Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study
Abstract
1. Introduction
1.1. Importance of Selenium
1.2. Selenium Intakes and Older Adults
1.3. Potential Mechanisms of Selenium Function
1.4. Aims and Hypotheses
2. Materials and Methods
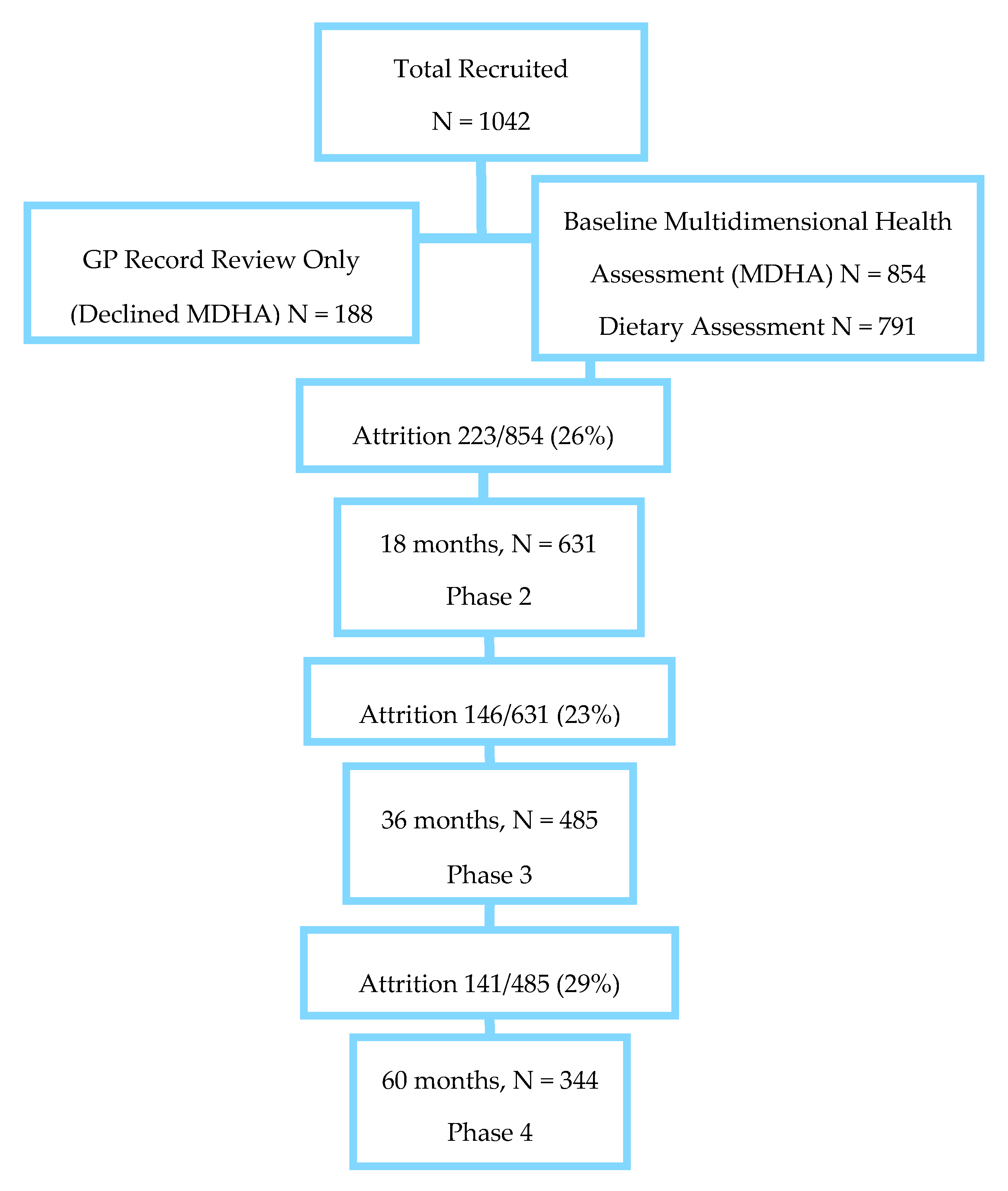
2.1. Study Population
2.2. Socioeconomic, Lifestyle and Health Measures
2.3. Musculoskeletal Function
2.4. Dietary Assessment
2.5. Statistical Analyses
2.6. Sensitivity Analysis
3. Results
3.1. Participant Characteristics
3.2. Food Intakes of Selenium
3.3. Musculoskeletal Performance
3.3.1. Cross-Sectional Analyses
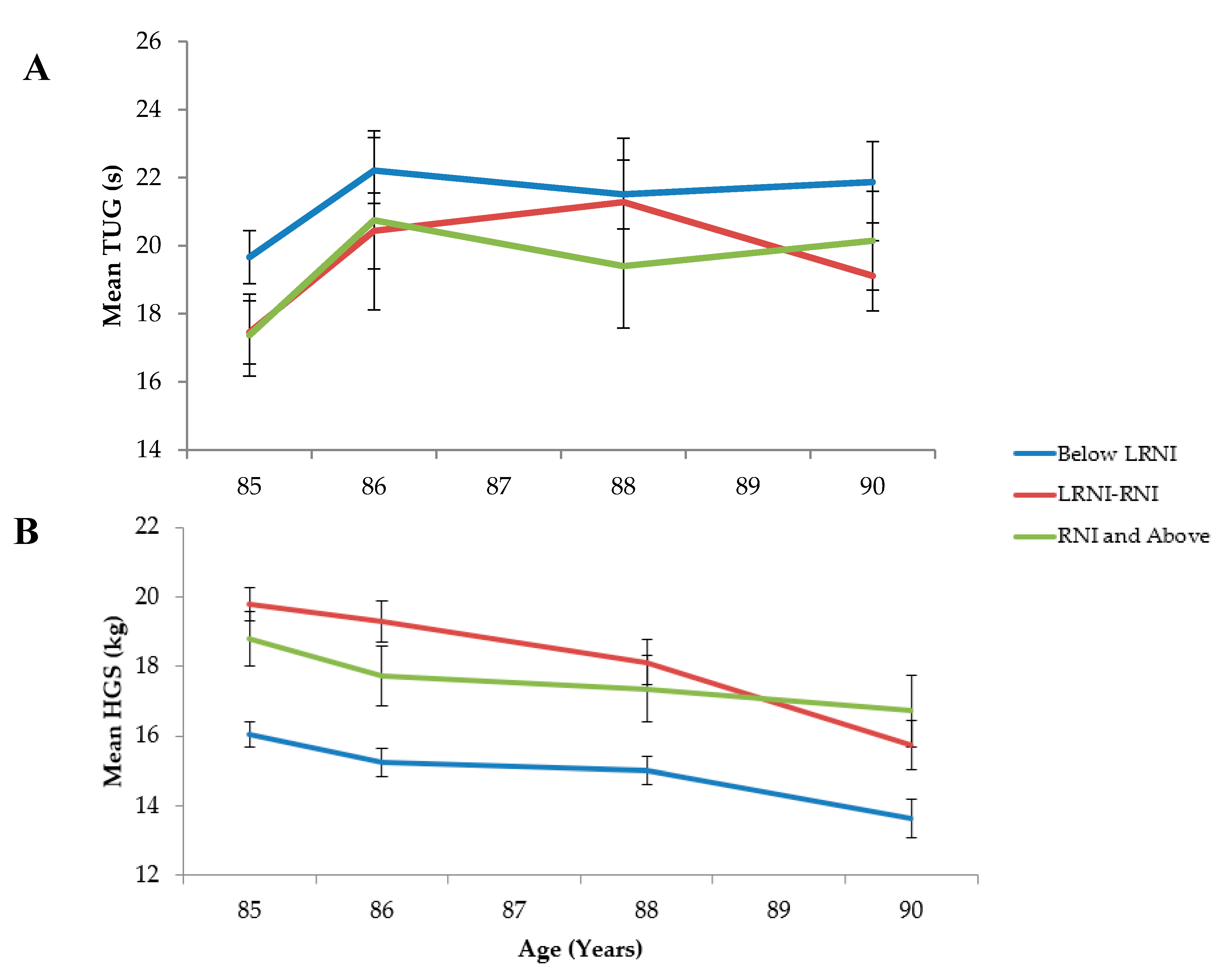
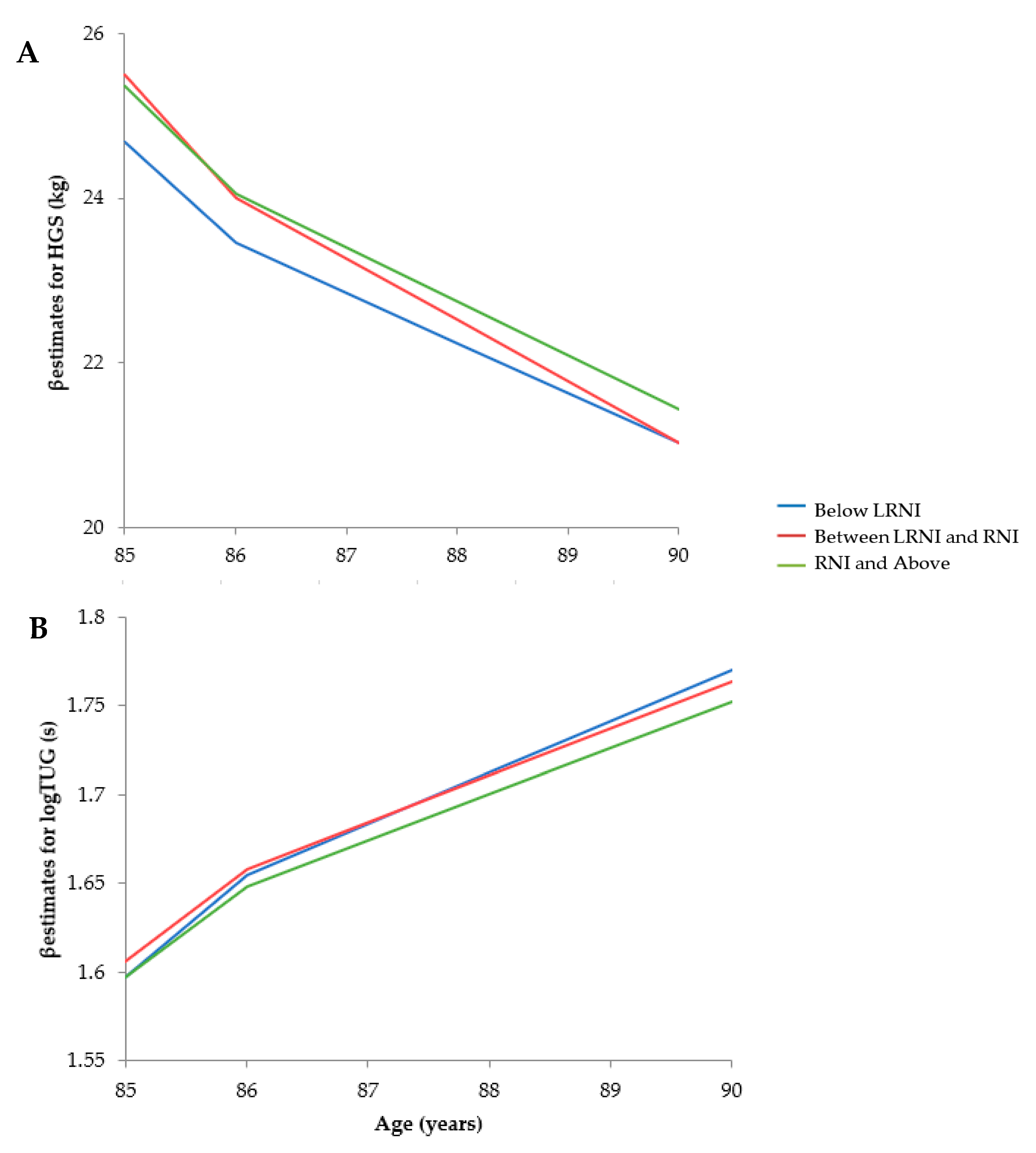
3.3.2. Prospective Analyses
Hand Grip Strength
Timed Up and Go
3.4. Sensitivity Analysis
4. Discussion
4.1. Summary
4.2. Selenium Intakes
4.3. Sources of Selenium
4.4. Potential Mechanistic Roles of Selenium
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Chariot, P.; Bignani, O. Skeletal Muscle Disorders Associated with Selenium Deficiency in Humans. Muscle Nerve 2003, 27, 662–668. [Google Scholar] [CrossRef]
- Moghadaszadeh, B.; Beggs, A.H. Selenoproteins and Their Impact On Human Health Through Diverse Physiological Pathways. Physiology 2006, 21, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation And Function Of Selenoproteins In Human Disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-Chain Selenium and Human Health: Emphasis On Intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Roca-Perez, L.; Gil, C.; Cervera, M.L.; Gonzálvez, A.; Ramos-Miras, J.; Pons, V.; Bech, J.; Boluda, R. Selenium And Heavy Metals Content In Some Mediterranean Soils. J. Geochem. Explor. 2010, 107, 110–116. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Selenium In Soils And Crops, Its Deficiencies In Livestock And Humans: Implications For Management. Commun. Soil Sci. Plant. Anal. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium Deficiency Risk Predicted To Increase Under Future Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.H.; Bu, Y.; Ren, C.Z.; Li, J.Z.; Han, J.J.; Tao, C.; Zhang, K.; Wang, X.X.; Lu, G.X.; et al. Effects Of Selenium Fertilizer On Grain Yield, Se Uptake And Distribution In Common Buckwheat (Fagopyrum Esculentum Moench). Plant Soil Environ. 2016, 61, 371–377. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.J.; Breward, N.; et al. Biofortification Of UK Food Crops With Selenium. Proc. Nutr. Soc. 2006, 65, 169–181. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition (SACN). Position Statement on Selenium and Health May 2013, 1–97. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/339431/SACN_Selenium_and_Health_2013.pdf (accessed on 5 April 2020).
- Yamamuro, T. Kashin-Beck Disease: A Historical Overview. Int. Orthop. 2001, 25, 134–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, G.; Ge, K.; Chen, J.; Chen, X. Selenium-related endemic diseases and the daily selenium requirement of humans. World Rev. Nutr. Diet. 1988, 55, 98–152. [Google Scholar]
- Rayman, M.P. The Use Of High-Selenium Yeast To Raise Selenium Status: How Does It Measure Up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, Ð.M.; Lasheras, C. Food Intake And Serum Selenium Concentration In Elderly People. Ann. Nutr. Metab. 2006, 50, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated With Frailty In Older Adults: A Systematic Review And Meta-Analysis Of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Margetts, M.B.; Thompson, R.L.; Elia, M.; Jackson, A.A. Prevalence Of Risk Of Undernutrition Is Associated With Poor Health Status In Older People In The UK. Eur. J. Clin. Nutr. 2003, 57, 69–74. [Google Scholar] [CrossRef]
- Viñas, B.R.; Barba, L.R.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; De Groot, L.C.; van’t Veer, P.; Matthys, C.; Majem, L.S. Projected Prevalence Of Inadequate Nutrient Intakes In Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef]
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.; Luiking, Y.C.; de Groot, L.C. Micronutrient Intakes And Potential Inadequacies Of Community-Dwelling Older Adults: A Systematic Review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef]
- Brenneisen, P.; Steinbrenner, H.; Sies, H. Selenium, Oxidative Stress, And Health Aspects. Mol. Asp. Med. 2005, 26, 256–267. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A Review Of Dietary Selenium Intake And Selenium Status In Europe And The Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Ighodaro, M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) And Glutathione Peroxidase (GPX): Their Fundamental Role In The Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Jackson, M.; Strategies, J. For Reducing Oxidative Damage In Ageing Skeletal Muscle. Adv. Drug Deliv. Rev. 2009, 61, 1363–1368. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role Of Selenium In Inflammation And Immunity: From Molecular Mechanisms To Therapeutic Opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, B.; De Bleecker, J.L. Cytokines And Chemokines As Regulators Of Skeletal Muscle Inflammation: Presenting The Case Of Duchenne Muscular Dystrophy. Mediat. Inflamm. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; De Vita, F.; Lauretani, F.; Buttò, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G.P. IGF-1, The Cross Road Of The Nutritional, Inflammatory And Hormonal Pathways To Frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef]
- Walston, J.Q.; Xue, R.D.; Semba, L.; Ferrucci, A.R.; Cappola, M.; Ricks, J.G.; Fried, L.P. Serum Antioxidants, Inflammation, And Total Mortality In Older Women. Am. J. Epidemiol. 2005, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, F.; Alonzi, T.; Moretta, A.; Lazzaro, D.; Costa, P.; Poli, V.; Martini, A.; Ciliberto, G.; Fattori, E. Interleukin 6 Causes Growth Impairment In Transgenic Mice Through A Decrease In Insulin-Like Growth Factor-I. A Model For Stunted Growth In Children With Chronic Inflammation. J. Clin. Investig. 1997, 99, 643–650. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 Myokine Signaling In Skeletal Muscle: A Double-Edged Sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Barbieri, M.; Ferrucci, L.; Ragno, E.; Corsi, A.; Bandinelli, S.; Bonafè, M.; Olivieri, F.; Giovagnetti, S.; Franceschi, C.; Guralnik, J.M.; et al. Inflammation And The Effect Of IGF-I On Muscle Strength And Power In Older Persons. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E481–E487. [Google Scholar] [CrossRef]
- Jakob, F.; Becker, K.; Paar, E.; Ebert-Duemig, R.; Schütze, N. Expression and regulation of thioredoxin reductases and other selenoproteins in bone. Methods Enzymol. 2002, 347, 168–179. [Google Scholar]
- Duntas, L.H. Selenium And Inflammation: Underlying Anti-Inflammatory Mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef]
- Vaananen, H.; Kalervo, H.Z.; Mika, M.; Jussi, M.H. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar]
- Zhang, J.; Li, J.; Zhang, Z.; Sun, B.; Wang, R.; Jiang, Z.; Li, S.; Xu, S. Ubiquitous Expression Of Selenoprotein N Transcripts In Chicken Tissues And Early Developmental Expression Pattern In Skeletal Muscles. Biol. Trace Elem. Res. 2011, 146, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Cohen, H.J.; Lyons, J.M.; Curtis, T.W.; Thunberg, B.; Cochran, W.J.; Klish, W.J. Proximal Muscle Weakness And Selenium Deficiency Associated With Long Term Parenteral Nutrition. Am. J. Clin. Nutr. 1986, 43, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.A.; Andrew, W.; Coe, A.S.; Brian, D.L.; John, A.W. Symptomatic Selenium Deficiency In A Child On Home Parenteral Nutrition. J. Pediatric Gastroenterol. Nutr. 1988, 7, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, A.M.; Thomson, C.D.; McKenzie, J.M.; Robinson, M.F. Selenium Deficiency In Total Parenteral Nutrition. Am. J. Clin. Nutr. 1979, 32, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Ray, A.L.; Guralnik, J.M.; Ferrucci, L. Association Of Low Plasma Selenium Concentrations With Poor Muscle Strength In Older Community-Dwelling Adults: The Inchianti Study. Am. J. Clin. Nutr. 2007, 86, 347–352. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yang, K.C.; Chang, H.H.; Lee, L.T.; Lu, C.W.; Huang, K.C. Low Serum Selenium Level Is Associated With Low Muscle Mass In The Community-Dwelling Elderly. J. Am. Med. Dir. Assoc. 2014, 15, 807–811. [Google Scholar] [CrossRef]
- Martin, H.; Aihie Sayer, A.; Jameson, K.; Syddall, H.; Dennison, E.M.; Cooper, C.; Robinson, S. Does Diet Influence Physical Performance In Community-Dwelling Older People? Findings From The Hertfordshire Cohort Study. Age Ageing 2011, 40, 181–186. [Google Scholar] [CrossRef]
- Beck, J.; Ferrucci, L.; Sun, K.; Walston, J.; Fried, L.P.; Varadhan, R.; Semba, R.D. Low Serum Selenium Concentrations Are Associated With Poor Grip Strength Among Older Women Living In The Community. Biofactors 2007, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; de Groot, L.C.; Mijnarends, D.M.; de Vries, J.H.; Verlaan, S.; Meijboom, S.; Schols, J.M. Differences In Nutrient Intake And Biochemical Nutrient Status Between Sarcopenic And Nonsarcopenic Older Adults—Results From The Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Terry, J.; Aspray, J.M.; Bauer, T.C.; Jaimie, H.; Tom, R.H.; Jamie, S.M. Nutritional Status, Body Composition, And Quality Of Life In Community-Dwelling Sarcopenic And Non-Sarcopenic Older Adults: A Case-Control Study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef]
- Hoeg, A.; Gogakos, A.; Murphy, E.; Mueller, S.; Köhrle, J.; Reid, D.M.; Schomburg, L. Bone Turnover And Bone Mineral Density Are Independently Related To Selenium Status In Healthy Euthyroid Postmenopausal Women. J. Clin. Endocrinol. Metab. 2012, 97, 4061–4070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Xiao, J. Selenoproteins And Selenium Status In Bone Physiology And Pathology. Biochim. Et Biophys. Acta Gen. Subj. 2014, 1840, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Gregoire, B.R.; Zeng, H. Selenium Deficiency Decreases Antioxidative Capacity And Is Detrimental To Bone Microarchitecture In Mice. J. Nutr. 2012, 142, 1526–1531. [Google Scholar] [CrossRef]
- Min, Z.; Zhao, W.; Zhong, N.; Guo, Y.; Sun, M.; Wang, Q.; Han, Y. Abnormality Of Epiphyseal Plate Induced By Selenium Deficiency Diet In Two Generation DA Rats. APMIS 2015, 123, 697–705. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Egrise, D.; Nève, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Am. Soc. Bone Miner. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef]
- Hurt, H.D.; Cary, E.E.; Visek, W.J. Growth, Reproduction, And Tissue Concentrations Of Selenium In The Selenium-Depleted Rat. J. Nutr. 1971, 101, 761–766. [Google Scholar] [CrossRef]
- Taekema, D.G.; Gussekloo, J.; Maier, A.B.; Westendorp, R.G.; de Craen, A.J. Handgrip Strength As A Predictor Of Functional, Psychological And Social Health. A Prospective Population-Based Study Among The Oldest Old. Age Ageing 2010, 39, 331–337. [Google Scholar] [CrossRef]
- Frederiksen, H.; Hjelmborg, J.; Mortensen, J.; Mcgue, M.; Vaupel, J.W.; Christensen, K. Age Trajectories Of Grip Strength: Cross-Sectional And Longitudinal Data Among 8,342 Danes Aged 46 To 102. Ann. Epidemiol. 2006, 16, 554–562. [Google Scholar] [CrossRef]
- Bowling, A.P.; Edelmann, R.J.; Leaver, J.; Hoekel, T. Loneliness, Mobility, Well-Being And Social Support In A Sample Of Over 85 Year Olds. Personal. Individ. Differ. 1989, 10, 1189–1192. [Google Scholar] [CrossRef]
- Collerton, J.; Davies, K.; Jagger, C.; Kingston, A.; Bond, J.; Eccles, M.P.; Kirkwood, T.B. Health And Disease In 85 Year Olds: Baseline Findings From The Newcastle 85+ Cohort Study. BMJ 2009, 339, b4904. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (2006) BMI Classification. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 27 June 2020).
- Chandola, T.; Jenkinson, C. The New UK National Statistics Socio-Economic Classification (NS-SEC); Investigating Social Class Differences In Self-Reported Health Status. J. Public Health 2000, 22, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Innerd, P.; Catt, M.; Collerton, J.; Davies, K.; Trenell, M.; Kirkwood, T.B.; Jagger, C. A Comparison Of Subjective And Objective Measures Of Physical Activity From The Newcastle 85+ Study. Age Ageing 2015, 44, 691–694. [Google Scholar] [CrossRef]
- Jagger, C.; Collerton, J.C.; Davies, K.; Kingston, A.; Robinson, L.A.; Eccles, M.P.; Bond, J. Capability And Dependency In The Newcastle 85+ Cohort Study. Projections Of Future Care Needs. BMC Geriatr. 2011, 11. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review Of The Measurement Of Grip Strength In Clinical And Epidemiological Studies: Towards A Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Mathias, S.; Nayak, U.S. Isaacs Bernard. Balance in elderly patients: The get-up and go test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar]
- Greene, B.R.; Doheny, E.P.; O’Halloran, A.; Anne Kenny, R. Frailty Status Can Be Accurately Assessed Using Inertial Sensors And The TUG Test. Age Ageing 2013, 43, 406–411. [Google Scholar] [CrossRef]
- Adamson, A.J.; Collerton, J.; Davies, K.; Foster, E.; Jagger, C.; Stamp, E.; Kirkwood, T. Nutrition In Advanced Age: Dietary Assessment In The Newcastle 85+ Study. Eur. J. Clin. Nutr. 2009, 63, S6–S18. [Google Scholar] [CrossRef]
- Michael, N.; Mary, A.; James, M. Food Portion Sizes. 1997. Available online: https://www.nutritools.org/pdf/portion-sizes/FoodPortionSizes—AUser’sGuidetothePhotographicAtla.pdf (accessed on 2 February 2020).
- Robert Alexander McCance, Food Standards Agency. McCance and Widdowson’s The Composition of Foods, 6th Summary Edition; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Mendonça, N.; Hill, T.R.; Granic, A.; Davies, K.; Collerton, J.; Mathers, J.C.; Jagger, C. Macronutrient Intake And Food Sources In The Very Old: Analysis Of The Newcastle 85+ Study. Br. J. Nutr. 2016, 115, 2170–2180. [Google Scholar] [CrossRef]
- Mendonça, N.; Hill, T.R.; Granic, A.; Davies, K.; Collerton, J.; Mathers, J.C.; Jagger, C. Micronutrient Intake And Food Sources In The Very Old: Analysis Of The Newcastle 85+ Study. Br. J. Nutr. 2016, 116, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Siervo, M.; Bertoli, S.; Battezzati, A.; Wells, J.C.; Lara, J.; Ferraris, C.; Tagliabue, A. Accuracy Of Predictive Equations For The Measurement Of Resting Energy Expenditure In Older Subjects. Clin. Nutr. 2014, 33, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.; Mathers, J.C.; Sayer, A.A. Vitamin D Status, Muscle Strength And Physical Performance Decline In Very Old Adults: A Prospective Study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Mendonça, N.; Sayer, A.A.; Hill, T.R.; Davies, K.; Adamson, A.; Siervo, M.; Mathers, J.C.; Jagger, C.L. Protein Intake, Muscle Strength And Physical Performance In The Very Old: The Newcastle 85+ Study. Clin. Nutr. 2018, 37, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, N.; Granic, A.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Kingston, A.; Jagger, C. Protein Intake And Disability Trajectories In Very Old Adults: The Newcastle 85+ Study. J. Am. Geriatr. Soc. 2019, 67, 50–56. [Google Scholar] [CrossRef]
- Roberts Caireen, Steer Toni, Maplethorpe Natalie, Cox Lorna, Meadows Sarah, Nicholson Sonja, Page Polly, and Swan Gillian. National Diet and Nutrition Survey. Results from Years 7-8 (combined) of the Rolling Programme (2014/15 to 2015/16) Public Health England. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/699241/NDNS_results_years_7_and_8.pdf (accessed on 17 February 2020).
- Hughes, C.M.; Woodside, J.V.; McGartland, C.; Roberts, M.J.; Nicholls, D.P.; McKeown, P.P. Nutritional Intake And Oxidative Stress In Chronic Heart Failure. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 376–382. [Google Scholar] [CrossRef]
- De Jong, N.; Gibson, R.S.; Thomson, C.D.; Ferguson, E.L.; McKenzie, J.E.; Green, T.J.; Horwath, C.C. Selenium And Zinc Status Are Suboptimal In A Sample Of Older New Zealand Women In A Community-Based Study. J. Nutr. 2001, 131, 2677–2684. [Google Scholar] [CrossRef]
- EPIC Oxford. 2019. Publications. Available online: http://www.epic-oxford.org/ (accessed on 5 May 2020).
- Lane, J.A.; Oliver, S.E.; Appleby, P.N.; Lentjes, M.A.; Emmett, P.; Kuh, D.; Stephen, A.; Brunner, E.J.; Shipley, M.J.; Hamdy, F.C.; et al. Prostate Cancer Risk Related To Foods, Food Groups, Macronutrients And Micronutrients Derived From The UK Dietary Cohort Consortium Food Diaries. Eur. J. Clin. Nutr. 2016, 71, 274–283. [Google Scholar] [CrossRef]
- Diehr, P.H.; Thielke, S.M.; Newman, A.B.; Hirsch, C.; Tracy, R. Decline In Health For Older Adults: Five-Year Change In 13 Key Measures Of Standardized Health. J. Gerontol. Ser. A Psychol. Sci. Soc. Sci 2013, 68, 1059–1067. [Google Scholar] [CrossRef]
- McLaughlin, S.J.; Connell, C.M.; Heeringa, S.G.; Li, L.W.; Roberts, J.S. Successful Aging In The United States: Prevalence Estimates From A National Sample Of Older Adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2009, 65, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Farré, C.; Garre-Olmo, J.; Bonmatí-Tomàs, A.; Malagón-Aguilera, M.C.; Gelabert-Vilella, S.; Fuentes-Pumarola, C.; Juvinyà-Canal, D. Prevalence And Related Factors Of Active And Healthy Ageing In Europe According To Two Models: Results From The Survey Of Health, Ageing And Retirement In Europe (SHARE). PLoS ONE 2018, 13, e0206353. [Google Scholar] [CrossRef]
- Diaz-Alarcon, J.P.; Navarro-Alarcón, M.; de la Serrana, H.L.G.; Lopez-Martinez, M.C. Determination Of Selenium In Cereals, Legumes And Dry Fruits From Southeastern Spain For Calculation Of Daily Dietary Intake. Sci. Total Environ. 1996, 184, 183–189. [Google Scholar] [CrossRef]
- Tinggi, U.; Reilly, C.; Patterson, C.M. Determination Of Selenium In Foodstuffs Using Spectrofluorometry And Hydride Generation Atomic Absorption Spectrometry. J. Food Compos. Anal. 1992, 5, 269–280. [Google Scholar] [CrossRef]
- Berner, L.A.; Becker, G.; Wise, M.; Doi, J. Characterization Of Dietary Protein Among Older Adults In The United States: Amount, Animal Sources, And Meal Patterns. J. Acad. Nutr. Diet. 2013, 113, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Spungen, J.H.; Bi, X.; Barraj, L.M. Fresh And Fresh Lean Pork Are Substantial Sources Of Key Nutrients When These Products Are Consumed By Adults In The United States. Nutr. Res. 2011, 31, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High Compliance With Dietary Recommendations In A Cohort Of Meat Eaters, Fish Eaters, Vegetarians, And Vegans: Results From The European Prospective Investigation Into Cancer And Nutrition–Oxford Study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef]
- Finley, J.W. Bioavailability Of Selenium From Foods. Nutr. Rev. 2006, 64, 146–151. [Google Scholar] [CrossRef]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Diet And Its Relationship With Grip Strength In Community-Dwelling Older Men And Women: The Hertfordshire Cohort Study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef]
- Hagan, K.A.; Chiuve, S.E.; Stampfer, M.J.; Katz, J.N.; Grodstein, F. Greater Adherence To The Alternative Healthy Eating Index Is Associated With Lower Incidence Of Physical Function Impairment In The Nurses’ Health Study. J. Nutr. 2016, 146, 1341–1347. [Google Scholar] [CrossRef]
- Robinson, S.M.; Jameson, K.A.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Aihie Sayer, A. Clustering Of Lifestyle Risk Factors And Poor Physical Function In Older Adults: The Hertfordshire Cohort Study. J. Am. Geriatr. Soc. 2013, 61, 1684–1691. [Google Scholar] [CrossRef]
- Xu, B.; Houston, D.K.; Locher, J.L.; Ellison, K.J.; Gropper, S.; Buys, D.R.; Zizza, C.A. Higher Healthy Eating Index-2005 Scores Are Associated With Better Physical Performance. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2011, 67A, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative Protein Damage Is Associated With Poor Grip Strength Among Older Women Living In The Community. J. Appl. Physiol. 2007, 103, 17–20. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Fried, L.P. Oxidative Stress And Severe Walking Disability Among Older Women. Am. J. Med. 2007, 120, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties Of The ‘Timed Up And Go’ Test: More Than Meets The Eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Carter, K.; Mhurchu, C.N.; Anderson, C.S. Selenium And Zinc In Hair And Toenails In Relation To The Physical And Mental Health Status Of Older Adults: The Freemasons Health Study In New Zealand. Australas. J. Ageing 2007, 26, 180–186. [Google Scholar] [CrossRef]
- Papp, L.V.; Holmgren, A.; Khanna, K.K. Selenium And Selenoproteins In Health And Disease. Antioxid. Redox Signal. 2010, 12, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From Selenium To Selenoproteins: Synthesis, Identity, And Their Role In Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress And Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Rederstorff, M.; Krol, A.; Lescure, A. Understanding The Importance Of Selenium And Selenoproteins In Muscle Function. Cell. Mol. Life Sci. 2005, 63, 52–59. [Google Scholar] [CrossRef]
- Rederstorff, M.; Castets, P.; Arbogast, S.; Lainé, J.; Vassilopoulos, S.; Beuvin, M.; Dubourg, O.; Vignaud, A.; Ferry, A.; Krol, A.; et al. Increased Muscle Stress-Sensitivity Induced By Selenoprotein N Inactivation In Mouse: A Mammalian Model For SEPN1-Related Myopathy. PLoS ONE 2011, 6, e23094. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Protection Against Reactive Oxygen Species By Selenoproteins. Biochim. Et Biophys. Acta Gen. Subj. 2009, 1790, 1478–1485. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The Contribution Of Reactive Oxygen Species To Sarcopenia And Muscle Ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive Oxygen Species Are Signalling Molecules For Skeletal Muscle Adaptation. Exp. Physiol. 2009, 95, 1–9. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, And Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative Stress In Aging-Matters Of The Heart And Mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal Muscle Aging: Influence Of Oxidative Stress And Physical Exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L.V. Age-Induced Oxidative Stress: How Does It Influence Skeletal Muscle Quantity And Quality? J. Appl. Physiol. 2016, 121, 1047–1052. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Elena, V. Amino Acid Metabolism And Regulatory Effects In Aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef]
- Taylor, A.H.; Cable, N.T.; Faulkner, G.; Hillsdon, M.; Narici, M.; Van Der Bij, A.K. Physical Activity And Older Adults: A Review Of Health Benefits And The Effectiveness Of Interventions. J. Sports Sci. 2004, 22, 703–725. [Google Scholar] [CrossRef]
- Brownie, S. Why Are Elderly Individuals At Risk Of Nutritional Deficiency? Int. J. Nurs. Pract. 2006, 12, 110–118. [Google Scholar] [CrossRef]
- Pagmantidis, V.; Bermano, G.; Villette, S.; Broom, I.; Arthur, J.; Hesketh, J. Effects Of Se-Depletion On Glutathione Peroxidase And Selenoprotein W Gene Expression In The Colon. FEBS Lett. 2004, 579, 792–796. [Google Scholar] [CrossRef]
- Arbogast, S.; Ana, F.S. Protection Against Oxidative Stress: Selenoprotein, N. As A Novel Player At The Crossroads Of Redox Signaling And Calcium Homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef]
- Ujjawal, G.H.; Nagaraja, T.P.; Prabhu, S.K. Selenoproteins And Their Role In Oxidative Stress And Inflammation. Curr. Chem. Biol. 2013, 7, 65–73. [Google Scholar] [CrossRef]
- Davies, K.A.; Kingston, L.R.J.; Hughes, J.M.; Hunt, S.A.H.; Barker, J.; Edwards, J.; Collerton, C.J.; Thomas, B.L.K. Improving Retention Of Very Old Participants In Longitudinal Research: Experiences From The Newcastle 85+ Study. PLoS ONE 2014, 9, e108370. [Google Scholar] [CrossRef] [PubMed]
- Jeruszka-Bielak, M.; Kollajtis-Dolowy, A.; Santoro, A.; Ostan, R.; Berendsen, A.A.; Jennings, A.; Meunier, N.; Marseglia, A.; Caumon, E.; Gillings, R.; et al. Are Nutrition-Related Knowledge And Attitudes Reflected In Lifestyle And Health Among Elderly People? A Study Across Five European Countries. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Mendonça, N.; Kingston, A.; Granic, A.; Jagger, C. Protein Intake And Transitions Between Frailty States And To Death In Very Old Adults: The Newcastle 85+ Study. Age Ageing 2019, 49, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Westbury, L.D.; Cooper, R.; Kuh, D.; Ward, K.; Syddall, H.E.; Sayer, A.A.; Cooper, C. Adult Lifetime Diet Quality And Physical Performance In Older Age: Findings From A British Birth Cohort. J. Gerontol. Ser. A 2017, 73, 1532–1537. [Google Scholar] [CrossRef]
- O’Kane, S.M.; Pourshahidi, L.K.; Mulhern, M.S.; Weir, R.R.; Hill, S.; O’Reilly, J.; Kmiotek, D.; Deitrich, C.; Mackle, E.M.; Fitzgerald, E.; et al. The Effect Of Processing And Seasonality On The Iodine And Selenium Concentration Of Cow’s Milk Produced In Northern Ireland (NI): Implications For Population Dietary Intake. Nutrients 2018, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Levander, O.A. The Need For Measures Of Selenium Status. J. Am. Coll. Toxicol. 1986, 5, 37–44. [Google Scholar] [CrossRef]
- Bratakos, M.S.; Zafiropoulos, T.F.; Siskos, P.A.; Ioannou, P.V. Selenium Losses On Cooking Greek Foods. Int. J. Food Sci. Technol. 2007, 23, 585–590. [Google Scholar] [CrossRef]
- Keck, A.-S.; John, W.F. Database Values Do Not Reflect Selenium Contents Of Grain, Cereals, And Other Foods Grown Or Purchased In The Upper Midwest Of The United States. Nutr. Res. 2006, 26, 17–22. [Google Scholar] [CrossRef]
- Navarro, M.; Lopez, H.; Ruiz, M.L.; Gonzalez, S.; Perez, V.; Lopez, M.C. Determination Of Selenium In Serum By Hydride Generation Atomic Absorption Spectrometry For Calculation Of Daily Dietary Intake. Sci. Total Environ. 1995, 175, 245–252. [Google Scholar] [CrossRef]
- Gibson, R.S.; Martinez, O.B.; MacDonald, A.C. The Zinc, Copper, And Selenium Status Of A Selected Sample Of Canadian Elderly Women. J. Gerontol. 1985, 40, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Rannem, T.; Ladefoged, K.; Hylander, E.; Christiansen, J.; Laursen, H.; Kristensen, J.H.; Linstow, M.; Beyer, N.; Liguori, R.; Dige-Petersen, H.; et al. The Effect Of Selenium Supplementation On Skeletal And Cardiac Muscle In Selenium-Depleted Patients. J. Parenter. Enter. Nutr. 1995, 19, 351–355. [Google Scholar] [CrossRef] [PubMed]
| Food Group | |
|---|---|
| 1 | cereal and cereal products |
| 2 | milk and milk products |
| 3 | eggs and egg dishes |
| 4 | oils and fat spreads |
| 5 | meat and meat products |
| 6 | fish and fish dishes |
| 7 | vegetables |
| 8 | potatoes |
| 9 | savoury snacks |
| 10 | nuts and seeds |
| 11 | fruits |
| 12 | sugar, preserves and confectionery |
| 13 | non-alcoholic beverages |
| 14 | alcoholic beverages |
| 15 | miscellaneous |
| Characteristic | All Participants | Low Se (< 40 µg/d) | Moderate Se (40–59, 40–74 µg/d) | Optimal Se (≥ 60, ≥ 75 µg/d) | p |
|---|---|---|---|---|---|
| Socio-demographic factors | n = 791 | n = 417 | n = 261 | n = 113 | |
| Women % (n) | 61.8 (489) | 72.9 (304) | 46.7 (122) | 55.7 (63) | < 0.001 a |
| Men % (n) | 38.1 (302) | 27.1 (113) | 53.5 (139) | 44.2 (50) | |
| Years of education % (n) | 0.838 c | ||||
| 0–9 | 64.1 (501) | 65.4 (270) | 64.1 (164) | 59.8 (67) | |
| 10–11 | 23.4 (183) | 22.3 (92) | 24.2 (62) | 25.9 (29) | |
| ≥ 12 | 12.4 (97) | 12.3 (51) | 11.7 (30) | 14.3 (16) | |
| Occupational class % (n) | 0.427 c | ||||
| Managerial and Professional | 26.2 (270) | 33.3 (139) | 31.8 (83) | 32.7 (37) | |
| Intermediate | 11.0 (113) | 13.9 (58) | 11.5 (30) | 18.6 (21) | |
| Routine and Manual | 40.3 (415) | 47.2 (197) | 52.1 (136) | 46.0 (52) | |
| Living in Institution | 0.149 c | ||||
| Yes | 8.7 (69) | 7.4 (31) | 8.8 (23) | 13.3 (15) | |
| No | 91.3 (722) | 92.6 (386) | 91.2 (238) | 86.7 (98) | |
| Diet-related factors | |||||
| Diet change in past year % (n) | 0.502 c | ||||
| Yes | 6.9 (53) | 5.9 (24) | 8.0 (20) | 8.1 (9) | |
| No | 93.1 (718) | 94.1 (385) | 92.0 (231) | 91.9 (102) | |
| Selenium μg/d (Median, IQR) | 39.5, 29.7 | 27.3, 13.9 | 51.4, 12.6 | 87.5, 31.2 | < 0.001 a |
| Total energy kCal (M, SD) | 1678.6, 507.6 | 1492.9, 455.0 | 1841.5, 463.2 | 1987.9, 513.9 | < 0.001 a |
| Carbohydrate | 201.5, 63.2 | 181.9, 58.3 | 218.6, 60.9 | 228.6, 63.4 | < 0.001 a |
| Fat | 69.5, 26.6 | 61.9, 24.0 | 74.7, 25.4 | 83.4, 29.3 | < 0.001 a |
| Protein | 64.9, 22.6 | 53.2, 15.9 | 72.1, 20.6 | 84.4, 26.5 | < 0.001 a |
| Misreporting food intake b % (n) | < 0.001 c | ||||
| Yes | 17.0 (124) | 23.5 (89) | 8.1 (20) | 14.3 (15) | |
| No | 83.0 (606) | 76.5 (290) | 91.9 (226) | 85.7 (90) | |
| Lifestyle factors | |||||
| Smoking % (n) | 0.033 c | ||||
| Non-Smoker | 94.3 (745) | 94.5 (393) | 96.2 (251) | 89.4 (101) | |
| Current Smoker | 5.7 (45) | 5.5 (23) | 3.8 (10) | 10.6 (12) | |
| Current alcohol intake % (n) | 0.712 c | ||||
| Yes | 70.9 (380) | 70.4 (197) | 73.0 (127) | 68.3 (56) | |
| No | 29.1 (156) | 29.6 (83) | 27.0 (47) | 31.7 (26) | |
| Physical activity (PA) % (n) | 0.398 c | ||||
| Low (score 0–1) | 22.1 (176) | 24.0 (100) | 19.5 (51) | 22.1 (25) | |
| Moderate (score 2–6) | 40.7 (343) | 44.7 (186) | 42.7 (111) | 40.7 (46) | |
| High (score 7–18) | 37.2 (270) | 31.3 (130) | 37.7 (98) | 37.2 (42) | |
| Selenium Supplement Use | 0.860 c | ||||
| Yes | 3.2 (25) | 3.4 (14) | 2.7 (7) | 3.5 (4) | |
| No | 96.8 (766) | 96.6 (403) | 97.3 (254) | 96.5 (109) | |
| Health-related factors | |||||
| Self-rated health | 0.270 c | ||||
| Excellent/Very Good | 32.0 (330) | 37.6 (157) | 44.1 (115) | 40.7 (46) | |
| Good | 30.1 (310) | 35.7 (149) | 35.2 (92) | 40.7 (46) | |
| Fair/Poor | 17.9 (184) | 24.2 (101) | 19.2 (50) | 17.7 (20) | |
| Disability Score % (n) n = 785 | 0.036c | ||||
| No Disability | 20.5 (161) | 16.7 (69) | 23.8 (62) | 27.0 (30) | |
| Low (score 1–6) | 52.1 (409) | 54.7 (226) | 51.0 (133) | 45.0 (50) | |
| Moderate (score 7–12) | 18.6 (146) | 17.7 (73) | 19.5 (51) | 19.8 (22) | |
| High (score 13–17) | 8.8 (69) | 10.9 (45) | 5.7 (15) | 8.1 (9) | |
| GDS score (M, SD) | 3.3, 2.4 | 3.3, 2.3 | 3.3, 2.3 | 3.4, 2.6 | 0.713 a |
| Cognitive Impairment (M, SD) | 0.948 a | ||||
| Normal (26–30 SMMSE score) | 27.3, 2.7 | 27.3, 2.8 | 27.3, 2.6 | 27.4, 2.6 | |
| Free T4 in pmol/L (M, SD) | 15.6, 2.6 | 15.7, 2.7 | 15.5, 2.4 | 15.8, 2.9 | 0.778 a |
| Free T3 in pmol/L (M, SD) | 4.6, 0.5 | 4.5, 0.5 | 4.6, 0.6 | 4.6, 0.5 | 0.002 a |
| Phase 1 Arthritis in hands % (n) | 0.202 c | ||||
| Yes | 7.1 (55) | 8.6 (35) | 5.9 (15) | 4.5 (5) | |
| No | 92.9 (719) | 91.4 (371) | 94.1 (241) | 95.5 (107) | |
| Phase 1 Walking Aids % (n) | 0.046 c | ||||
| Yes | 17.8 (131) | 20.9 (81) | 13.2 (32) | 17.1 (18) | |
| No | 82.2 (604) | 79.1 (306) | 86.8 (211) | 82.9 (87) | |
| HGS Phase 1 (M, SD) | 18.7, 7.7 | 16.8, 7.2 | 20.8, 7.5 | 20.0, 7.8 | < 0.001 b |
| TUG Phase 1 (M, SD) | 17.3, 13.1 | 18.3, 13.5 | 16.7, 14.2 | 15.1, 6.8 | 0.009 a |
| Anthropometry | |||||
| BMI (M, SD) | 24.5, 4.4 | 24.6, 4.7 | 24.6, 4.0 | 24.0, 4.0 | 0.636 a |
| Physical Performance | All Participants | Low Se | Moderate Se | Optimal Se | p |
|---|---|---|---|---|---|
| Hand Grip Strength | |||||
| Baseline (n) | 813 | 401 | 254 | 112 | |
| kg (M, SD) | 17.5, 7.7 | 16.0, 7.1 | 19.8, 7.8 | 18.8, 8.2 | < 0.001 a |
| Hand arthritis, % (n) yes | 0.1 (55) | 63.6 (35) | 27.3 (15) | 0.1 (5) | 0.202 b |
| Follow-up at 1.5 years (n) | 605 | 306 | 198 | 90 | |
| kg (M, SD) | 16.9, 7.8 | 15.3, 6.9 | 19.3, 8.3 | 17.7, 8.2 | < 0.001 a |
| Follow-up at 3 years (n) | 452 | 226 | 148 | 71 | |
| kg (M, SD) | 16.4, 7.3 | 15.0, 6.3 | 18.1, 7.9 | 17.4, 7.9 | < 0.001 a |
| Follow-up at 5 years (n) | 294 | 140 | 97 | 54 | |
| kg (M, SD) | 14.9, 7.0 | 13.7, 6.5 | 15.7, 7.1 | 16.7, 7.6 | 0.008 a |
| Timed Up-and-Go | |||||
| Baseline (n) | 747 | 387 | 243 | 105 | |
| s (M, SD) | 18.7, 14.7 | 19.7, 15.4 | 17.5, 14.5 | 17.4, 12.3 | 0.009 c |
| Use of walking aids, % (n) yes | 17.8 (131) | 61.8 (81) | 24.4 (32) | 13.7 (18) | 0.046 b |
| Follow-up at 1.5 years (n) | 547 | 277 | 184 | 80 | |
| s (M, SD) | 21.4, 17.1 | 22.2, 16.1 | 20.4, 15.1 | 20.7, 23.6 | 0.095 c |
| Use of walking aids, % (n) yes | 16.6 (90) | 56.7 (51) | 27.8 (25) | 15.6 (14) | 0.407 b |
| Follow-up at 3 years (n) | 402 | 199 | 134 | 63 | 0.098 c |
| s (M, SD) | 21.5, 18.8 | 21.5, 14.2 | 21.3, 21.8 | 19.4, 14.5 | |
| Use of walking aids, % (n) yes | 17.7 (70) | 57.1 (40) | 30.3 (21) | 12.9 (9) | 0.421 b |
| Follow-up at 5 years (n) | 274 | 134 | 86 | 49 | |
| s (M, SD) | 20.8, 12.2 | 21.9, 13.8 | 19.1, 9.7 | 20.1, 10.2 | 0.529 c |
| Use of walking aids, % (n) yes | 26.1 (71) | 49.3 (35) | 29.6 (21) | 21.1 (15) | 0.733 b |
| Outcome | Variable | Model 3 | |
|---|---|---|---|
| β (SE) | p | ||
| HGS (kg) | Intercept | 25.36 (33.11) | 0.444 |
| ALL INDIVIDUALS | Se intake group | ||
| Low Se | −0.69 (0.65) | 0.292 | |
| Moderate Se | 0.14 (0.64) | 0.829 | |
| Decline | |||
| Time | −1.31 (0.17) | < 0.001 | |
| Slopes (rate of decline) | |||
| Se intake × Time | |||
| Low Se × Time | 0.09 (0.20) | 0.650 | |
| Moderate Se | −0.18 (0.21) | 0.387 | |
| HGS (kg) | Intercept | 125.81 (59.65) | 0.036 |
| MEN | Se intake group | ||
| Low Se | 1.19 (1.19) | 0.317 | |
| Moderate Se | 0.94 (1.07) | 0.384 | |
| Decline | |||
| Time | −1.69 (0.31) | < 0.001 | |
| Slopes (rate of decline) | |||
| Se intake × Time | |||
| Low Se × Time | −0.25 (0.39) | 0.513 | |
| Moderate Se | −0.16 (0.37) | 0.669 | |
| HGS (kg) | Intercept | −23.82 (34.80) | 0.045 |
| WOMEN | Se intake group | ||
| Low Se | −0.91 (0.69) | 0.184 | |
| Moderate Se | −0.08 (0.73) | 0.915 | |
| Decline | |||
| Time | −1.05 (0.18) | < 0.001 | |
| Slopes (rate of decline) | |||
| Se intake × Time | |||
| Low Se × Time | 0.10 (0.21) | 0.626 | |
| Moderate Se | −0.05 (0.23) | 0.826 | |
| TUG (log10-s) | Intercept | 1.65 (1.08) | 0.126 |
| ALL INDIVIDUALS | Se intake group | ||
| Low Se | −0.002 (0.024) | 0.364 | |
| Moderate Se | −0.009 (0.024) | 0.722 | |
| Decline | |||
| Time | 0.051 (0.010) | < 0.001 | |
| Slopes (rate of decline) | |||
| Se intake × Time | |||
| Low Se × Time | 0.020 (0.012) | 0.091 | |
| Moderate Se | 0.013 (0.013) | 0.301 | |
| TUG (log10-s) | Intercept | −2.949 (3.239) | 0.365 |
| MEN | Se intake group | ||
| Low Se | −0.016 (0.045) | 0.730 | |
| Moderate Se | 0.031(0.041) | 0.457 | |
| Decline | |||
| Time | 0.054 (0.013) | < 0.001 | |
| Slopes (rate of decline) | |||
| Se intake × Time | |||
| Low Se × Time | 0.002 (0.016) | 0.917 | |
| Moderate Se | −0.010 (0.015) | 0.507 | |
| TUG (log10-s) | Intercept | 0.629 (2.305) | 0.785 |
| WOMEN | Se intake group | ||
| Low Se | −0.023 (0.037) | 0.541 | |
| Moderate Se | −0.029 (0.039) | 0.462 | |
| Decline | |||
| Time | 0.042 (0.010) | < 0.001 | |
| Slopes (rate of decline) | |||
| Se intake × Time | |||
| Low Se × Time | 0.006 (0.011) | 0.577 | |
| Moderate Se | 0.003 (0.012) | 0.801 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, G.; Mendonça, N.; Jagger, C.; Walsh, J.; Eastell, R.; Mathers, J.C.; Hill, T.R. Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study. Nutrients 2020, 12, 2068. https://doi.org/10.3390/nu12072068
Perri G, Mendonça N, Jagger C, Walsh J, Eastell R, Mathers JC, Hill TR. Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study. Nutrients. 2020; 12(7):2068. https://doi.org/10.3390/nu12072068
Chicago/Turabian StylePerri, Giorgia, Nuno Mendonça, Carol Jagger, Jennifer Walsh, Richard Eastell, John C. Mathers, and Tom R. Hill. 2020. "Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study" Nutrients 12, no. 7: 2068. https://doi.org/10.3390/nu12072068
APA StylePerri, G., Mendonça, N., Jagger, C., Walsh, J., Eastell, R., Mathers, J. C., & Hill, T. R. (2020). Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study. Nutrients, 12(7), 2068. https://doi.org/10.3390/nu12072068





