Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake by Ethnicity, Income, and Education Level in the United States: NHANES 2003–2014
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participant Characteristics
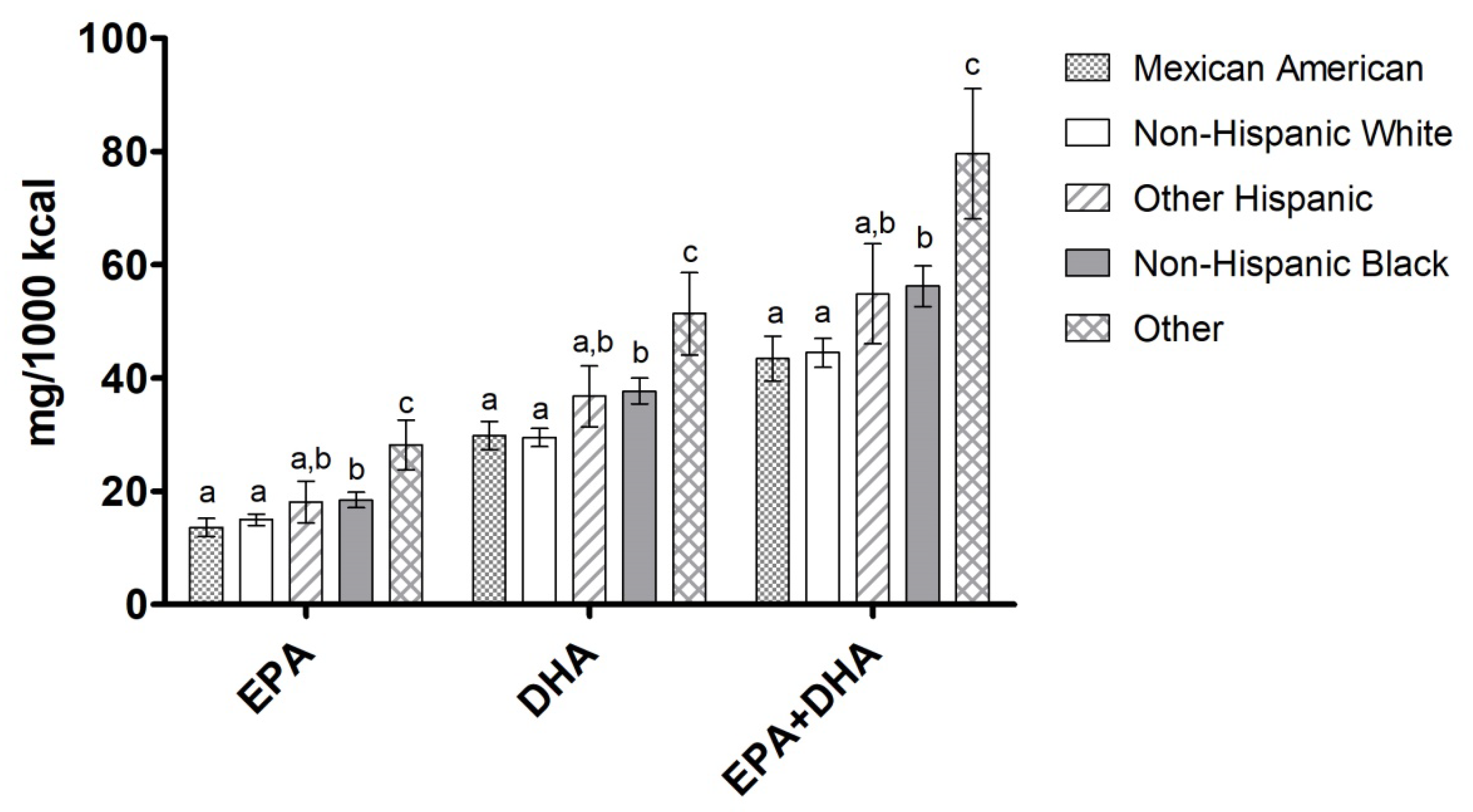
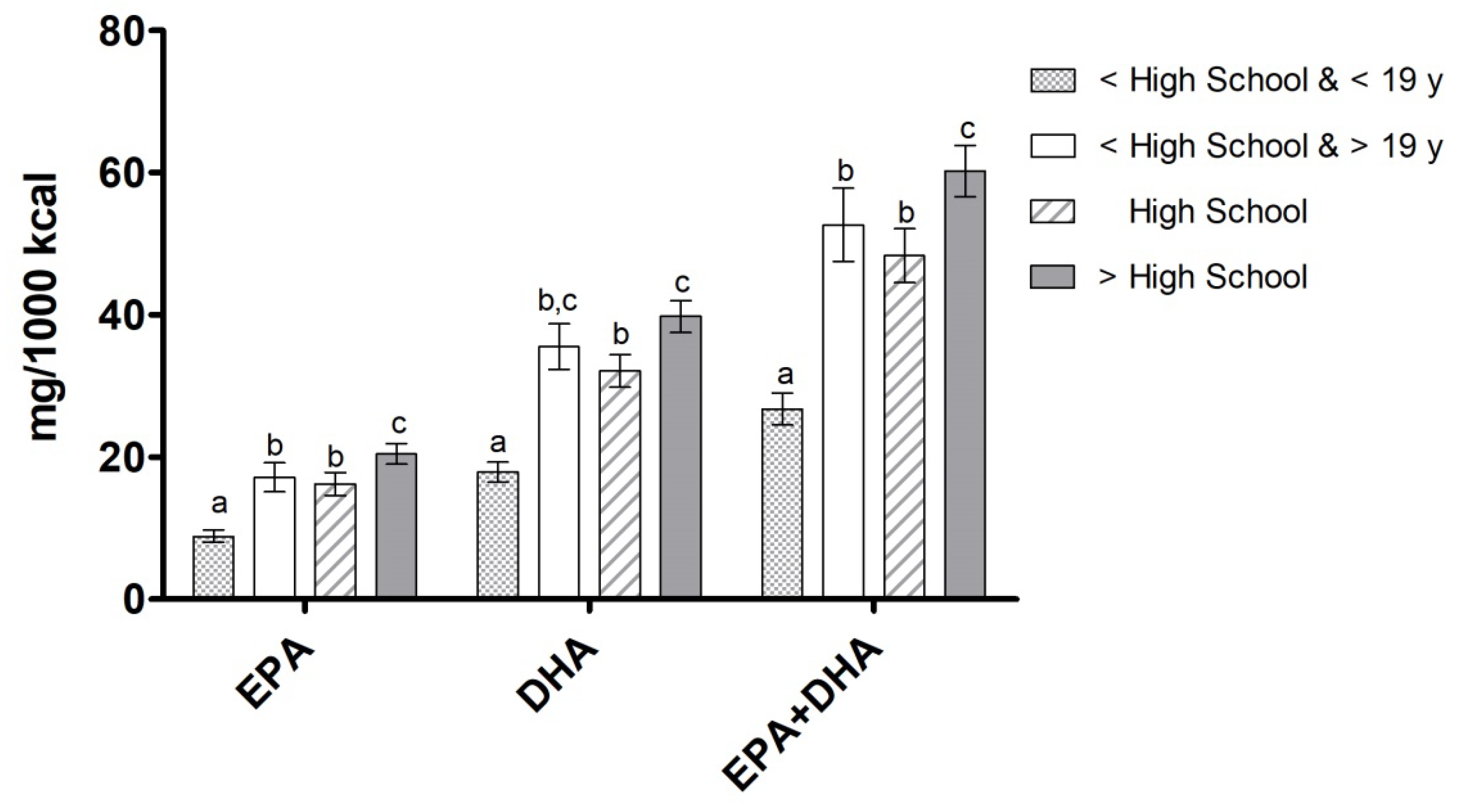
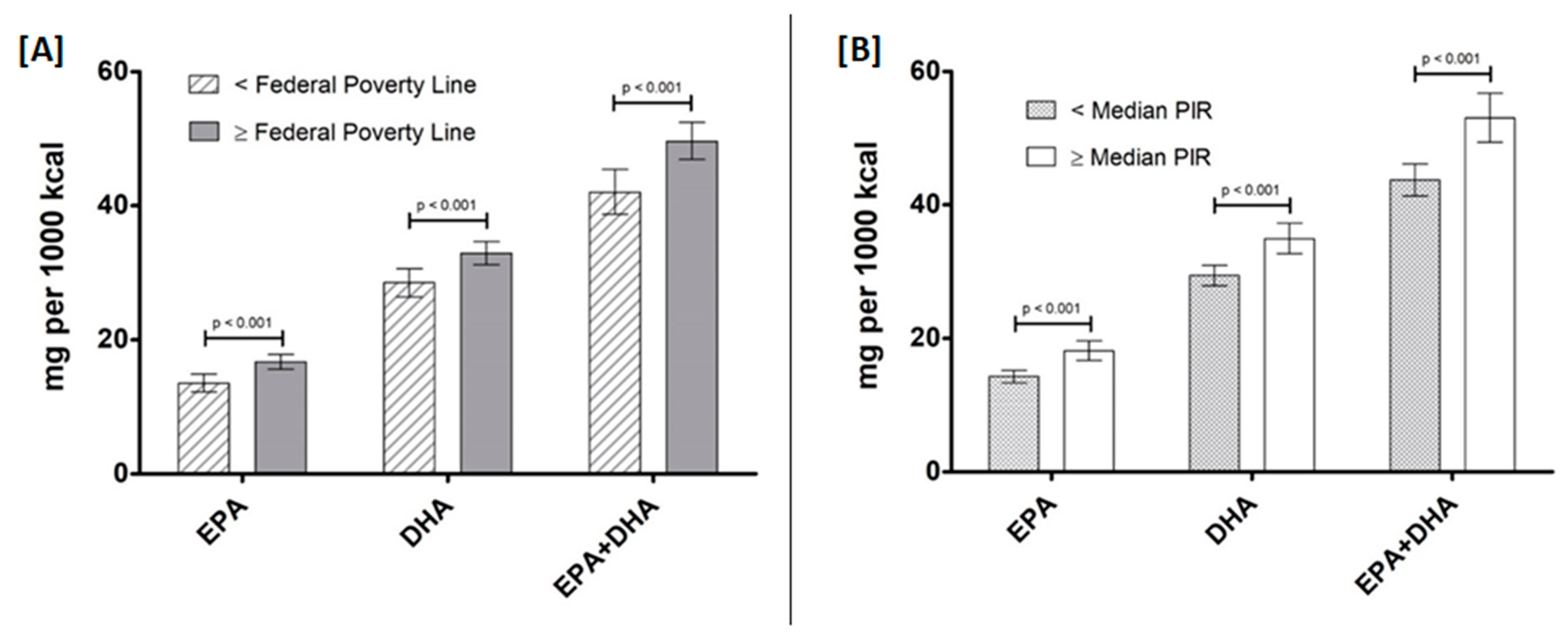
3.2. EPA, DHA, and EPA+DHA Intake by Ethnicity, Education Level, and Income
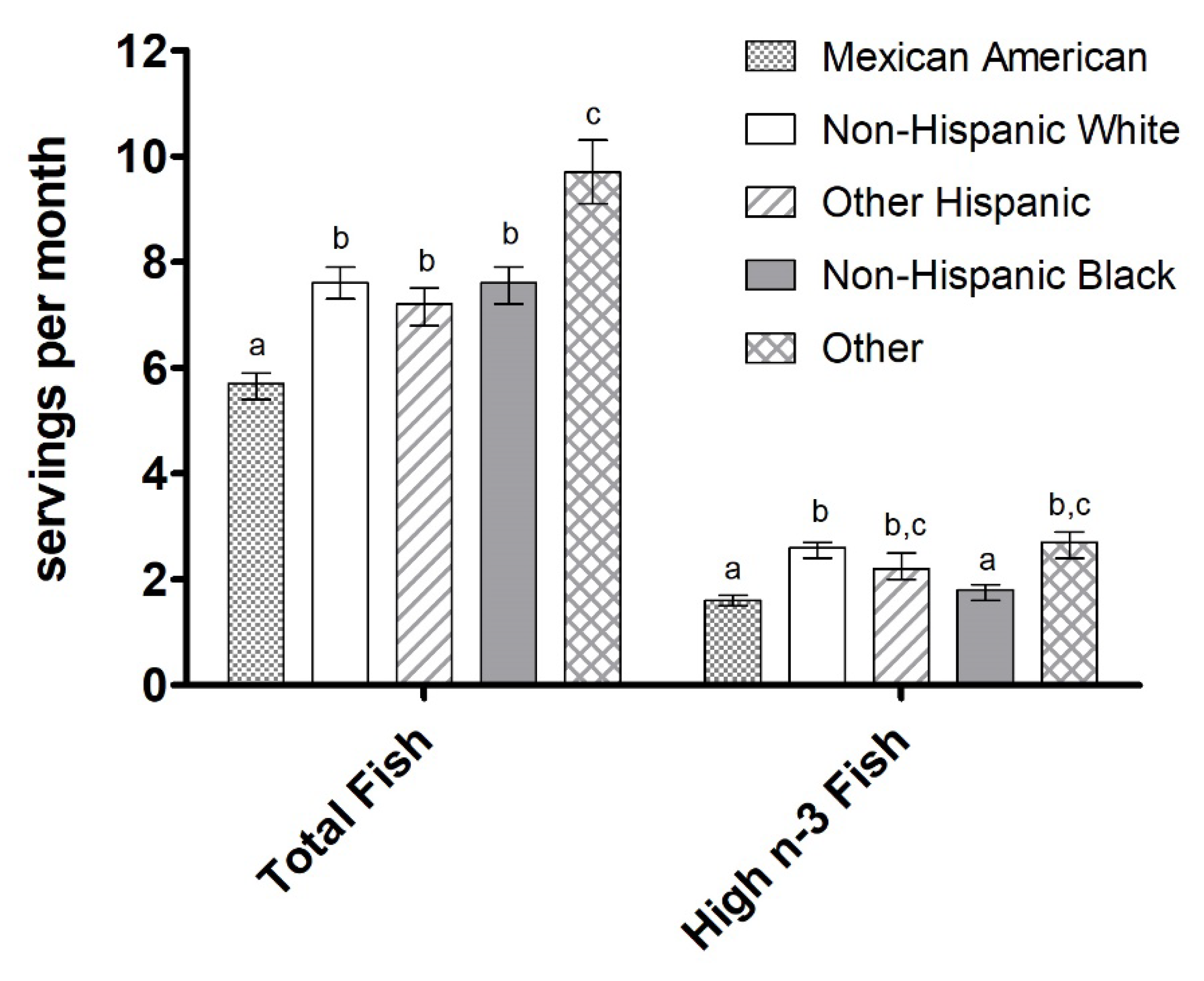
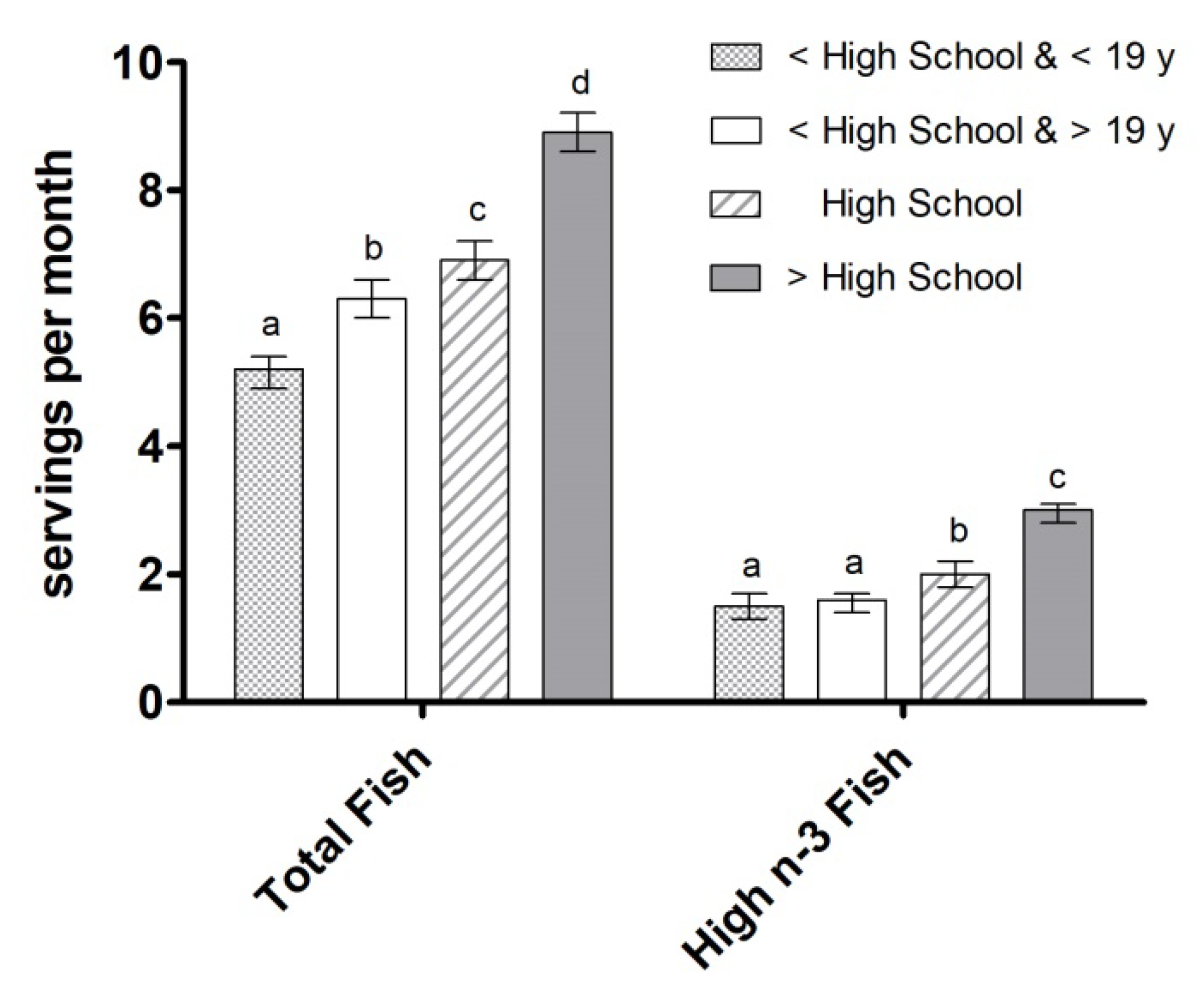
3.3. Fish Intake by Ethnicity, Education Level, and Income
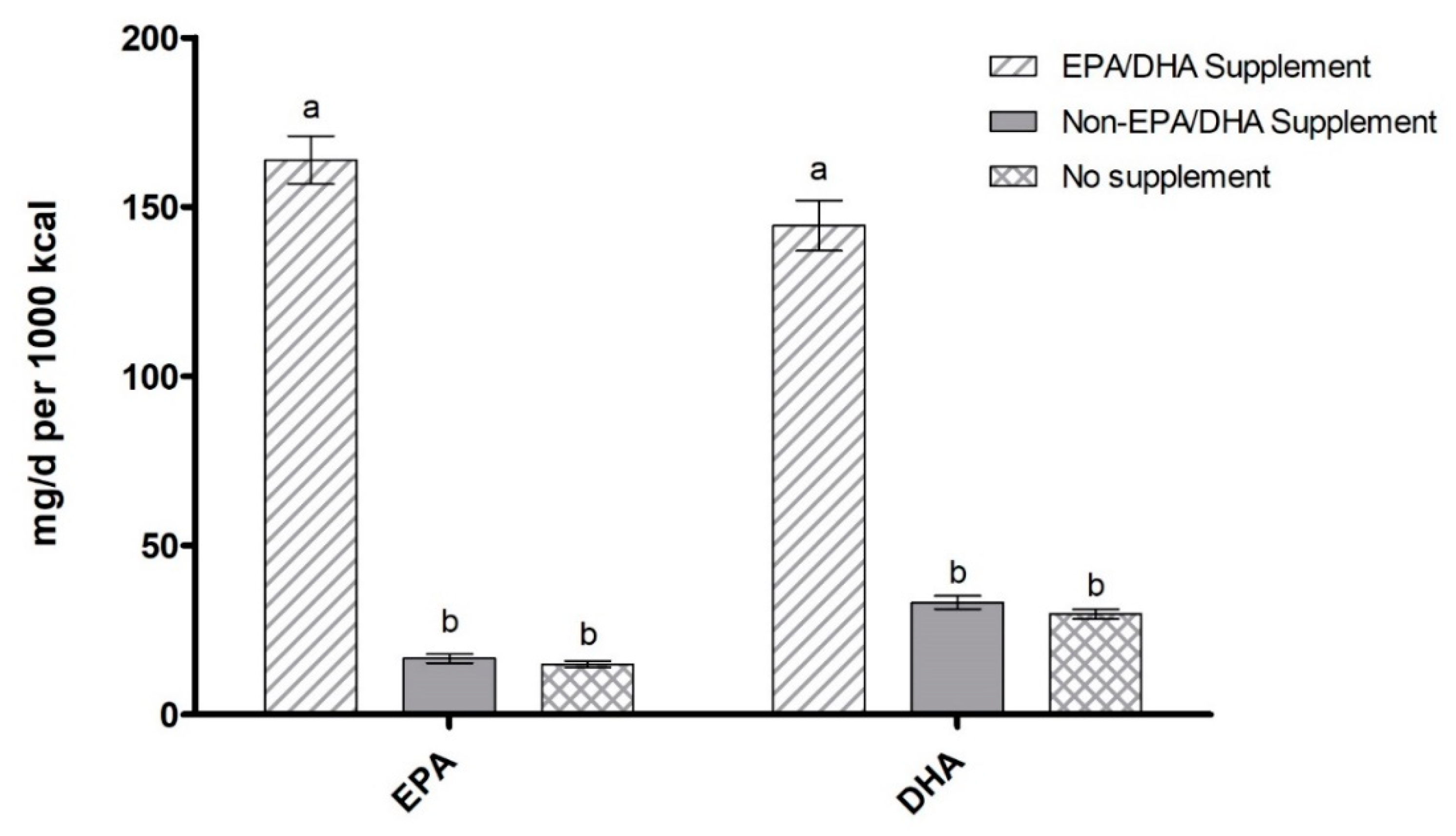
3.4. EPA/DHA-Containing Supplement Use by Ethnicity, Education Level, and Income
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thota, R.N. Science behind the cardio-metabolic benefits of omega-3 polyunsaturated fatty acids: Biochemical effects vs. clinical outcomes. Food Funct. 2018, 6, 3576–3596. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American heart association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef]
- Roncaglioni, M.C.; Risk and Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [PubMed]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Salem, N., Jr.; Palmblad, J. ω-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. 2013, 4, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Christen, W.G.; Schaumberg, D.A.; Glynn, R.J.; Buring, J.E. Dietary omega-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch. Ophthalmol. 2011, 129, 921–929. [Google Scholar] [CrossRef]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; De Jong, P.T.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Shulkin, M. n-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: A systematic review and meta-analysis. J. Nutr. 2018, 148, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.D.; Campos, M.M. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.D.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomarkers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107 (Suppl. 2), S159–S170. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition; WHO: Geneva, Switzerland, 10–14 November 2008; Available online: http://www.who.int/nutrition/topics/FFA_interim_recommendations/en/ (accessed on 30 September 2019).
- Omega-3 Fatty Acids. Fact Sheet for Health Professionals. 2019. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/ (accessed on 30 September 2019).
- Simopoulos, A.P.; Leaf, A.; Salem, N. Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Office of Dietary Supplements—Omega-3 Fatty Acids. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/ (accessed on 29 August 2019).
- Ervin, R.B.; Wright, J.D.; Wang, C.Y.; Kennedy-Stephenson, J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Advanced Data 2004, 348, 1–6. [Google Scholar]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids. 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Papamandjaris, A.A. Lipids: Cellular metabolism. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W.M.I., Ed.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 132–148. [Google Scholar]
- Harris, W. Omega-3 fatty acids. In Encyclopedia of Dietary Supplements, 2nd ed.; Coates, P.M., Betz, J.M., Blackman, M.R., Cragg, C.M., Levine, M., Moss, J., White, J.D., Eds.; Informa Healthcare: London, UK; New York, NY, USA, 2010; pp. 577–586. [Google Scholar]
- Burdge, G.C.; Wooton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18, 3n-3) to eicosapentaenoic acid (20, 5n-3) and docosahexaenoic acid (22, 6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Stessy Bisselou, K.; Kusi Appiah, A.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 fatty acid intake by age, gender, and pregnancy status in the United States: National health and nutrition examination survey 2003–2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: Potential for deficiency. Nutrients 2017, 9, 197. [Google Scholar] [CrossRef]
- Richter, C.K.; Bisselou, K.S.; Nordgren, T.M.; Smith, L.; Appiah, A.K.; Hein, N.; Anderson-Berry, A.; Kris-Etherton, P.; Hanson, C.; Skulas-Ray, A.C. N-3 Docosapentaenoic acid intake and relationship with plasma long-chain N-3 fatty acid concentrations in the United States: NHANES 2003–2014. Lipids 2019, 54, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Julián-Serrano, S.; Dodd, K.; Angleró, I.; Emenaker, N. Ethnic Disparities in EPA and DHA Intake in US Adults: Results from the NHANES 2011–2014 (P04-107-19). Curr. Dev. Nutr. 2019, 3, 107–119. [Google Scholar] [CrossRef]
- Jahns, L.; Raatz, S.K.; Johnson, L.K.; Kranz, S.; Silverstein, J.T.; Picklo, M.J. Intake of seafood in the US varies by age, income, and education level but not by race-ethnicity. Nutrients 2014, 6, 6060–6075. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 31 October 2019).
- R Package Version 3 32. Survey: Analysis of Complex Survey Samples; R Foundation for Statistical Computing: Vienna, Austria, 2017.
- Lumley, T. Analysis of complex survey samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 31 October 2019).
- Cowan, A.E.; Jun, S.; Gahche, J.J.; Tooze, J.A.; Dwyer, J.T.; Eicher-Miller, H.A.; Bhadra, A.; Guenther, P.M.; Potischman, N.; Dodd, K.W.; et al. Dietary supplement use differs by socioeconomic and health-related characteristics among U.S.; Adults, NHANES 2011–2014. Nutrients 2018, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Farmery, A.K.; Hendrie, G.A.; O’Kane, G. Sociodemographic variation in consumption patterns of sustainable and nutritious seafood in Australia. Front. Nutr. 2018, 5, 118. [Google Scholar] [CrossRef]
- Guesnet, P.; Alessandri, J.M. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—Implications for dietary recommendations. Biochimie 2011, 93, 7–12. [Google Scholar] [CrossRef]
- Emmett, P.M.; Jones, L.R.; Golding, J. Pregnancy diet and associated outcomes in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73, 154–174. [Google Scholar] [CrossRef]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P.; Eliassen, J.C.; Alfieri, D.; Weber, W.; Jarvis, K.; DelBello, M.P.; et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 2010, 91, 1060–1067. [Google Scholar] [CrossRef]
- Boucher, O. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am. J. Clin. Nutr. 2011, 93, 120. [Google Scholar] [CrossRef]
- Fuentes-Albero, M. Omega-3 long-chain polyunsaturated fatty acids intake in children with attention deficit and hyperactivity disorder. Brain Sci. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- American Heart Association. Keep Saying Yes to Fish Twice a Week for Heart Health. 2018. Available online: http://newsroom.heart.org/news/keep-saying-yes-to-fish-twice-a-week-for-heart-health (accessed on 13 September 2019).
- Dangardt, F.; Osika, W.; Chen, Y.; Nilsson, U.; Gan, L.M.; Gronowitz, E.; Strandvik, B.; Friberg, P. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis 2010, 212, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.W.; Cheatham, C.L. Omega-6/omega-3 fatty acid intake of children and older adults in the U.S.: Dietary intake in comparison to current dietary recommendations and the Healthy Eating Index. Lipids Health Dis. 2018, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G. Short update on docosapentaenoic acid: A bioactive long-chain n-3 fatty acid. Curr. Opin. Clin. Nutr. Metab. Care. 2016, 19, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G. Docosapentaenoic acid (22, 5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Skulas-Ray, A. Red blood cell Docosapentaenoic acid (DPA n-3) is inversely associated with triglycerides and C-reactive protein (CRP) in healthy adults and dose-dependently increases following n-3 fatty acid supplementation. Nutrients 2015, 7, 6390–6404. [Google Scholar] [CrossRef]
- Murakami, K. Prevalence and characteristics of misreporting of energy intake in US adults: NHANES 2003–2012. Br. J. Nutr. 2015, 114, 1294–1303. [Google Scholar] [CrossRef]
- Sprauge, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]


| World Health Organization [14] | Age | AI (Adequate Intake; per day) |
| DHA | 12–24 months | 10–12 mg/kg |
| EPA+DHA | 2–4 years | 100–150 mg |
| 4–6 years | 150–200 mg | |
| 6–10 years | 200–250 mg | |
| Adults | 200–250 mg | |
| National Institutes of Health [18] | Age | Male/Female (per day) |
| EPA+DHA | 1–3 years | 70 mg/70 mg |
| 4–8 years | 90 mg/90 mg | |
| 9–13 years | 120 mg/100 mg | |
| 14–18 years | 160 mg/110 mg | |
| 19–50 years | 160 mg/110 mg | |
| 51 years | 160 mg/110 mg |
| Characteristics | N | Mean (SE) | |
| Age (years) | 44,585 | 37.3 (22.1) | |
| Calories (kcal) | 44,482 | 2064 (793.6) | |
| Poverty-Income Ratio | 41,692 | 2.9 (1.7) | |
| EPA intake (mg/1000 kcal) | 44,482 | 16.3 (44.4) | |
| DHA intake (mg/1000 kcal) | 44,482 | 32.3 (68.6) | |
| EPA+DHA intake (mg/1000 kcal) | 44,482 | 48.5 (110.9) | |
| Total fish intake (servings/30 days) | 29,033 | 7.5 (6.4) | |
| Fish high in n-3 LCPUFA intake (servings/30 days) | 24,909 | 2.4 (3.4) | |
| Fish low in n-3 LCPUFA intake (servings/30 days) | 29,033 | 5.4 (4.7) | |
| Characteristics | N | % | |
| Gender | Male | 22,056 | 49.0 |
| Female | 22,529 | 51.0 | |
| Race/Ethnicity | Non-Hispanic Black | 10,453 | 12.0 |
| Non-Hispanic White | 17,809 | 66.7 | |
| Mexican-American | 9212 | 9.9 | |
| Other Hispanic | 3588 | 5.0 | |
| Other | 3523 | 6.4 | |
| Education | <high school and <19 years old | 12,442 | 19.3 |
| <high school | 6370 | 13.0 | |
| high school | 6601 | 19.5 | |
| >high school | 13,645 | 48.2 | |
| Income | <median PIR | 26,602 | 49.8 |
| ≥median PIR | 15,090 | 50.2 | |
| <FPL | 10,770 | 16.6 | |
| ≥FPL | 30,922 | 83.4 | |
| Reported EPA/DHA Supplement Use (%) | ||
|---|---|---|
| Total Population | 2420 | |
| Education | <high school and <19 y | 148 (1.5%) |
| <high school | 310 (5.5%) | |
| High school | 458 (7.3%) | |
| >high school | 1445 (11.3%) | |
| Race/Ethnicity | Non-Hispanic Black | 330 (3.2%) |
| Non-Hispanic White | 1388 (9.0%) | |
| Mexican American | 223 (3.0%) | |
| Other Hispanic | 207 (5.4%) | |
| Other | 272 (7.4%) | |
| Income | <median PIR | 990 (4.7%) |
| ≥median PIR | 1262 (10.0%) | |
| <FPL | 224 (2.6%) | |
| ≥FPL | 2028 (8.3%) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cave, C.; Hein, N.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Bisselou, K.S.; Appiah, A.K.; Kris-Etherton, P.; Skulas-Ray, A.C.; Thompson, M.; et al. Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake by Ethnicity, Income, and Education Level in the United States: NHANES 2003–2014. Nutrients 2020, 12, 2045. https://doi.org/10.3390/nu12072045
Cave C, Hein N, Smith LM, Anderson-Berry A, Richter CK, Bisselou KS, Appiah AK, Kris-Etherton P, Skulas-Ray AC, Thompson M, et al. Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake by Ethnicity, Income, and Education Level in the United States: NHANES 2003–2014. Nutrients. 2020; 12(7):2045. https://doi.org/10.3390/nu12072045
Chicago/Turabian StyleCave, Caleb, Nicholas Hein, Lynette M. Smith, Ann Anderson-Berry, Chesney K. Richter, Karl Stessy Bisselou, Adams Kusi Appiah, Penny Kris-Etherton, Ann C. Skulas-Ray, Maranda Thompson, and et al. 2020. "Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake by Ethnicity, Income, and Education Level in the United States: NHANES 2003–2014" Nutrients 12, no. 7: 2045. https://doi.org/10.3390/nu12072045
APA StyleCave, C., Hein, N., Smith, L. M., Anderson-Berry, A., Richter, C. K., Bisselou, K. S., Appiah, A. K., Kris-Etherton, P., Skulas-Ray, A. C., Thompson, M., Nordgren, T. M., Hanson, C., & Thoene, M. (2020). Omega-3 Long-Chain Polyunsaturated Fatty Acids Intake by Ethnicity, Income, and Education Level in the United States: NHANES 2003–2014. Nutrients, 12(7), 2045. https://doi.org/10.3390/nu12072045






