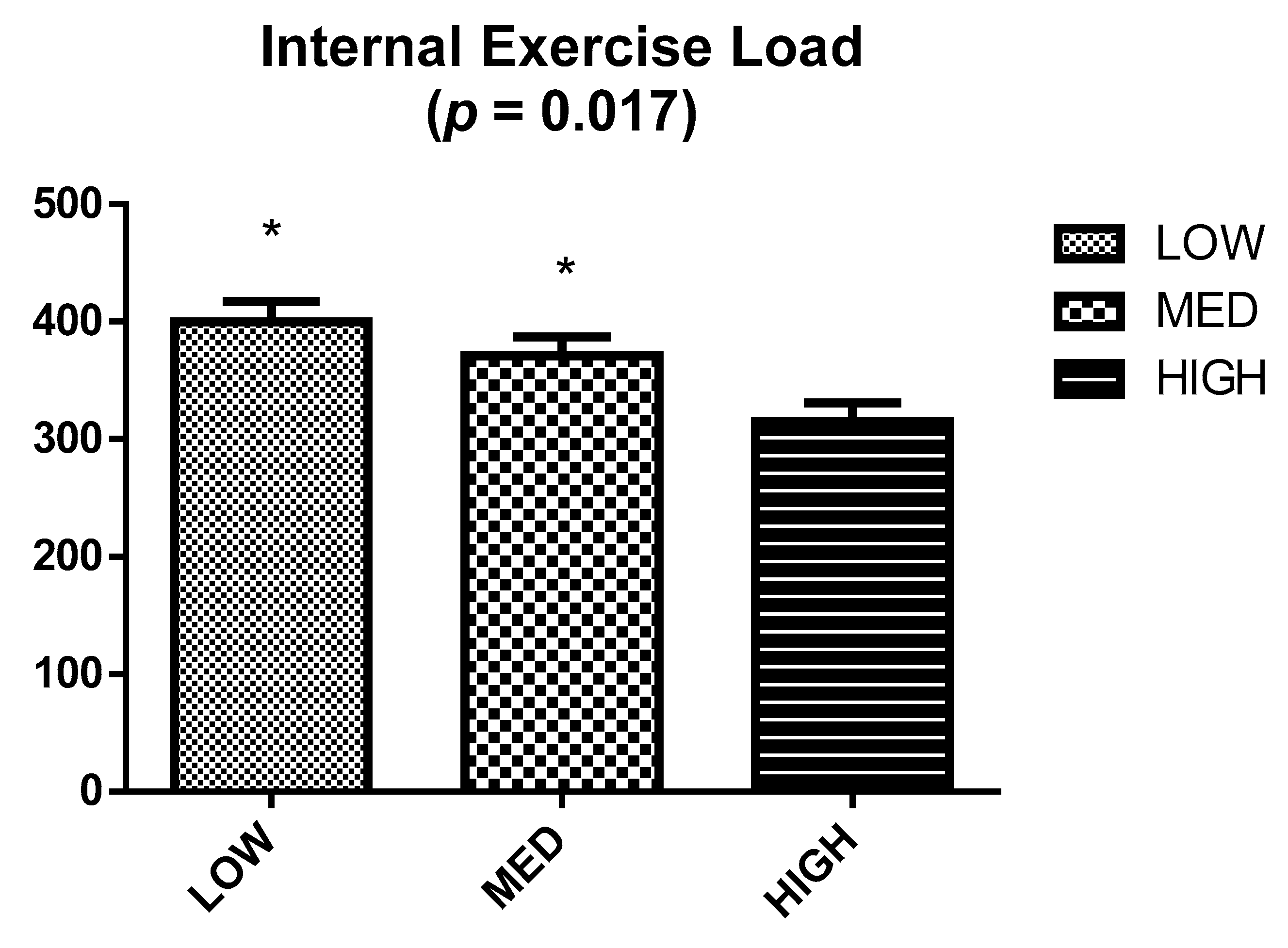
2.4. Internal Exercise Load
2.5. Anthropometry and Body Composition
2.6. Statistical Data Analyses
3. Results
4. Discussion
4.1. Limitations, Strengths and Future Lines of Research
4.2. Practical Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, M.D.; Ong, J.C.; Wang, G. Historical Analysis of Participation in 161 km Ultramarathons in North America. Int. J. Hist. Sport 2010, 27, 1877–1891. [Google Scholar] [CrossRef]
- Fornasiero, A.; Savoldelli, A.; Fruet, D.; Boccia, G.; Pellegrini, B.; Schena, F. Physiological intensity profile, exercise load and performance predictors of a 65-km mountain ultra-marathon. J. Sports Sci. 2018, 36, 1287–1295. [Google Scholar] [CrossRef]
- Millet, G.Y.; Tomazin, K.; Verges, S.; Vincent, C.; Bonnefoy, R.; Boisson, R.C.; Gergelé, L.; Féasson, L.; Martin, V. Neuromuscular consequences of an extreme mountain ultra-marathon. PLoS ONE 2011, 6, e17059. [Google Scholar] [CrossRef]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and Pathophysiological Responses to Ultramarathon Running in Non-elite Runners. Front. Physiol. 2019, 10, 1300. [Google Scholar] [CrossRef]
- Martinez, S.; Aguilo, A.; Rodas, L.; Lozano, L.; Moreno, C.; Tauler, P. Energy, macronutrient and water intake during a mountain ultramarathon event: The influence of distance. J. Sports Sci. 2018, 36, 333–339. [Google Scholar] [CrossRef]
- Millet, G.P.; Millet, G.Y. Ultramarathon is an outstanding model for the study of adaptive responses to extreme load and stress. BMC Med. 2012, 10, 77. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring Training Load to Understand Fatigue in Athletes. Sports Med. 2014, 44, 139–147. [Google Scholar] [CrossRef]
- Fernandes, J.; Lamb, K.; Twist, C. Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise. J. Funct. Morphol. Kinesiol. 2018, 3, 45. [Google Scholar] [CrossRef]
- King, A.J.; O’Hara, J.P.; Morrison, D.J.; Preston, T.; King, R.F.G.J. Carbohydrate dose influences liver and muscle glycogen oxidation and performance during prolonged exercise. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Stellingwerff, T.; Hodgson, A.B.; Randell, R.; Pöttgen, K.; Res, P.; Jeukendrup, A.E. Nutritional intake and gastrointestinal problems during competitive endurance events. Med. Sci. Sports Exerc. 2012, 44, 344–351. [Google Scholar] [CrossRef]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44, 25–33. [Google Scholar] [CrossRef]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New strategies in sport nutrition to increase exercise performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef]
- Costa, R.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.; Costa, V.; Gibson, P. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef]
- King, A.J.; O’Hara, J.P.; Arjomandkhah, N.C.; Rowe, J.; Morrison, D.J.; Preston, T.; King, R.F.G.J. Liver and muscle glycogen oxidation and performance with dose variation of glucose-fructose ingestion during prolonged (3 h) exercise. Eur. J. Appl. Physiol. 2019, 119, 1157–1169. [Google Scholar] [CrossRef]
- Viribay, A.; Arribalzaga, S.; Mielgo-Ayuso, J.; Castañeda-Babarro, A.; Seco-Calvo, J.; Urdampilleta, A. Effects of 120 g/h of Carbohydrates Intake during a Mountain Marathon on Exercise-Induced Muscle Damage in Elite Runners. Nutrients 2020, 12, 1367. [Google Scholar] [CrossRef]
- Khong, T.K.; Selvanayagam, V.S.; Sidhu, S.K.; Yusof, A. Role of carbohydrate in central fatigue: A systematic review. Scand. J. Med. Sci. Sports 2017, 27, 376–384. [Google Scholar] [CrossRef]
- Stewart, R.D.; Duhamel, T.A.; Foley, K.P.; Ouyang, J.; Smith, I.C.; Green, H.J. Protection of muscle membrane excitability during prolonged cycle exercise with glucose supplementation. J. Appl. Physiol. 2007, 103, 331–339. [Google Scholar] [CrossRef]
- Millet, G.Y.; Martin, V.; Martin, A.; Vergès, S. Electrical stimulation for testing neuromuscular function: From sport to pathology. Eur. J. Appl. Physiol. 2011, 111, 2489–2500. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Giandolini, M.; Gimenez, P.; Temesi, J.; Arnal, P.J.; Martin, V.; Rupp, T.; Morin, J.B.; Samozino, P.; Millet, G.Y. Effect of the Fatigue Induced by a 110-km Ultramarathon on Tibial Impact Acceleration and Lower Leg Kinematics. PLoS ONE 2016, 11, e0151687. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J. Muscle glycogen and cell function—Location, location, location. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 4), 34–40. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J.; Saltin, B.; Holmberg, H.-C. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J. Physiol. 2011, 589, 711–725. [Google Scholar] [CrossRef]
- Doyle, J.A.; Sherman, W.M.; Strauss, R.L. Effects of eccentric and concentric exercise on muscle glycogen replenishment. J. Appl. Physiol. 1993, 74, 1848–1855. [Google Scholar] [CrossRef]
- Gavin, J.P.; Myers, S.D.; Willems, M.E.T. Effect of eccentric exercise with reduced muscle glycogen on plasma interleukin-6 and neuromuscular responses of musculus quadriceps femoris. J. Appl. Physiol. 2016, 121, 173–184. [Google Scholar] [CrossRef]
- Burke, L.M.; van Loon, L.J.C.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2017, 122, 1055–1067. [Google Scholar] [CrossRef]
- O’Reilly, K.P.; Warhol, M.J.; Fielding, R.A.; Frontera, W.R.; Meredith, C.N.; Evans, W.J. Eccentric exercise-induced muscle damage impairs muscle glycogen repletion. J. Appl. Physiol. 1987, 63, 252–256. [Google Scholar] [CrossRef]
- Carroll, T.J.; Taylor, J.L.; Gandevia, S.C. Recovery of central and peripheral neuromuscular fatigue after exercise. J. Appl. Physiol. 2017, 122, 1068–1076. [Google Scholar] [CrossRef]
- Alghannam, A.F.; Gonzalez, J.T.; Betts, J.A. Restoration of Muscle Glycogen and Functional Capacity: Role of Post-Exercise Carbohydrate and Protein Co-Ingestion. Nutrients 2018, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Jeukendrup, A.E. Optimal composition of fluid-replacement beverages. Compr. Physiol. 2014, 4, 575–620. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 1–21. [Google Scholar] [CrossRef]
- Saunders, M.J.; Kane, M.D.; Todd, M.K. Effects of a carbohydrate-protein beverage on cycling endurance and muscle damage. Med. Sci. Sports Exerc. 2004, 36, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Stevenson, E.; Hayes, P.R.; Robson-Ansley, P.; Howatson, G. Effect of milk-based carbohydrate-protein supplement timing on the attenuation of exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2010, 35, 270–277. [Google Scholar] [CrossRef]
- Hall, A.H.; Leveritt, M.D.; Ahuja, K.D.K.; Shing, C.M. Coingestion of carbohydrate and protein during training reduces training stress and enhances subsequent exercise performance. Appl. Physiol. Nutr. Metab. 2013, 38, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon, triathlon, and road cycling. J. Sports Sci. 2011, 29. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional considerations for single-stage ultra-marathon training and racing. J. Int. Soc. Sports Nutr. 2019, 16, 1–58. [Google Scholar] [CrossRef]
- Smith, J.W.; Pascoe, D.D.; Passe, D.H.; Ruby, B.C.; Stewart, L.K.; Baker, L.B.; Zachwieja, J.J. Curvilinear dose-response relationship of carbohydrate (0-120 g.h(-1)) and performance. Med. Sci. Sports Exerc. 2013, 45, 336–341. [Google Scholar] [CrossRef]
- Australian Institute of Sport. ABCD Classification System. 2017. Available online: https://www.ausport.gov.au/ais/nutrition/supplements/classification (accessed on 10 July 2020).
- Trommelen, J.; Fuchs, C.J.; Beelen, M.; Lenaerts, K.; Jeukendrup, A.E.; Cermak, N.M.; Van Loon, L.J.C. Fructose and sucrose intake increase exogenous carbohydrate oxidation during exercise. Nutrients 2017, 9, 167. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-González, J.; Refoyo, I.; León-Guereño, P.; Cordova, A.; Del Coso, J. Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race. Nutrients 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Rosell, D.; Mora Custodio, R.; Franco-Márquez, F.; Yáñez García, J.; Badillo, J.J. Traditional vs. Sport-Specific Vertical Jump Tests: Reliability, Validity, and Relationship with the Legs Strength and Sprint Performance in Adult and Teen Soccer and Basketball Players. J. Strength Cond. Res. 2016, 31, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.; Kennedy, R.; Wallace, E. Familiarization, validity and smallest detectable difference of the isometric squat test in evaluating maximal strength. J. Sports Sci. 2018, 36, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Luhtanen, P.; Komi, P.V. A simple method for measurement of mechanical power in jumping. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 273–282. [Google Scholar] [CrossRef]
- Landart, A.; Cámara, J.; Urdampilleta, A.; Santos-Concejero, J.; Gomez, J.; Yanci, J. Análisis de la fatiga neuromuscular y cardiovascular tras disputar una maratón de montaña. RICYDE Rev. Int. Cienc. Deport. 2019, 16, 43–56. [Google Scholar] [CrossRef]
- Hartmann, H.; Wirth, K.; Klusemann, M. Analysis of the load on the knee joint and vertebral column with changes in squatting depth and weight load. Sports Med. 2013, 43, 993–1008. [Google Scholar] [CrossRef]
- Balsalobre-Fernandez, C.; Marchante, D.; Munoz-Lopez, M.; Jimenez, S.L. Validity and reliability of a novel iPhone app for the measurement of barbell velocity and 1RM on the bench-press exercise. J. Sports Sci. 2018, 36, 64–70. [Google Scholar] [CrossRef]
- Foster, C.; Daines, E.; Hector, L.; Snyder, A.C.; Welsh, R. Athletic performance in relation to training load. Wis. Med. J. 1996, 95, 370–374. [Google Scholar]
- Borresen, J.; Lambert, M.I. Quantifying training load: A comparison of subjective and objective methods. Int. J. Sports Physiol. Perform. 2008, 3, 16–30. [Google Scholar] [CrossRef]
- Lee, R.C.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef]
- Balducci, P.; Clémençon, M.; Trama, R.; Blache, Y.; Hautier, C. Performance Factors in a Mountain Ultramarathon. Int. J. Sports Med. 2017, 38, 819–826. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Boon, H.; Gijsen, A.; Stegen, J.; Kuipers, H.; Loon, L. Carbohydrate supplementation during prolonged cycling spares muscle glycogen but does not affect intramyocellular lipid use. Pflug. Arch. 2007, 454, 635–647. [Google Scholar] [CrossRef]
- Stellingwerff, T. Competition nutrition practices of elite ultramarathon runners. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 93–99. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; McLaughlin, J. Carbohydrate ingestion during exercise: Effects on performance, training adaptations and trainability of the gut. Nestle Nutr. Inst. Workshop Ser. 2011, 69, 1–17. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef]
- Millet, G.Y.; Martin, V.; Temesi, J. The role of the nervous system in neuromuscular fatigue induced by ultra-endurance exercise. Appl. Physiol. Nutr. Metab. 2018, 43, 1151–1157. [Google Scholar] [CrossRef]
- Hill, C.A.; Thompson, M.W.; Ruell, P.A.; Thom, J.M.; White, M.J. Sarcoplasmic reticulum function and muscle contractile character following fatiguing exercise in humans. J. Physiol. 2001, 531, 871–878. [Google Scholar] [CrossRef]
- Kyparos, A.; Matziari, C.; Albani, M.; Arsos, G.; Sotiriadou, S.; Deligiannis, A. A decrease in soleus muscle force generation in rats after downhill running. Can. J. Appl. Physiol. 2001, 26, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef]
- Allen, D.G.; Lannergren, J.; Westerblad, H. Muscle cell function during prolonged activity: Cellular mechanisms of fatigue. Exp. Physiol. 1995, 80, 497–527. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Holmberg, H.-C.; Schrøder, H.D.; Saltin, B.; Ortenblad, N. Human skeletal muscle glycogen utilization in exhaustive exercise: Role of subcellular localization and fibre type. J. Physiol. 2011, 589, 2871–2885. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, F.V.; Ørtenblad, N.; Pedersen, T.H.; Jørgensen, R.; Nielsen, O.B. Lactate per se improves the excitability of depolarized rat skeletal muscle by reducing the Cl- conductance. J. Physiol. 2010, 588, 4785–4794. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of Muscle Glycogen Metabolism during Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Smith, F.E.; Thelwall, P.E.; Taylor, R.; Stevenson, E.J.; Trenell, M.I.; Cermak, N.M.; van Loon, L.J.C. Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1032–E1039. [Google Scholar] [CrossRef]
- Widrick, J.J.; Costill, D.L.; McConell, G.K.; Anderson, D.E.; Pearson, D.R.; Zachwieja, J.J. Time course of glycogen accumulation after eccentric exercise. J. Appl. Physiol. 1992, 72, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Pascoe, D.D.; Fink, W.J.; Robergs, R.A.; Barr, S.I.; Pearson, D. Impaired muscle glycogen resynthesis after eccentric exercise. J. Appl. Physiol. 1990, 69, 46–50. [Google Scholar] [CrossRef]
- Burke, L.M.; Jeukendrup, A.E.; Jones, A.M.; Mooses, M. Contemporary Nutrition Strategies to Optimize Performance in Distance Runners and Race Walkers. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 117–129. [Google Scholar] [CrossRef]
- Nielsen, J.; Krustrup, P.; Nybo, L.; Gunnarsson, T.P.; Madsen, K.; Schrøder, H.D.; Bangsbo, J.; Ortenblad, N. Skeletal muscle glycogen content and particle size of distinct subcellular localizations in the recovery period after a high-level soccer match. Eur. J. Appl. Physiol. 2012, 112, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
| Variables | LOW | MED | HIGH | p |
|---|---|---|---|---|
| Race Time (min) | 278.2 ± 43.6 | 284.1 ± 40.0 | 271.7 ± 41.7 | 0.063 |
| Age (years) | 37.8 ± 9.4 | 37.2 ± 5.4 | 38.0 ± 6.8 | 0.639 |
| Height (cm) | 175.6 ± 10.3 | 172.3 ± 7.0 | 174.2 ± 3.5 | 0.361 |
| Weight (kg) | 71.8 ± 10.3 | 66.6 ± 10.1 | 67.4 ± 11.1 | 0.607 |
| BMI | 23.3 ± 2.9 | 22.4 ± 2.6 | 22.1 ± 3.0 | 0.747 |
| ∑6S (mm) | 58.8 ± 21.5 | 55.4 ± 21.6 | 43.7 ± 21.6 | 0.467 |
| Muscle Mass (kg) | 29.8 ± 4.7 | 28.4 ± 5.1 | 30.4 ± 3.2 | 0.412 |
| Study Time | LOW | MED | HIGH | p |
|---|---|---|---|---|
| ABKJT (s) | ||||
| T1 | 0.54 ± 0.05 | 0.53 ± 0.06 | 0.53 ± 0.05 | 0.867 |
| T2 | 0.51 ± 0.05 * | 0.50 ± 0.04 * | 0.53 ± 0.04 | 0.867 |
| ABKH (cm) | ||||
| T1 | 36.57 ± 6.36 | 34.86 ± 7.37 | 34.46 ± 6.55 | 0.861 |
| T2 | 32.42 ± 6.52 * | 31.06 ± 4.97 * | 34.47 ± 4.78 | 0.584 |
| HST1-RM (kg) | ||||
| T1 | 103.32 ± 35.67 | 109.07 ± 33.62 | 97.17 ± 8.60 | 0.991 |
| T2 | 81.66 ± 32.42 | 91.91 ± 25.54 | 90.09 ± 14.20 | 0.659 |
| HSTSpeed (m/s) | ||||
| T1 | 0.65 ± 0.06 | 0.63 ± 0.10 | 0.57 ± 0.14 | 0.728 |
| T2 | 0.60 ± 0.11 | 0.50 ± 0.09 * | 0.55 ± 0.18 | 0.370 |
| Study Time | LOW | MED | HIGH | p |
|---|---|---|---|---|
| Time (s) | ||||
| T1 | 102.8 ± 38.3 | 104.0 ± 48.1 | 108.0 ± 46.5 | 0.962 |
| T2 | 89.8 ± 37.1 | 87.9 ± 39.4 * | 110.1 ± 48.4 | 0.537 |
| Lactate (mmol/L) | ||||
| T1 | 7.45 ± 1.42 | 5.80 ± 0.91 | 6.79 ± 2.30 | 0.200 |
| T2 | 5.65 ± 1.27 * | 4.79 ± 1.35 | 6.70 ± 2.07 | 0.131 |
| HR max (bpm) | ||||
| T1 | 184.8 ± 14.2 | 186.0 ± 11.0 | 179.6 ± 9.0 | 0.791 |
| T2 | 174.7 ± 11.0 * | 173.7 ± 8.1 * | 174.9 ± 7.7 | 0.970 |
| BORG | ||||
| T1 | 18.83 ± 1.17 | 18.43 ± 1.40 | 18.29 ± 0.76 | 0.008 |
| T2 | 18.83 ± 0.98 & | 18.71 ± 1.38 & | 17.00 ± 1.00 * | 0.028 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urdampilleta, A.; Arribalzaga, S.; Viribay, A.; Castañeda-Babarro, A.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of 120 vs. 60 and 90 g/h Carbohydrate Intake during a Trail Marathon on Neuromuscular Function and High Intensity Run Capacity Recovery. Nutrients 2020, 12, 2094. https://doi.org/10.3390/nu12072094
Urdampilleta A, Arribalzaga S, Viribay A, Castañeda-Babarro A, Seco-Calvo J, Mielgo-Ayuso J. Effects of 120 vs. 60 and 90 g/h Carbohydrate Intake during a Trail Marathon on Neuromuscular Function and High Intensity Run Capacity Recovery. Nutrients. 2020; 12(7):2094. https://doi.org/10.3390/nu12072094
Chicago/Turabian StyleUrdampilleta, Aritz, Soledad Arribalzaga, Aitor Viribay, Arkaitz Castañeda-Babarro, Jesús Seco-Calvo, and Juan Mielgo-Ayuso. 2020. "Effects of 120 vs. 60 and 90 g/h Carbohydrate Intake during a Trail Marathon on Neuromuscular Function and High Intensity Run Capacity Recovery" Nutrients 12, no. 7: 2094. https://doi.org/10.3390/nu12072094
APA StyleUrdampilleta, A., Arribalzaga, S., Viribay, A., Castañeda-Babarro, A., Seco-Calvo, J., & Mielgo-Ayuso, J. (2020). Effects of 120 vs. 60 and 90 g/h Carbohydrate Intake during a Trail Marathon on Neuromuscular Function and High Intensity Run Capacity Recovery. Nutrients, 12(7), 2094. https://doi.org/10.3390/nu12072094








