Normalized Indices Derived from Visceral Adipose Mass Assessed by Magnetic Resonance Imaging and Their Correlation with Markers for Insulin Resistance and Prediabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetic Resonance Imaging
| ● | VAT/m | VAT/body height | [L/m] |
| ● | VAT/m2 | VAT/body height2 | [L/m2] |
| ● | VAT/m3 | VAT/body height3 | [L/m3] |
| ● | %VAT | VAT/total adipose tissue | [%] * |
| ● | VAT/TLT | VAT/total lean tissue | [%] * |
| ● | VAT/WEI | VAT/body weight | [L/kg] |
2.2. Anthropometric Parameters and Metabolic Measurements
2.3. Statistical Analyses
3. Results
3.1. Gender Related Characteristics of Subjects in the TDFS and UKBB
3.2. Determinants of Insulin Resistance and Impaired Glucose Metabolism in the TDFS
3.3. Determinants of Impaired Glucose Metabolism in the UKBB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yanovski, S.Z.; Yanovski, J.A. Obesity prevalence in the United States—up, down, or sideways? N. Engl. J. Med. 2011, 364, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Nour, N.N. Obesity in resource-poor nations. Rev. Obstet. Gynecol. 2010, 3, 180–184. [Google Scholar] [PubMed]
- Jørgensen, M.E. Obesity and diabetes-an Arctic challenge. Int. J. Circumpolar Health 2010, 69, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Odgen, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; Lamb, M.M.; Flegal, K.M. Prevalence of high body mass index in US children and adolescents 2007–2008. JAMA 2010, 303, 242–249. [Google Scholar] [CrossRef]
- Ninomiya, J.K.; L’Italien, G.; Criqui, M.H.; Whyte, J.L.; Gamst, A.; Chen, R.S. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation 2004, 109, 42–46. [Google Scholar] [CrossRef]
- Artham, S.M.; Lavie, C.J.; Patel, H.M.; Ventura, H.O. Impact of obesity on the risk of heart failure and its prognosis. J. Cardiometab. Syndr. 2008, 3, 155–161. [Google Scholar] [CrossRef]
- Yatsuya, H.; Li, Y.; Hilawe, E.H.; Ota, A.; Wang, C.; Chiang, C.; Zhang, Y.; Uemura, M.; Osako, A.; Ozaki, Y.; et al. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ. J. 2014, 78, 2807–2818. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Björntorp, P. Abdominal obesity and the metabolic syndrome. Ann. Med. 1992, 24, 465–468. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, S.; Gokulakrishnan, K.; Velmurugan, K.; Deepa, M.; Mohan, V. Visceral & subcutaneous abdominal fat in relation to insulin resistance & metabolic syndrome in non-diabetic south Indians. Indian J. Med. Res. 2010, 131, 629–635. [Google Scholar] [PubMed]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef]
- Stefan, N.; Kantartzis, K.; Machann, J.; Schick, F.; Thamer, C.; Rittig, K.; Balletshofer, B.; Machicao, F.; Fritsche, A.; Häring, H.-U. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008, 168, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess body fat in obese and normal-weight subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Thamer, C.; Schnoedt, B.; Haap, M.; Häring, H.-U.; Claussen, C.D.; Stumvoll, M.; Fritsche, A.; Schick, F. Standardized assessment of whole body adipose tissue topography by MRI. J. Magn. Reason. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2005, 21, 455–462. [Google Scholar] [CrossRef]
- Kullberg, J.; Johansson, L.; Ahlström, H.; Courivaud, F.; Koken, P.; Eggers, H.; Börnert, P. Automated assessment of whole-body adipose tissue depots from continuously moving bed MRI: A feasibility study. J. Magn. Reason. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2009, 30, 185–193. [Google Scholar] [CrossRef]
- Middleton, M.S.; Haufe, W.; Hooker, J.; Borga, M.; Dahlqvist Leinhard, O.; Romu, T.; Tunón, P.; Hamilton, G.; Wolfson, T.; Gamst, A.; et al. Quantifying Abdominal Adipose Tissue and Thigh Muscle Volume and Hepatic Proton Density Fat Fraction: Repeatability and Accuracy of an MR Imaging-based, Semiautomated Analysis Method. Radiology 2017, 283, 438–449. [Google Scholar] [CrossRef]
- Thörmer, G.; Bertram, H.H.; Garnov, N.; Peter, V.; Schütz, T.; Shang, E.; Blüher, M.; Kahn, T.; Busse, H. Software for automated MRI-based quantification of abdominal fat and preliminary evaluation in morbidly obese patients. J. Magn. Reason. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2013, 37, 1144–1150. [Google Scholar] [CrossRef]
- Würslin, C.; Machann, J.; Rempp, H.; Claussen, C.D.; Yang, B.; Schick, F. Topography mapping of whole body adipose tissue using A fully automated and standardized procedure. J. Magn Reson Imaging Off. J. Int. Soc. Magn. Reson. Med. 2010, 31, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Matthews, P.M.; Bamberg, F.; Bluemke, D.A.; Francis, J.M.; Friedrich, M.G.; Leeson, P.; Nagel, E.; Plein, S.; Rademakers, F.E.; et al. Imaging in population science: Cardiovascular magnetic resonance in 100,000 participants of UK Biobank-rationale, challenges and approaches. J. Cardiovasc. Magn. Reson. 2013, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, F.; Kauczor, H.U.; Weckbach, S.; Schlett, C.L.; Forsting, M.; Ladd, S.C.; Greiser, K.H.; Weber, M.A.; Schulz-Menger, J.; Niendorf, T.; et al. German National Cohort MRI Study Investigators. Whole-Body MR Imaging in the German National Cohort: Rationale, Design, and Technical Background. Radiology 2015, 277, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Moewes, D.; Koch, M.; Müller, H.P.; Jacobs, G.; Kassubek, J.; Lieb, W.; Nöthlings, U. MRI-determined total volumes of visceral and subcutaneous abdominal and trunk adipose tissue are differentially and sex-dependently associated with patterns of estimated usual nutrient intake in a northern German population. Am. J. Clin. Nutr. 2015, 101, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Thamer, C.; Stefan, N.; Schwenzer, N.F.; Kantartzis, K.; Häring, H.-U.; Claussen, C.D.; Fritsche, A.; Schick, F. Follow-up Whole-Body Assessment of Adipose Tissue Compartments during a Lifestyle Intervention in a Large Cohort at Increased Risk for Type 2 Diabetes. Radiology 2010, 257, 353–363. [Google Scholar] [CrossRef]
- Kantartzis, K.; Machann, J.; Schick, F.; Rittig, K.; Machicao, F.; Fritsche, A.; Häring, H.-U.; Stefan, N. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 2011, 54, 864–868. [Google Scholar] [CrossRef]
- Machann, J.; Bongers, M.; Fritsche, A.; Häring, H.U.; Nikolaou, K.; Schick, F. MR-derived indices for identification of quantity and distribution of adipose tissue-age-and gender related differences in a cohort at increased risk for metabolic diseases. Proc. Int. Soc. Mag. Reason. Med. 2016, 24, 1057. [Google Scholar]
- Ulbrich, E.J.; Nanz, D.; Dahlqvist Leinhard, O.; Marcon, M.; Fischer, M.A. Whole-body adipose tissue and lean muscle volumes and their distribution across gender and age: MR-derived normative values in a normal-weight Swiss population. Magn. Reson. Med. 2018, 79, 449–458. [Google Scholar] [CrossRef]
- Linge, J.; Borga, M.; West, J.; Tuthill, T.; Miller, M.R.; Dumitriu, A.; Thomas, E.L.; Romu, T.; Tunón, P.; Bell, J.D.; et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity 2018, 26, 1785–1795. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Hu, F.B.; Schulze, M.B. Divergent associations of height with cardiometabolic disease and cancer: Epidemiology, pathophysiology, and global implications. Lancet Diabetes Endocrinol. 2016, 4, 457–467. [Google Scholar] [CrossRef]
- Stefan, N.; Schäfer, S.; Machicao, F.; Machann, J.; Schick, F.; Claussen, C.D.; Stumvoll, M.; Häring, H.-U.; Fritsche, A. Liver fat and insulin resistance are independently associated with the -514C>T polymorphism of the hepatic lipase gene. J. Clin. Endocrinol. Metab. 2005, 90, 4238–4243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Dahlqvist Leinhard, O.; Romu, T.; Collins, R.; Garratt, S.; Bell, J.D.; Borga, M.; Thomas, L. Feasibility of MR-based body composition analysis in large scale population studies. PLoS ONE 2016, 11, e0163332. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gallagher, D.; Mayer, L.; Beetsch, J.; Pietrobelli, A. Scaling of human body composition to statu.re: New insights into body mass index. Am. J. Clin. Nutr. 2007, 86, 82–91. [Google Scholar] [CrossRef]
- Machann, J.; Thamer, C.; Schnoedt, B.; Stefan, N.; Stumvoll, M.; Häring, H.-U.; Claussen, C.D.; Fritsche, A.; Schick, F. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: A whole body MRI/MRS study. Magn. Reson. Mater. Phys. Biol. Med. 2005, 18, 128–137. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Silva, A.M.; Chen, J.; Gallagher, D.; Sardinha, L.B.; Allison, D.B.; Heymsfield, S.B. Sexual dimorphism of adipose tissue distribution across the lifespan: A cross-sectional whole-body magnetic resonance imaging study. Nutr. Metab. 2009, 16, 6–17. [Google Scholar] [CrossRef]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects with Prediabetes, Subjects With Diabetes, and Normal Control Subjects From the General Population: The KORA-MRI Study. Diabetes 2017, 66, 158–169. [Google Scholar] [CrossRef]
- Gold, E.B.; Bromberger, J.; Crawford, S.; Samuels, S.; Greendale, G.A.; Harlow, S.D.; Skurnick, J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am. J. Epidemiol. 2001, 153, 865–874. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Häring, H.-U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017, 26, 292–300. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Fallah, F.; Machann, J.; Martirosian, P.; Bamberg, F.; Schick, F.; Yang, B. Comparison of T1-weighted 2D TSE, 3D SPGR, and two-point 3D Dixon MRI for automated segmentation of visceral adipose tissue at 3 Tesla. Magn. Reson. Mater. Phys. 2017, 30, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Springer, F.; Ehehalt, S.; Sommer, J.; Ballweg, V.; Machann, J.; Binder, G.; Claussen, C.D.; Schick, F.; DISKUS-Study Group. Predicting volumes of metabolically important whole-body adipose tissue compartments in overweight and obese adolescents by different MRI approaches and anthropometry. Eur. J. Radiol. 2012, 81, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.; Springer, F.; Machann, J.; Schick, F.; Fritsche, A.; Häring, H.-U.; Blumenstock, G.; Ranke, M.B.; Stefan, N.; Binder, G.; et al. Relationships of body composition and liver fat content with insulin resistance in obesity-matched adolescents and adults. Obesity 2014, 22, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
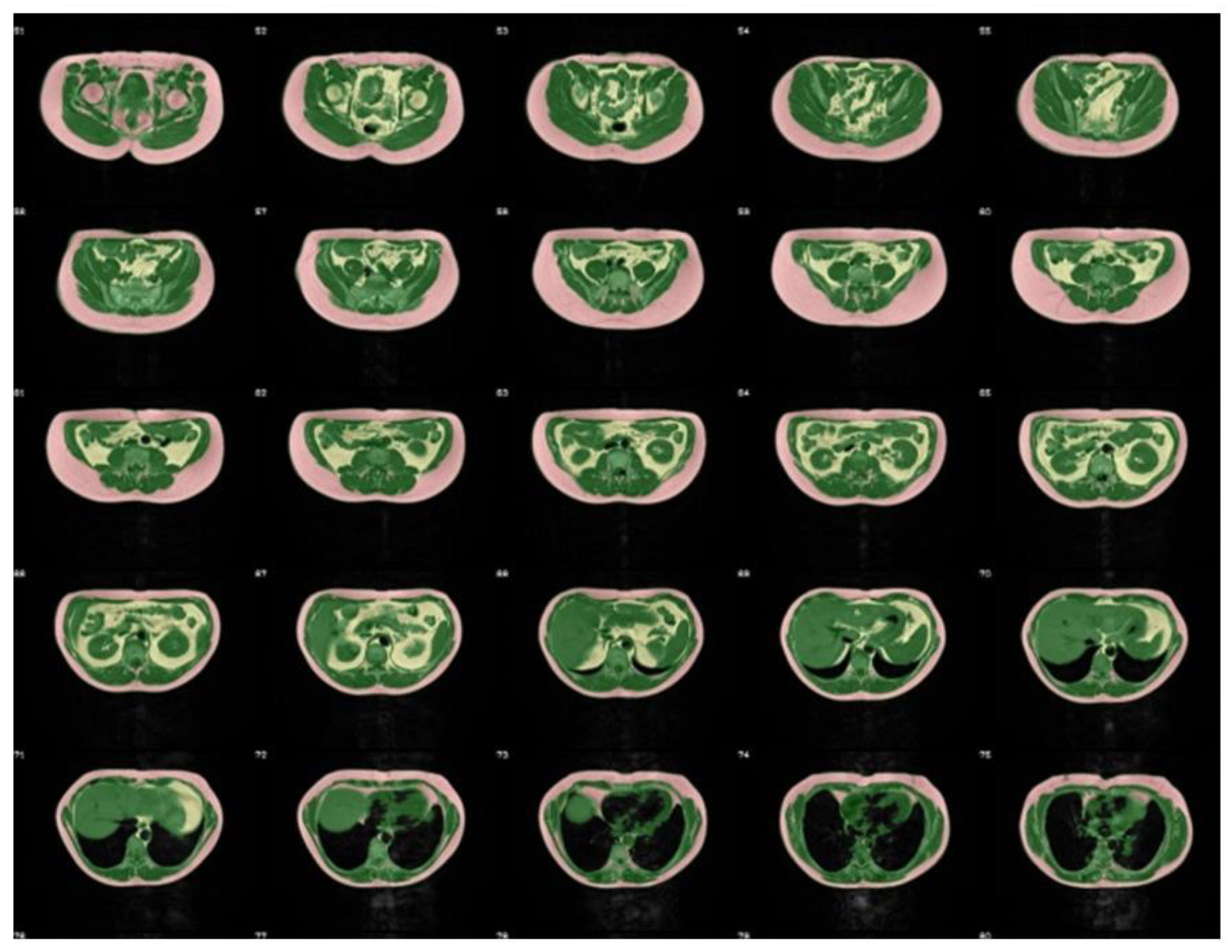
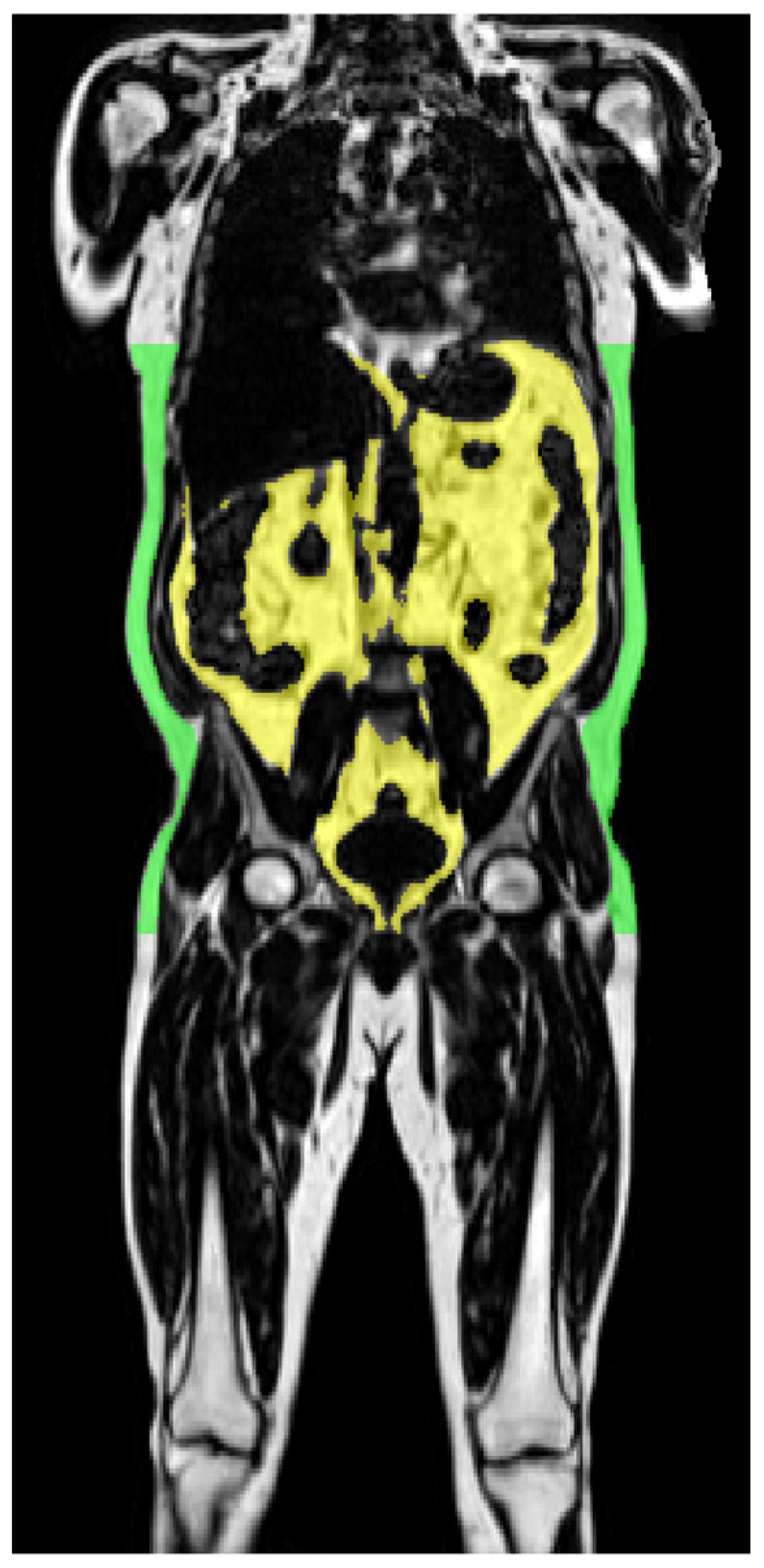
| a | |||
| Characteristics | Estimates | F-Ratio | p |
| log VAT (l) | −0.217 | 140.837 | <0.0001 |
| log BMI | −0.552 | 42.603 | <0.0001 |
| Sex | 0.067 | 51.331 | <0.0001 |
| log Hip Circumference | 0.518 | 16.295 | <0.0001 |
| log Age | 0.083 | 11.153 | 0.0009 |
| log WHR | 0 | 1.421 | 0.2354 |
| log Waist Circumference | 0 | 1.382 | 0.2400 |
| b | |||
| Characteristics | Estimates | Wald/Score ChiSq | Prob > Chi-Square |
| log age | −2.627 | 94.072 | <0.0001 |
| log VAT (l) | −0.811 | 10.311 | 0.0013 |
| Sex | −0.339 | 14.761 | 0.0001 |
| log WHR | 0 | 3.004 | 0.083 |
| log BMI | 0 | 0.083 | 0.773 |
| log Waist circumference | 0 | 2.662 | 0.103 |
| log Hip circumference | 0 | 2.612 | 0.106 |
| n | Age [Years] | BMI [kg/m2] | WC [cm] | HC [cm] | WHR | VAT [L] | VAT/m [L/m] | VAT/m2 [L/m2] | VAT/m3 [L/m3] | %VAT | VAT/TLT | VAT/WEI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| females | ||||||||||||||
| TDFS | ISIMats | 801 | 0.013 | 0.274 | 0.301 | 0.186 | 0.159 | 0.355 | 0.363 | 0.369 | 0.375 | 0.194 | 0.349 | 0.316 |
| TDFS | HbA1c | 801 | 0.235 | 0.028 | 0.060 | 0.028 | 0.039 | 0.144 | 0.155 | 0.164 | 0.178 | 0.151 | 0.156 | 0.160 |
| UKBB | HbA1c | 4774 | 0.071 | 0.023 | 0.035 | 0.012 | 0.035 | 0.039 | 0.044 | 0.045 | 0.046 | 0.037 | 0.042 | 0.041 |
| males | ||||||||||||||
| TDFS | ISIMats | 494 | 0.034 | 0.288 | 0.274 | 0.200 | 0.123 | 0.293 | 0.299 | 0.302 | 0.305 | 0.051 | 0.267 | 0.224 |
| TDFS | HbA1c | 494 | 0.254 | 0.031 | 0.040 | 0.016 | 0.028 | 0.123 | 0.133 | 0.141 | 0.148 | 0.107 | 0.143 | 0.145 |
| UKBB | HbA1c | 4791 | 0.026 | 0.026 | 0.028 | 0.011 | 0.028 | 0.036 | 0.039 | 0.041 | 0.043 | 0.014 | 0.043 | 0.035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machann, J.; Stefan, N.; Wagner, R.; Fritsche, A.; Bell, J.D.; Whitcher, B.; Häring, H.-U.; Birkenfeld, A.L.; Nikolaou, K.; Schick, F.; et al. Normalized Indices Derived from Visceral Adipose Mass Assessed by Magnetic Resonance Imaging and Their Correlation with Markers for Insulin Resistance and Prediabetes. Nutrients 2020, 12, 2064. https://doi.org/10.3390/nu12072064
Machann J, Stefan N, Wagner R, Fritsche A, Bell JD, Whitcher B, Häring H-U, Birkenfeld AL, Nikolaou K, Schick F, et al. Normalized Indices Derived from Visceral Adipose Mass Assessed by Magnetic Resonance Imaging and Their Correlation with Markers for Insulin Resistance and Prediabetes. Nutrients. 2020; 12(7):2064. https://doi.org/10.3390/nu12072064
Chicago/Turabian StyleMachann, Jürgen, Norbert Stefan, Robert Wagner, Andreas Fritsche, Jimmy D. Bell, Brandon Whitcher, Hans-Ulrich Häring, Andreas L. Birkenfeld, Konstantin Nikolaou, Fritz Schick, and et al. 2020. "Normalized Indices Derived from Visceral Adipose Mass Assessed by Magnetic Resonance Imaging and Their Correlation with Markers for Insulin Resistance and Prediabetes" Nutrients 12, no. 7: 2064. https://doi.org/10.3390/nu12072064
APA StyleMachann, J., Stefan, N., Wagner, R., Fritsche, A., Bell, J. D., Whitcher, B., Häring, H.-U., Birkenfeld, A. L., Nikolaou, K., Schick, F., & Thomas, E. L. (2020). Normalized Indices Derived from Visceral Adipose Mass Assessed by Magnetic Resonance Imaging and Their Correlation with Markers for Insulin Resistance and Prediabetes. Nutrients, 12(7), 2064. https://doi.org/10.3390/nu12072064






