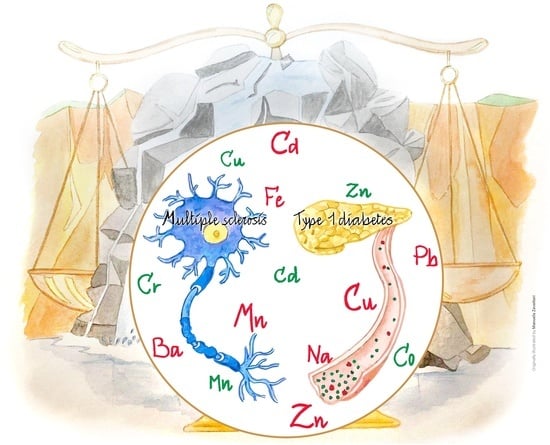
Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk
Abstract
1. Introduction
2. Autoimmunity
2.1. Multiple Sclerosis
2.2. Type I Diabetes
3. Trace Elements
- potentially toxic elements, e.g., lead (Pb), cadmium (Cd), fluorine (F), mercury (Hg), arsenic (As), aluminum (Al), barium (Ba), lithium (Li), tin (Sn);
- elements of probable physiological importance, e.g., manganese (Mn), silicon (Si), nickel (Ni), boron (B), vanadium (V);
- essential elements, e.g., chromium (Cr), copper (Cu), zinc (Zn), selenium (Se), molybdenum (Mb), cobalt (Co), iodine (I).
3.1. Potentially Toxic Elements
3.1.1. Lead
3.1.2. Cadmium
3.1.3. Barium
3.1.4. Lithium
3.1.5. Mercury
3.2. Likely Essential Elements
Silicon
3.3. Essential Elements
3.3.1. Zinc
3.3.2. Copper
3.3.3. Chromium
4. Legislation of the Elements in Water and Food
- Directive 98/83/CE, relating to the quality of water intended for human consumption [122,123,124], was transposed in Italy, with the emanation of Legislative Decree No. 31/2001, which defines “water devoted to human consumption” as
- -
- all treated or untreated waters, intended for drinking, culinary or food preparation or for other domestic uses, regardless of their origin, whether they are supplied through a distribution network, by means of tanks, in bottles or containers;
- -
- all waters used in a food business for the manufacture, treatment, conservation or placing on the market of products or substances intended for human consumption.
- Directive 2003/40/CE determines the list, the concentration limits and the labeling indications for the elements and compounds of natural mineral waters, as well as the conditions of use of ozone-enriched air for the treatment of natural mineral waters and spring waters. This Directive has been transposed in Italy with the Ministerial Decree of 29 December 2003. It imposes less restrictive limits on some elements and compounds of mineral water, especially with regard to those substances that can be dangerous to health.
- ○
- “water subjected to an ozone-enriched air oxidation technique”, if this technique is used to remove iron, manganese, sulfur and arsenic residues;
- ○
- “it contains more than 1.5 mg/L of fluorine: regular consumption by infants and children under the age of 7 is not appropriate”.
- Regulation n. 1881/2006 CE defines the maximum values of some contaminants in food.
- Regulation CE 333/2007 deals with the methods of sampling and analysis for the official control of the maximum values of metals/metalloids;
- Council Directive 96/23/CE covers the surveillance of residues of chemical elements in food of animal origin;
- Regulation (UE) n. 1169/2011 details the provision of food information to consumers: mandatory information on the label;
- Regulation (UE) N. 432/2012 relates to the compilation of a list of permitted health claims on food products, for vitamins, minerals and other substances, e.g., “Zinc contributes to the normal function of the immune system”;
- Directive CE 2009/54 details the use and marketing of natural mineral waters.This directive does not apply
- (a)
- to waters which are medicinal products within the meaning of Directive 2001/83/EC of the European Parliament and of the Council, of 6 November 2001, on the Community code relating to medicinal products for human use;
- (b)
- to natural mineral waters used for healing purposes at the source in thermal or hydrothermal establishments.
- D. Lgs. N. 28, 15/02/2016: Implementation of Council Directive 2013/51/EURATOM, of 22 October 2013, which establishes requirements for the protection of the population’s health with regard to radioactive substances present in water devoted to human consumption.
- Decree 10/02/2015: Assessment criteria of the characteristics of natural mineral waters.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- López-Abente, G.; Locutura-Rupérez, J.; Fernández-Navarro, P.; Martín-Méndez, I.; Bel-Lan, A.; Núñez, O. Compositional analysis of topsoil metals and its associations with cancer mortality using spatial misaligned data. Environ. Geochem. Health 2018, 40, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, R.; Stern, P.; Dawood, I.; Cheema, S. Metals and Disease: A Global Primary Health Care Perspective. J. Toxicol. 2011, 2011, 319136. [Google Scholar] [CrossRef] [PubMed]
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019. [Google Scholar]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Griem, P.; Gleichmann, E. Metal ion induced autoimmunity. Curr. Opin. Immunol. 1995, 7, 831–838. [Google Scholar] [CrossRef]
- Schiraldi, M.; Monestier, M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009, 30, 502–509. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, S. Structural basis of metal hypersensitivity. Immunol. Res. 2013, 55, 83–90. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of Selected Vitamins and Trace Elements to Immune Function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Russell, M.W. Mucosal Surfaces: Immunological Protection. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–12. [Google Scholar]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Bottini, N.; Vang, T.; Cucca, F.; Mustelin, T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin. Immunol. 2006, 18, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Fowell, D.; Powrie, F.; Saoudi, A.; Seddon, B.; Heath, V.; Mason, D. The role of subsets of CD4+ T cells in autoimmunity. In T Cell Subsets in Infectious and Autoimmune Diseases; John Wiley & Sons, Ltd.: Chichester, UK, 1995; pp. 173–182. [Google Scholar]
- Jäger, A.; Kuchroo, V.K. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand. J. Immunol. 2010, 72, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Burris, T.P. Th17 cells in Type 1 diabetes: A future perspective. Diabetes Manag. 2015, 5, 247–250. [Google Scholar] [CrossRef]
- Kennedy Bedoya, S.; Lam, B.; Lau, K.; Larkin III, J. Th17 Cells in Immunity and Autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789. [Google Scholar]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH 17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH 17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Kondělková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med. (Hradec Kralove) 2010, 53, 73–77. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Lückel, C.; Picard, F.; Raifer, H.; Campos Carrascosa, L.; Guralnik, A.; Zhang, Y.; Klein, M.; Bittner, S.; Steffen, F.; Moos, S.; et al. IL-17+ CD8+ T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat. Commun. 2019, 10, 5722. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Jin, H.; Xu, Y. T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 1027. [Google Scholar] [CrossRef]
- Zhao, C.; Chu, Y.; Liang, Z.; Zhang, B.; Wang, X.; Jing, X.; Hao, M.; Wang, Y.; An, J.; Zhang, X.; et al. Low dose of IL-2 combined with rapamycin restores and maintains the long-term balance of Th17/Treg cells in refractory SLE patients. BMC Immunol. 2019, 20, 32. [Google Scholar] [CrossRef]
- Nistala, K.; Wedderburn, L.R. Th17 and regulatory T cells: Rebalancing pro-and anti-inflammatory forces in autoimmune arthritis. Rheumatol. 2009, 48, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Putzki, N.; Limmroth, V.; Remus, R.; Lindemann, M.; Knop, D.; Mueller, N.; Hardt, C.; Kreuzfelder, E.; Grosse-Wilde, H. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J. Neuroimmunol. 2006, 180, 178–184. [Google Scholar] [CrossRef]
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015, 125, 4212–4222. [Google Scholar] [CrossRef]
- Stejskal, V. Allergy and Autoimmunity Caused by Metals: A Unifying Concept. In Vaccines and Autoimmunity; Wiley-Blackwell: New York, NY, USA, 2014; pp. 57–63. ISBN 9781118663721. [Google Scholar]
- Gilmore, C.P.; Donaldson, I.; Bö, L.; Owens, T.; Lowe, J.; Evangelou, N. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: A comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J. Neurol. Neurosurg. Psychiatry 2009, 80, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef]
- Orton, S.M.; Herrera, B.M.; Yee, I.M.; Valdar, W.; Ramagopalan, S.V.; Sadovnick, A.D.; Ebers, G.C. Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 2006, 5, 932–936. [Google Scholar] [CrossRef]
- Multiple Sclerosis International Federation. Atlas of MS 2013: Mapping Multiple Sclerosis around the World; MSI Federation: London, UK, 2013. [Google Scholar]
- Urru, S.A.M.; Antonelli, A.; Sechi, G.M. Prevalence of multiple sclerosis in Sardinia: A systematic cross-sectional multi-source survey. Mult. Scler. J. 2020, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Baranzini, S.E.; Oksenberg, J.R. The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years. Trends Genet. 2017, 33, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.E.; Iizumi, T.; Battaglia, T.; Liu, M.; Perez-Perez, G.I.; Herbert, J.; Blaser, M.J. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci. Rep. 2019, 9, 16396. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitán, M.I. Multiple sclerosis and environmental factors: The role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol. Scand. 2015, 132, 46–55. [Google Scholar] [CrossRef]
- Russell, R.D.; Lucas, R.; Brennan, V.; Sherriff, J.L.; Begley, A.; Black, L.J.; Chapman, C.; Coulthard, A.; Dear, K.; Dwyer, T.; et al. Reported changes in dietary behavior following a first clinical diagnosis of central nervous system demyelination. Front. Neurol. 2018, 9, 161. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef]
- Rosenkranz, E.; Maywald, M.; Hilgers, R.D.; Brieger, A.; Clarner, T.; Kipp, M.; Plümäkers, B.; Meyer, S.; Schwerdtle, T.; Rink, L. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J. Nutr. Biochem. 2016, 29, 116–123. [Google Scholar] [CrossRef]
- Etemadifar, M.; Mehrabi, B.; Kiani-Peykani, R.; Abtahi, S.H.; Nekouie-Isfahani, K.; Ramagopalan, S.V.; Fereidan-Esfahani, M. Soil heavy metals are associated with the distribution of multiple sclerosis in Isfahan, Iran. Acta Neurol. Scand. 2016, 134, 292–299. [Google Scholar] [CrossRef]
- Monti, M.C.; Guido, D.; Montomoli, C.; Sardu, C.; Sanna, A.; Pretti, S.; Lorefice, L.; Marrosu, M.G.; Valera, P.; Cocco, E. Is geo-environmental exposure a risk factor for multiple sclerosis? A population-based cross-sectional study in South-Western Sardinia. PLoS ONE 2016, 11, e0163313. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; Socci, C.; Stabilini, A.; Valle, A.; Monti, P.; Piemonti, L.; Nano, R.; Olek, S.; Maffi, P.; Scavini, M.; et al. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 2011, 60, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Ryba-Stanisbawowska, M.; Skrzypkowska, M.; Myvliwska, J.; Myvliwiec, M. The Serum IL-6 Profile and Treg/Th17 Peripheral Cell Populations in Patients with Type 1 Diabetes. Mediators Inflamm. 2013, 2013, 205284. [Google Scholar]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef] [PubMed]
- Krischer, J.P.; Lynch, K.F.; Schatz, D.A.; Ilonen, J.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.X.; Simell, O.G.; Toppari, J.; et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: The TEDDY study. Diabetologia 2015, 58, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Ilonen, J.; Hammais, A.; Laine, A.P.; Lempainen, J.; Vaarala, O.; Veijola, R.; Simell, O.; Knip, M. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013, 62, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, D.; zu Castell, W.; Bonifacio, E.; Rewers, M.; Hagopian, W.A.; She, J.X.; Lernmark, A.; Toppari, J.; Vehik, K.; Williams, A.J.K.; et al. Time-resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 2019, 68, 119–130. [Google Scholar] [CrossRef]
- Chiang, J.L.; Maahs, D.M.; Garvey, K.C.; Hood, K.K.; Laffel, L.M.; Weinzimer, S.A.; Wolfsdorf, J.I.; Schatz, D. Type 1 diabetes in children and adolescents: A position statement by the American Diabetes Association. Diabetes Care 2018, 41, 2026–2044. [Google Scholar] [CrossRef]
- Melendez-Ramirez, L.Y.; Richards, R.J.; Cefalu, W.T. Complications of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 625–640. [Google Scholar] [CrossRef]
- Contu, D.; Morelli, L.; Zavattari, P.; Lampis, R.; Angius, E.; Frongia, P.; Murru, D.; Maioli, M.; Francalacci, P.; Todd, J.A.; et al. Sex-related bias and exclusion mapping of the nonrecombinant portion of chromosome Y in human type 1 diabetes in the isolated founder population of Sardinia. Diabetes 2002, 51, 3573–3576. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Herr, M.; Dudbridge, F.; Zavattari, P.; Cucca, F.; Guja, C.; March, R.; Duncan Campbell, R.; Barnett, A.H.; Bain, S.C.; Todd, J.A.; et al. Evaluation of fine mapping strategies for a multifactorial disease locus: Systematic linkage and association analysis of IDDM1 in the HLA region on chromosome 6p21. Hum. Mol. Genet. 2000, 9, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Zavattari, P.; Lampis, R.; Motzo, C.; Loddo, M.; Mulargia, A.; Whalen, M.; Maioli, M.; Angius, E.; Todd, J.A.; Cucca, F. Conditional linkage disequilibrium analysis of a complex disease superlocus, IDDM1 in the HLA region, reveals the presence of independent modifying gene effects influencing the type 1 diabetes risk encoded by the major HLA-DQB1,-DRB1 disease loci. Hum. Mol. Genet. 2001, 10, 881–889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noble, J.A.; Valdes, A.M.; Varney, M.D.; Carlson, J.A.; Moonsamy, P.; Fear, A.L.; Lane, J.A.; Lavant, E.; Rappner, R.; Louey, A.; et al. HLA class I and genetic susceptibility to type 1 diabetes: Results from the type 1 diabetes genetics consortium. Diabetes 2010, 59, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M.; Cook, M.; Klitz, W.; Thomson, G.; Erlich, H.A. The role of HLA class II genes in insulin-dependent diabetes mellitus: Molecular analysis of 180 Caucasian, multiplex families. Am. J. Hum. Genet. 1996, 59, 1134–1148. [Google Scholar] [PubMed]
- Zavattari, P.; Lampis, R.; Mulargia, A.; Loddo, M.; Angius, E.; Todd, J.A.; Cucca, F. Confirmation of the DRB1-DQB1 loci as the major component of IDDM1 in the isolated founder population of Sardinia. Hum. Mol. Genet. 2000, 9, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D.; Howson, J.M.M.; Smyth, D.; Walker, N.M.; Stevens, H.; Yang, J.H.M.; She, J.X.; Eisenbarth, G.S.; Rewers, M.; Todd, J.A.; et al. Confirmation of novel type 1 diabetes risk loci in families. Diabetologia 2012, 55, 996–1000. [Google Scholar] [CrossRef]
- Rewers, M.; Ludvigsson, J. Environmental risk factors for type 1 diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef]
- Oilinki, T.; Otonkoski, T.; Ilonen, J.; Knip, M.; Miettinen, P. Prevalence and characteristics of diabetes among Somali children and adolescents living in Helsinki, Finland. Pediatr. Diabetes 2012, 13, 176–180. [Google Scholar] [CrossRef]
- Söderström, U.; Åman, J.; Hjern, A. Being born in Sweden increases the risk for type 1 diabetes—A study of migration of children to Sweden as a natural experiment. Acta Paediatr. Int. J. Paediatr. 2012, 101, 73–77. [Google Scholar] [CrossRef]
- Squitti, R.; Negrouk, V.; Perera, M.; Llabre, M.M.; Ricordi, C.; Rongioletti, M.C.A.; Mendez, A.J. Serum copper profile in patients with type 1 diabetes in comparison to other metals. J. Trace Elem. Med. Biol. 2019, 56, 156–161. [Google Scholar] [CrossRef]
- Adewumi, M.T.; Njoku, C.H.; Saidu, Y.; Abubakar, M.K.; Shehu, R.A.; Bilbis, L.S. Serum Chromium, Copper and Manganese Levels of Diabetic Subjects in Katsina, Nigeria. Asian J. Biochem. 2007, 2, 284–288. [Google Scholar]
- Forte, G.; Bocca, B.; Peruzzu, A.; Tolu, F.; Asara, Y.; Farace, C.; Oggiano, R.; Madeddu, R. Blood metals concentration in type 1 and type 2 diabetics. Biol. Trace Elem. Res. 2013, 156, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Cheng, N.; Bryden, N.A.; Polansky, M.M.; Cheng, N.; Chi, J.; Feng, J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 1997, 46, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Korc, M. Manganese action on pancreatic protein synthesis in normal and diabetic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 1983, 8, G628–G634. [Google Scholar] [CrossRef] [PubMed]
- Valera, P.; Zavattari, P.; Albanese, S.; Cicchella, D.; Dinelli, E.; Lima, A.; De Vivo, B. A correlation study between multiple sclerosis and type 1 diabetes incidences and geochemical data in Europe. Environ. Geochem. Health 2014, 36, 79–98. [Google Scholar] [CrossRef]
- Valera, P.; Zavattari, P.; Sanna, A.; Pretti, S.; Marcello, A.; Mannu, C.; Targhetta, C.; Bruno, G.; Songini, M. Zinc and Other Metals Deficiencies and Risk of Type 1 Diabetes: An Ecological Study in the High Risk Sardinia Island. PLoS ONE 2015, 10, e0141262. [Google Scholar] [CrossRef]
- Samuelsson, U.; Oikarinen, S.; Hyöty, H.; Ludvigsson, J. Low zinc in drinking water is associated with the risk of type 1 diabetes in children. Pediatr. Diabetes 2011, 12, 156–164. [Google Scholar] [CrossRef]
- Martinez-Zamudio, R.; Ha, H.C. Environmental epigenetics in metal exposure. Epigenetics 2011, 6, 820–827. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Wang, D.; Baccarelli, A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2012, 41, 79–105. [Google Scholar] [CrossRef]
- Abedi-Valugerdi, M.; Hu, H.; Gö, G.; Mö, M. Mercury-induced anti-nucleolar autoantibodies can transgress the membrane of living cells in vivo and in vitro. Int. Immunol. 1999, 11, 605–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffman, H.N.; Phyliky, R.L.; Fleming, C.R. Zinc-induced copper deficiency. Gastroenterology 1988, 94, 508–512. [Google Scholar] [CrossRef]
- Marth, E.; Barth, S.; Jelovcan, S. Influence of cadmium on the immune system. Description of stimulating reactions. Cent. Eur. J. Public Health 2000, 8, 40–44. [Google Scholar]
- Zeng, H.; Uthus, E.O.; Combs, G.F. Mechanistic aspects of the interaction between selenium and arsenic. J. Inorg. Biochem. 2005, 99, 1269–1274. [Google Scholar] [CrossRef]
- Gaddipati, J.P.; Rajeshkumar, N.V.; Grove, J.C.; Maharaj, S.V.M.; Centeno, J.A.; Maheshwari, R.K.; Jonas, W.B. Low-Dose Cadmium Exposure Reduces Human Prostate Cell Transformation in Culture and Up-Regulates Metallothionein and MT-1G mRNA. Nonlinearity Biol. Toxicol. Med. 2003, 1, 154014203914343. [Google Scholar] [CrossRef] [PubMed]
- Herkovits, J.; Perez-Coll, C.S. Increased resistance against cadmium toxicity by means of pretreatment with low cadmium/zinc concentrations in Bufo arenarum embryos. Biol. Trace Elem. Res. 1995, 49, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Bogden, J.D. The Essential Trace Elements and Minerals. In Clinical Nutrition of the Essential Trace Elements and Minerals; Humana Press: Totowa, NJ, USA, 2000; pp. 3–9. [Google Scholar]
- Martinez-Finley, E.J.; Chakraborty, S.; Fretham, S.J.B.; Aschner, M. Cellular transport and homeostasis of essential and nonessential metals. Metallomics 2012, 4, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.; Vega, R.; Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 2006, 12, RA57–RA65. [Google Scholar]
- Goyer, R.A. Transplacental transport of lead. Environ. Health Perspect. 1990, 89, 101–105. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity, a review of the literature. Part I: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar]
- Fenga, C.; Gangemi, S.; Di Salvatore, V.; Falzone, L.; Libra, M. Immunological effects of occupational exposure to lead (Review). Mol. Med. Rep. 2017, 15, 3355–3360. [Google Scholar] [CrossRef] [PubMed]
- Dobrakowski, M.; Boroń, M.; Czuba, Z.P.; Kasperczyk, A.; Machoń-Grecka, A.; Kasperczyk, S. Cytokines related to three major types of cell-mediated immunity in short- and long-term exposures to lead compounds. J. Immunotoxicol. 2016, 13, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Krocova, Z.; MacEla, A.; Kroca, M.; Hernychova, L. The immunomodulatory effect(s) of lead and cadmium on the cells of immune system in vitro. Toxicol. In Vitro 2000, 14, 33–40. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Canada, A.T.; Sacco, C. Trace Elements and Public Health. Annu. Rev. Public Health 1985, 6, 131–146. [Google Scholar] [CrossRef] [PubMed]
- McNeill, I.R.; Isoardi, K.Z. Barium poisoning: An uncommon cause of severe hypokalemia. Toxicol. Commun. 2019, 3, 88–90. [Google Scholar] [CrossRef]
- De Sarno, P.; Axtell, R.C.; Raman, C.; Roth, K.A.; Alessi, D.R.; Jope, R.S. Lithium Prevents and Ameliorates Experimental Autoimmune Encephalomyelitis. J. Immunol. 2008, 181, 338–345. [Google Scholar] [CrossRef]
- Mehri, A. Trace elements in human nutrition (II)—An update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar]
- WHO. Environmental Health Criteria 236 PRINCIPLES AND METHODS FOR ASSESSING AUTOIMMUNITY ASSOCIATED WITH EXPOSURE TO First Draft Prepared by the World Health Organization Collaborating; World Health Organization: Geneva, Switzerland, 2006; Volume 4, pp. 1–4. [Google Scholar]
- Bagenstose, L.M.; Mentink-Kane, M.M.; Brittingham, A.; Mosser, D.M.; Monestier, M. Mercury enhances susceptibility to murine leishmaniasis. Parasite Immunol. 2001, 23, 633–640. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Devine, P.J. Mercury—Are we studying the right endpoints and mechanisms. Fuel Process. Technol. 2000, 65, 35–42. [Google Scholar] [CrossRef]
- Havarinasab, S.; Lambertsson, L.; Qvarnström, J.; Hultman, P. Dose-response study of thimerosal-induced murine systemic autoimmunity. Toxicol. Appl. Pharmacol. 2004, 194, 169–179. [Google Scholar] [CrossRef]
- Mayeux, J.M.; Escalante, G.M.; Christy, J.M.; Pawar, R.D.; Kono, D.H.; Pollard, K.M. Silicosis and Silica-Induced Autoimmunity in the Diversity Outbred Mouse. Front. Immunol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Miller, F.W.; Alfredsson, L.; Costenbader, K.H.; Kamen, D.L.; Nelson, L.M.; Norris, J.M.; De Roos, A.J. Epidemiology of environmental exposures and human autoimmune diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J. Autoimmun. 2012, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Zandman-Goddard, G.; Blank, M.; Ehrenfeld, M.; Gilburd, B.; Peter, J.; Shoenfeld, Y. A comparison of autoantibody production in asymptomatic and symptomatic women with silicone breast implants. J. Rheumatol. 1999, 26, 73–77. [Google Scholar] [PubMed]
- Soriano, A.; Butnaru, D.; Shoenfeld, Y. Long-term inflammatory conditions following silicone exposure: The expanding spectrum of the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Clin. Exp. Rheumatol. 2014, 32, 151–154. [Google Scholar] [PubMed]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc status and autoimmunity: A systematic review and meta-analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Hajo, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Bonaventura, P.; Lamboux, A.; Albarède, F.; Miossec, P. A Feedback Loop between Inflammation and Zn Uptake. PLoS ONE 2016, 11, e0147146. [Google Scholar] [CrossRef]
- Guttek, K.; Wagenbrett, L.; Reinhold, A.; Grüngreiff, K.; Reinhold, D. Zinc aspartate suppresses proliferation and Th1/Th2/Th17 cytokine production of pre-activated human T cells in vitro. J. Trace Elem. Med. Biol. 2018, 49, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, S.H.; Jhun, J.; Seo, H.B.; Jung, K.A.; Yang, C.W.; Park, S.H.; Cho, M. La A Combination with Probiotic Complex, Zinc, and Coenzyme Q10 Attenuates Autoimmune Arthritis by Regulation of Th17/Treg Balance. J. Med. Food 2018, 21, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, C.; Fukada, T.; Kanamoto, M.; Ohashi, W.; Hojyo, S.; Atsumi, T.; Ueda, N.; Azuma, I.; Hirota, H.; Murakami, M.; et al. Zinc suppresses T h 17 development via inhibition of STAT3 activation. Int. Immunol. 2010, 22, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.; Choi, Y.H.; Hwang, Y.; Kim, D.H.; Cho, S.; Hong, S.J.; Lee, W.W. Inhibition of interleukin-1β-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology 2015, 146, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Miyai, T.; Fujishiro, H.; Kawamura, M.; Yasuda, T.; Hijikata, A.; Bin, B.H.; Irié, T.; Tanaka, J.; Atsumi, T.; et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl. Acad. Sci. USA 2014, 111, 11786–11791. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Guttek, K.; Grüngreiff, K.; Thielitz, A.; Bühling, F.; Reinhold, A.; Brocke, S.; Reinhold, D. Oral zinc aspartate treats experimental autoimmune encephalomyelitis. BioMetals 2014, 27, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 7918–7924. [Google Scholar] [CrossRef]
- Sedighi, B.; Ebrahimi, H.A.; Haghdoost, A.A.; Abotorabi, M. Comparison of serum levels of copper and zinc among multiple sclerosis patients and control group. Iran. J. Neurol. 2013, 12, 125. [Google Scholar]
- Sitasawad, S.; Deshpande, M.; Katdare, M.; Tirth, S.; Parab, P. Beneficial effect of supplementation with copper sulfate on STZ-diabetic mice (IDDM). Diabetes Res. Clin. Pract. 2001, 52, 77–84. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001.
- Gad, S.C. Acute and chronic systemic chromium toxicity. Sci. Total Environ. 1989, 86, 149–157. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.; Seth, P.; Chaturvedi, U. Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000, 26, 22–27. [Google Scholar] [PubMed]
- Brown, R.O.; Forloines-Lynn, S.; Cross, R.E.; Heizer, W.D. Chromium deficiency after long-term total parenteral nutrition. Dig. Dis. Sci. 1986, 31, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida Regionali. Available online: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=4458&area=acque_potabili&menu=norme (accessed on 29 May 2020).
- Toto, T.; Attiva, C.; Valentini, V.; Zampetti, G. Acque in Deroga. Le Deroghe per le Acque Potabili: L’evoluzione del Problema, i Territori Coinvolti, la Mancata Informazione ai Cittadini e gli Interventi Necessari. 2012. Available online: https://www.legambiente.it/sites/default/files/docs/dossier_derogheacquepotabili_2012.pdf (accessed on 13 July 2020).
- World Health Organization Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017.
- Cicchella, D.; Albanese, S.; De Vivo, B.; Dinelli, E.; Giaccio, L.; Lima, A.; Valera, P. Trace elements and ions in Italian bottled mineral waters: Identification of anomalous values and human health related effects. J. Geochem. Explor. 2010, 107, 336–349. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; De Vivo, B.; Albanese, S.; Cicchella, D.; Valera, P. Hydrogeochemical analysis on Italian bottled mineral waters: Effects of geology. J. Geochem. Explor. 2010, 107, 317–335. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and trace elements in tap water from Italy. J. Geochem. Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Comparative study between bottled mineral and tap water in Italy. J. Geochem. Explor. 2012, 112, 368–389. [Google Scholar] [CrossRef]
- Lodi, M.B.; Fanari, F.; Fanti, A.; Desogus, F.; Getaneh, W.; Mazzarella, G.; Valera, P. Preliminary Study and Numerical Investigation of an Electrostatic Unit for the Removal of Fluoride from Thermal Water of Ethiopian Rift Valley. IEEE J. Multiscale Multiphysics Comput. Tech. 2020, 5, 72–82. [Google Scholar] [CrossRef]

| Threshold Concentration Values for Mineral Water and Water Devoted to Human Consumption | |||||||
|---|---|---|---|---|---|---|---|
| DM 29/12/2003 (Italy) Mineral Water | D.L. 31/2001 (Italy) Water Devoted to Human Consumption | Directive EU 2003/40/CE Mineral Water | Directive EU 1998/83/CE Water Devoted to Human Consumption | EPA (USA) Guidelines | WHO Guidelines | D. LGS. 152/06 (Italy) Groundwater | |
| Ec (μS/cm) | - | 2500 (g.l.) | - | 2500 (g.l.) | - | - | - |
| pH | - | ≥6.5–9.5 (g.l.) | - | ≥6.5–9.5 (g.l.) | ≥6.5–8.5 | - | - |
| Aluminum (μg/L) | - | 200 (g.l.) | - | 200 (g.l.) | - | 200 | 200 |
| Ammonium (mg/L) | - | 0.5 (g.l.) | - | 0.5 (g.l.) | - | - | <0.05 |
| Antimony (μg/L) | 5 | 5 | 5 | 5 | 6 | 20 | 5 |
| Arsenic (μg/L) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Barium (μg/L) | 1000 | - | 1000 | - | 2000 | 1300 | - |
| Beryllium (μg/L) | - | - | - | - | 4 | - | 4 |
| Boron (μg/L) | 5000 | 1000 | - | 1000 | - | 2400 | 1000 |
| Cadmium (μg/L) | 3 | 5 | 3 | 5 | 5 | 3 | 5 |
| Chlorides (mg/L) | - | 250 (g.l.) | - | 250 (g.l.) | - | 250 | - |
| Chromium (μg/L) | 50 | 50 | 50 | 50 | 100 | 50 | 50 |
| Iron (μg/L) | - | 200 (g.l.) | - | 200 (g.l.) | 200 | - | 200 |
| Fluorides (mg/L) | 5 (1.5 *) | 1.5 | 5 | 1.5 | 4 | 1.5 | 1.5 |
| Phosphorus (mg/L) | - | - | - | 5 | - | - | - |
| Lead (μg/L) | 10 | 10 | 10 | 10 | 15 | 10 | 10 |
| Manganese (μg/L) | 500 | 50 (g.l.) | 500 | 50 (g.l.) | - | 400 | 50 |
| Mercury (μg/L) | 1 | 1 | 1 | 1 | 2 | 6 | 1 |
| Molybdenum (μg/L) | - | - | - | - | - | 70 | - |
| Nickel (μg/L) | 20 | - | 20 | 20 | - | 70 | 20 |
| Nitrates (mg/L) | 45 (10 *) | 50 | 50 | 50 | 10 | 50 | - |
| Nitrites (mg/L) | 0.02 | 0.5 | 0.1 | 0.5 | 1 | 3 | 0.5 |
| Copper (μg/L) | 1000 | 1000 | 1000 | 2000 | 1300 | 2000 | 1000 |
| Selenium (μg/L) | 10 | 10 | 10 | 10 | 50 | 40 | 10 |
| Sodium (mg/L) | - | 200 (g.l.) | - | 200 (g.l.) | - | 200 | - |
| Sulphates (mg/L) | - | 250 (g.l.) | - | 250 (g.l.) | - | 500 | 250 |
| Thallium (μg/L) | - | - | - | - | 0.5/2 | - | 2 |
| Uranium (μg/L) | - | - | - | - | 30 | 30 | - |
| Vanadium (μg/L) | - | 140 | - | - | - | - | - |
| Zinc (μg/L) | - | - | - | - | - | 3000 | 3000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients 2020, 12, 2074. https://doi.org/10.3390/nu12072074
Cannas D, Loi E, Serra M, Firinu D, Valera P, Zavattari P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients. 2020; 12(7):2074. https://doi.org/10.3390/nu12072074
Chicago/Turabian StyleCannas, Daniela, Eleonora Loi, Matteo Serra, Davide Firinu, Paolo Valera, and Patrizia Zavattari. 2020. "Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk" Nutrients 12, no. 7: 2074. https://doi.org/10.3390/nu12072074
APA StyleCannas, D., Loi, E., Serra, M., Firinu, D., Valera, P., & Zavattari, P. (2020). Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients, 12(7), 2074. https://doi.org/10.3390/nu12072074