The Impact of a Slow-Release Large Neutral Amino Acids Supplement on Treatment Adherence in Adult Patients with Phenylketonuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Methods for Therapy Adherence Assessment
2.3. Methods for Quality of Life Assessment
2.4. Statistical Analysis
3. Results
3.1. Biochemical Measurement
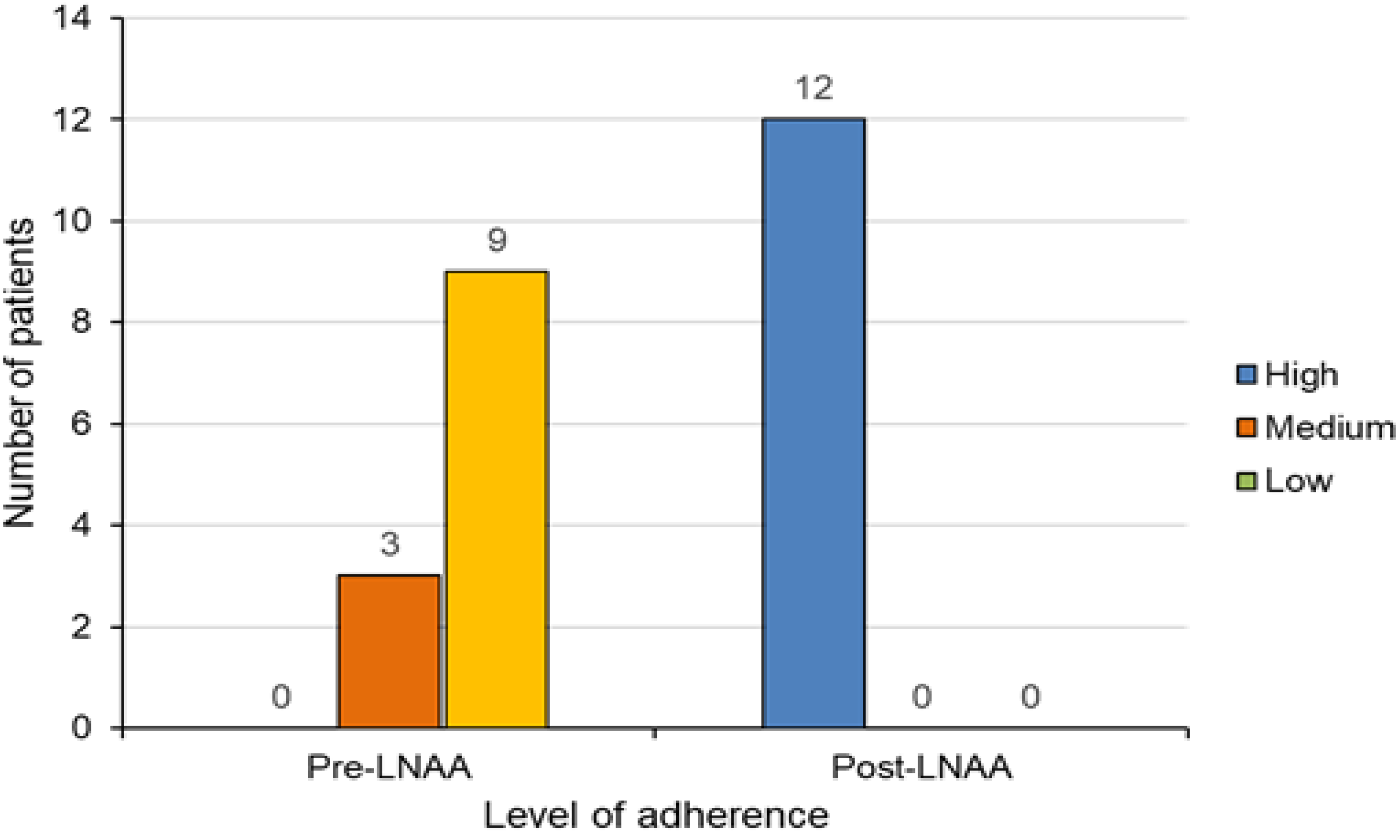
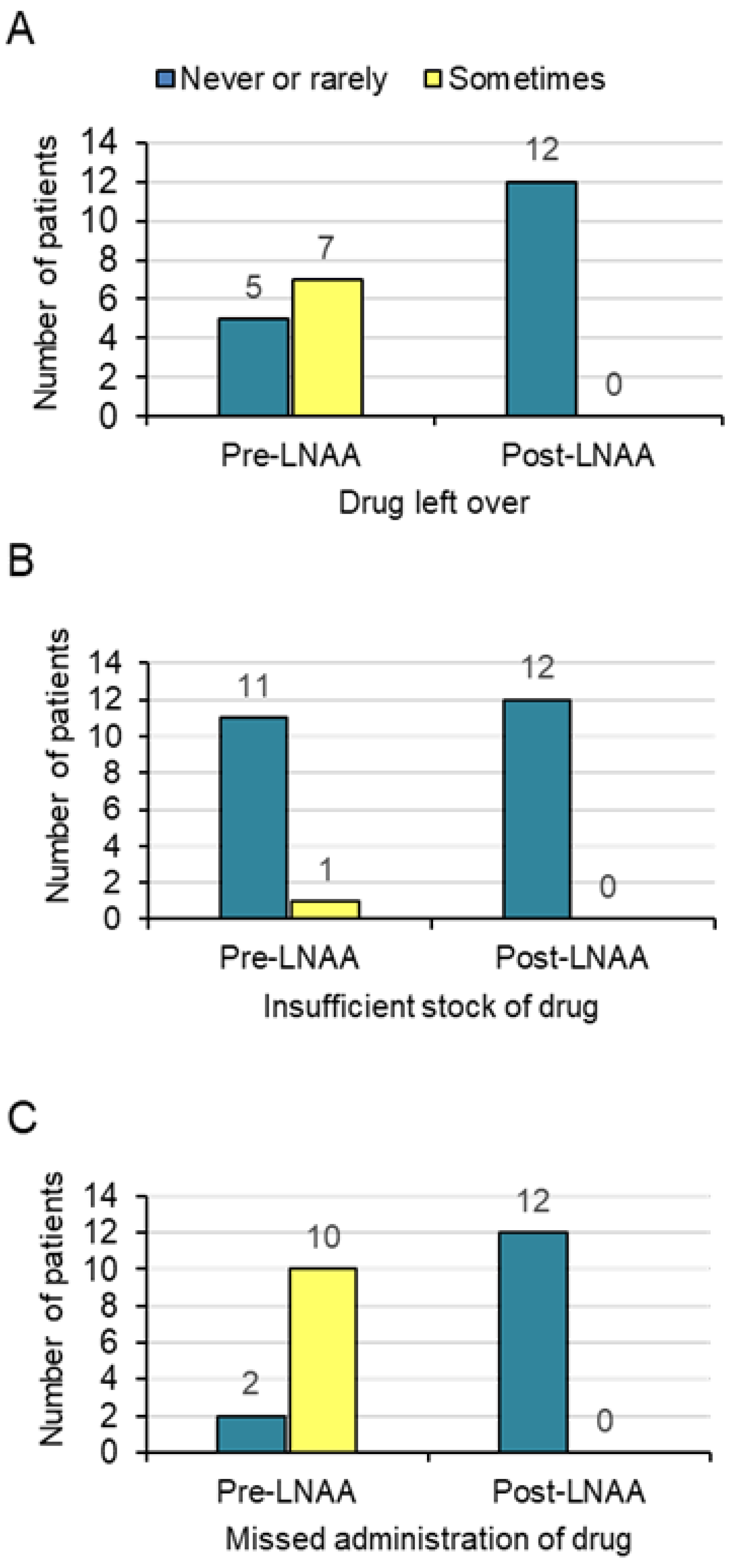
3.2. Medication Adherence Pre- and Post-LNAA
3.3. PKU-QoL Pre- and Post-LNAA Introduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blau, N.; Van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Blau, N. Genetics of Phenylketonuria: Then and Now. Hum. Mutat. 2016, 37, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Camp, K.M.; Parisi, M.A.; Acosta, P.B.; Berry, G.T.; Bilder, D.A.; Blau, N.; Bodamer, O.A.; Brosco, J.P.; Brown, C.S.; Burlina, A.; et al. Phenylketonuria Scientific Review Conference: State of the science and future research needs. Mol. Genet. Metab. 2014, 112, 87–122. [Google Scholar] [CrossRef] [PubMed]
- Van Wegberg, A.M.J.; Macdonald, A.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Gokmen-Ozel, H.; Van Rijn, M.; Burgard, P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 2010, 33, 665–670. [Google Scholar] [CrossRef]
- Enns, G.; Koch, R.; Brumm, V.; Blakely, E.; Suter, R.; Jurecki, E. Suboptimal outcomes in patients with PKU treated early with diet alone: Revisiting the evidence. Mol. Genet. Metab. 2010, 101, 99–109. [Google Scholar] [CrossRef]
- Van Spronsen, F.J.; Van Wegberg, A.M.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A.; et al. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [PubMed]
- Levy, H.; Lamppu, D.; Anastosoaie, V.; Baker, J.L.; DiBona, K.; Hawthorne, S.; Lindenberger, J.; Kinch, D.; Seymour, A.; McIlduff, M.; et al. 5-year retrospective analysis of patients with phenylketonuria (PKU) and hyperphenylalaninemia treated at two specialized clinics. Mol. Genet. Metab. 2020, 129, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, E.; Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Jurecki, E.; Cederbaum, S.; Kopesky, J.; Perry, K.; Rohr, F.; Sanchez-Valle, A.; Viau, K.; Sheinin, M.; Cohen-Pfeffer, J. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017, 120, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Tingley, K.; Chow, A.; Pallone, N.; Smith, M.; Rahman, A.; Chakraborty, P.; Geraghty, M.T.; Irwin, J.; Tessier, L.; et al. Outcomes in pediatric studies of medium-chain acyl-coA dehydrogenase (MCAD) deficiency and phenylketonuria (PKU): A review. Orphanet J. Rare Dis. 2020, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Medford, E.; Hare, D.J.; Wittkowski, A. Demographic and Psychosocial Influences on Treatment Adherence for Children and Adolescents with PKU: A Systematic Review. JIMD Reports 2017, 39, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Van Rijn, M.; Feillet, F.; Lund, A.; Bernstein, L.; Bosch, A.M.; Gizewska, M.; Van Spronsen, F.J. Adherence Issues in Inherited Metabolic Disorders Treated by Low Natural Protein Diets. Ann. Nutr. Metab. 2012, 61, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Cazzorla, C.; Bensi, G.; Biasucci, G.; Leuzzi, V.; Manti, F.; Musumeci, A.; Papadia, F.; Stoppioni, V.; Tummolo, A.; Vendemiale, M.; et al. Living with phenylketonuria in adulthood: The PKU ATTITUDE study. Mol. Genet. Metab. Rep. 2018, 16, 39–45. [Google Scholar] [CrossRef]
- Burlina, A.P.; Cazzorla, C.; Massa, P.; Polo, G.; Loro, C.; Gueraldi, D.; Burlina, A.P. Large Neutral Amino Acid Therapy Increases Tyrosine Levels in Adult Patients with Phenylketonuria: A Long-Term Study. Nutrients 2019, 11, 2541. [Google Scholar] [CrossRef]
- Scala, I.; Riccio, M.P.; Marino, M.; Bravaccio, C.; Parenti, G.; Strisciuglio, P. Large Neutral Amino Acids (LNAAs) Supplementation Improves Neuropsychological Performances in Adult Patients with Phenylketonuria. Nutrients 2020, 12, 1092. [Google Scholar] [CrossRef]
- Pena, M.J.; Pinto, A.; Daly, A.; Macdonald, A.; Azevedo, L.F.; Rocha, J.C.; Borges, N. The Use of Glycomacropeptide in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1794. [Google Scholar] [CrossRef]
- Burlina, A.; Blau, N. Effect of BH4 supplementation on phenylalanine tolerance. J. Inherit. Metab. Dis. 2008, 32, 40–45. [Google Scholar] [CrossRef]
- Longo, N.; Dimmock, D.; Levy, H.; Viau, K.; Bausell, H.; Bilder, D.A.; Burton, B.; Gross, C.; Northrup, H.; Rohr, F.; et al. Evidence- and consensus-based recommendations for the use of pegvaliase in adults with phenylketonuria. Genet. Med. 2018, 21, 1851–1867. [Google Scholar] [CrossRef]
- Christensen, H.N. Metabolism of Amino Acids and Proteins. Annu. Rev. Biochem. 1953, 22, 233–260. [Google Scholar] [CrossRef]
- Van Vliet, D.; Bruinenberg, V.M.; Mazzola, P.N.; Van Faassen, M.H.J.R.; De Blaauw, P.; Kema, I.P.; Heiner-Fokkema, M.R.; Van Anholt, R.D.; Van Der Zee, Y.A.; Van Spronsen, F.J. Large Neutral Amino Acid Supplementation Exerts Its Effect through Three Synergistic Mechanisms: Proof of Principle in Phenylketonuria Mice. PLoS ONE 2015, 10, e0143833. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.J.; De Groot, M.J.; Hoeksma, M.; Reijngoud, D.-J.; Van Rijn, M. Large neutral amino acids in the treatment of PKU: From theory to practice. J. Inherit. Metab. Dis. 2010, 33, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and Predictive Validity of a Self-reported Measure of Medication Adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Beyhaghi, H.; Reeve, B.B.; Rodgers, J.E.; Stearns, S.C. Psychometric Properties of the Four-Item Morisky Green Levine Medication Adherence Scale among Atherosclerosis Risk in Communities (ARIC) Study Participants. Value Health 2016, 19, 996–1001. [Google Scholar] [CrossRef]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the impact of phenylketonuria and its treatment on quality of life of patients and parents from seven European countries. Orphanet J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef]
- Regnault, A.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Benmedjahed, K.; Bosch, A.M. Development and psychometric validation of measures to assess the impact of phenylketonuria and its dietary treatment on patients’ and parents’ quality of life: The phenylketonuria—Quality of life (PKU-QOL) questionnaires. Orphanet J. Rare Dis. 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Brown, M.T.; Bussell, J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef]
- Cuevas, C.D.L. Towards a clarification of terminology in medicine taking behavior: Compliance, adherence and concordance are related although different terms with different uses. Curr. Clin. Pharmacol. 2011, 6, 74–77. [Google Scholar] [CrossRef]
- Schindeler, S.; Ghosh-Jerath, S.; Thompson, S.; Rocca, A.; Joy, P.; Kemp, A.; Rae, C.D.; Green, K.; Wilcken, B.; Christodoulou, J. The effects of large neutral amino acid supplements in PKU: An MRS and neuropsychological study. Mol. Genet. Metab. 2007, 91, 48–54. [Google Scholar] [CrossRef]
- Matalon, R.; Michals-Matalon, K.; Bhatia, G.; Grechanina, E.; Novikov, P.; McDonald, J.D.; Grady, J.; Tyring, S.K.; Guttler, F. Large neutral amino acids in the treatment of phenylketonuria (PKU). J. Inherit. Metab. Dis. 2006, 29, 732–738. [Google Scholar] [CrossRef]
- Matalon, R.; Michals-Matalon, K.; Bhatia, G.; Burlina, A.B.; Burlina, A.P.; Braga, C.; Fiori, L.; Giovannini, M.; Grechanina, E.; Novikov, P.; et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: Effect on blood phenylalanine. J. Inherit. Metab. Dis. 2007, 30, 153–158. [Google Scholar] [CrossRef]
- Concolino, D.; Mascaro, I.; Moricca, M.T.; Bonapace, G.; Matalon, K.; Trapasso, J.; Radhakrishnan, G.; Ferrara, C.; Matalon, R.; Strisciuglio, P. Long-term treatment of phenylketonuria with a new medical food containing large neutral amino acids. Eur. J. Clin. Nutr. 2016, 71, 51–55. [Google Scholar] [CrossRef] [PubMed]
- García, M.I.; Araya, G.; Coo, S.; Waisbren, S.E.; De La Parra, A. Treatment adherence during childhood in individuals with phenylketonuria: Early signs of treatment discontinuation. Mol. Genet. Metab. Rep. 2017, 11, 54–58. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Rokni, H.; Bailey, I.; Rohr, F.; Brown, T.; Warner-Rogers, J. Social factors and the meaning of food in adherence to medical diets: Results of a maternal phenylketonuria summer camp. J. Inherit. Metab. Dis. 1997, 20, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Stirratt, M.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.M.; Czajkowski, S.; Hilliard, M.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K.; et al. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.P.; Lachmann, R.H.; Manara, R.; Cazzorla, C.; Celato, A.; Van Spronsen, F.J.; Burlina, A. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: A systematic review. J. Inherit. Metab. Dis. 2019, 42, 209–219. [Google Scholar] [CrossRef]
| Patients | Gender | Age, Years | Molecular Analysis | Marital Status | Educational Level 1 | Professional Status | Leisure Time |
|---|---|---|---|---|---|---|---|
| 1 | M | 38 | c.473G>A/ c.1315+1G>A | Unmarried | 8 | Agriculture | Not specified |
| 2 | M | 33 | c.473G>A/ c.526C>T | Married | 18 | Insurance agent | Sport |
| 3 | M | 30 | c.473G>A/ c.473G>A | Married | 18 | Unemployed | Intellectual |
| 4 | M | 35 | c.842+3G>C in heterozygosis | Married | 13 | Office worker | Not specified |
| 5 | M | 26 | c.47_48delCT/ c.1315+2T>C | Unmarried | 18 | Freelance | Intellectual |
| 6 | F | 32 | c.842C>T/ c.1315+1G>A | Married | 18 | Teacher | Social activity |
| 7 | F | 28 | c.842C>T/ c.1315+1G>A | Unmarried | 13 | Office worker | Not specify |
| 8 | F | 22 | c.842C>T/ c.1315+1G>A | Unmarried | 13 | Unemployed | Social activity |
| 9 | M | 32 | c.1222C>T/ c.1315+1G>A | Unmarried | 13 | Sales | Not specify |
| 10 | M | 40 | c.782G>A/ c.782G>A | Married | 13 | Freelance | Sport |
| 11 | F | 20 | c.782G>A/ c.1066-11G>A | Unmarried | 13 | Unemployed | Not specify |
| 12 | F | 19 | c.1222C>T/ macro deletion in exon 3 | Married | 13 | Unemployed | Sport |
| Patient | 12 Months before LNAA | 12 Months after LNAA | ||||||
|---|---|---|---|---|---|---|---|---|
| Phe Values | Tyr Values | Phe/Tyr Values | DBS Frequency | Phe Values | Tyr Values | Phe/Tyr Values | DBS Frequency | |
| 1 | 790 ± 80 | 47 ± 8 | 17.2 ± 2.5 | 8 | 825 ± 114 | 68 ± 10 | 12.4 ± 2.4 | 17 |
| 2 | 1033 ± 198 | 54 ± 10 | 19.5 ± 4.4 | 5 | 1269 ± 265 | 65 ± 14 | 20.6 ± 7.6 | 14 |
| 3 | 983 ± 142 | 32 ± 7 | 31.5 ± 7.3 | 10 | 907 ± 166 | 60 ± 8 | 15.4 ± 3 | 20 |
| 4 | 790 ± 118 | 83 ± 14 | 9.7 ± 2.2 | 18 | 769 ± 144 | 98 ± 18 | 8 ± 1.4 | 34 |
| 5 | 838 ± 179 | 57 ± 12 | 15.7 ± 6.6 | 20 | 889 ± 149 | 79 ± 19 | 11.7 ± 2.7 | 24 |
| 6 | 880 ± 160 | 49 ± 11 | 18.3 ± 4 | 38 | 975 ± 148 | 68 ± 29 | 16 ± 5 | 39 |
| 7 | 779 ± 108 | 87 ± 35 | 10.6 ± 5 | 25 | 736 ± 93 | 86 ± 41 | 10.5 ± 4.9 | 27 |
| 8 | 628 ± 148 | 50 ± 13 | 13 ± 5.2 | 26 | 790 ± 147 | 59 ± 15 | 14 ± 4.5 | 29 |
| 9 | 808 ± 135 | 59 ± 7 | 19.9 ± 2.8 | 12 | 925 ± 139 | 79 ± 13 | 11.9 ± 1.8 | 18 |
| 10 | 842 ± 94 | 75 ± 12 | 11.5 ± 2.3 | 23 | 879 ± 91 | 108 ± 35 | 8.5 ± 1.9 | 36 |
| 11 | 765 ± 234 | 58 ± 11 | 13.4 ± 3.9 | 23 | 760 ± 124 | 60 ± 11 | 13 ± 2.7 | 24 |
| 12 | 823 ± 117 | 53 ± 19 | 16.9 ± 5 | 18 | 1000 ± 163 | 68 ± 17 | 15.2 ± 3.7 | 15 |
| Overall Patient Population | Prior to LNAA Treatment | During LNAA Treatment | p Value |
|---|---|---|---|
| Phe | 752 ± 143 | 894 ± 145 | 0.0522 |
| Tyr | 59 ± 13 | 75 ± 16 | 0.0195 |
| Phe/Tyr | 16± 4 | 12 ± 3 | 0.049 |
| Dimension | Item | p Value |
|---|---|---|
| Your Health | In the past 7 days, I became aggressive | 0.044 |
| Your PKU diet and supplements | In the past 7 days, it was hard to take my supplements several times a day | 0.042 |
| In the past 7 days, it was hard to only eat/drink what I should | 0.047 | |
| In the past 7 days, my supplements tasted: very good/good/ok, bad, very bad/I don’t take supplements | 0.017 | |
| In the past 7 days, I thought about eating food that was not on my diet | 0.047 | |
| Your daily life with PKU | Currently, it is inconvenient to carry my supplements with me when I am going on business trips or on holiday | 0.040 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlina, A.P.; Cazzorla, C.; Massa, P.; Loro, C.; Gueraldi, D.; Burlina, A.B. The Impact of a Slow-Release Large Neutral Amino Acids Supplement on Treatment Adherence in Adult Patients with Phenylketonuria. Nutrients 2020, 12, 2078. https://doi.org/10.3390/nu12072078
Burlina AP, Cazzorla C, Massa P, Loro C, Gueraldi D, Burlina AB. The Impact of a Slow-Release Large Neutral Amino Acids Supplement on Treatment Adherence in Adult Patients with Phenylketonuria. Nutrients. 2020; 12(7):2078. https://doi.org/10.3390/nu12072078
Chicago/Turabian StyleBurlina, Alessandro P., Chiara Cazzorla, Pamela Massa, Christian Loro, Daniela Gueraldi, and Alberto B. Burlina. 2020. "The Impact of a Slow-Release Large Neutral Amino Acids Supplement on Treatment Adherence in Adult Patients with Phenylketonuria" Nutrients 12, no. 7: 2078. https://doi.org/10.3390/nu12072078
APA StyleBurlina, A. P., Cazzorla, C., Massa, P., Loro, C., Gueraldi, D., & Burlina, A. B. (2020). The Impact of a Slow-Release Large Neutral Amino Acids Supplement on Treatment Adherence in Adult Patients with Phenylketonuria. Nutrients, 12(7), 2078. https://doi.org/10.3390/nu12072078







