Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention Study Design
2.3. Treatment Drinks
2.4. Blood Tests
2.5. Dietary Intake
2.6. Fecal Sample Collection and DNA Extraction
2.7. PCR Amplification and Illumina Sequencing
2.8. Classification of Microbiome
2.9. Preparation of Genomic DNA from Reference Strains and Fecal Samples
2.10. Real-Time Quantitative PCR
2.11. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Nutrient Intakes of 24 h Recall
3.3. Comparison of Hematological Profiles and Anthropometric Measurements
3.4. Comparison of the Center for Epidemiological Studies Depression Scale Scores
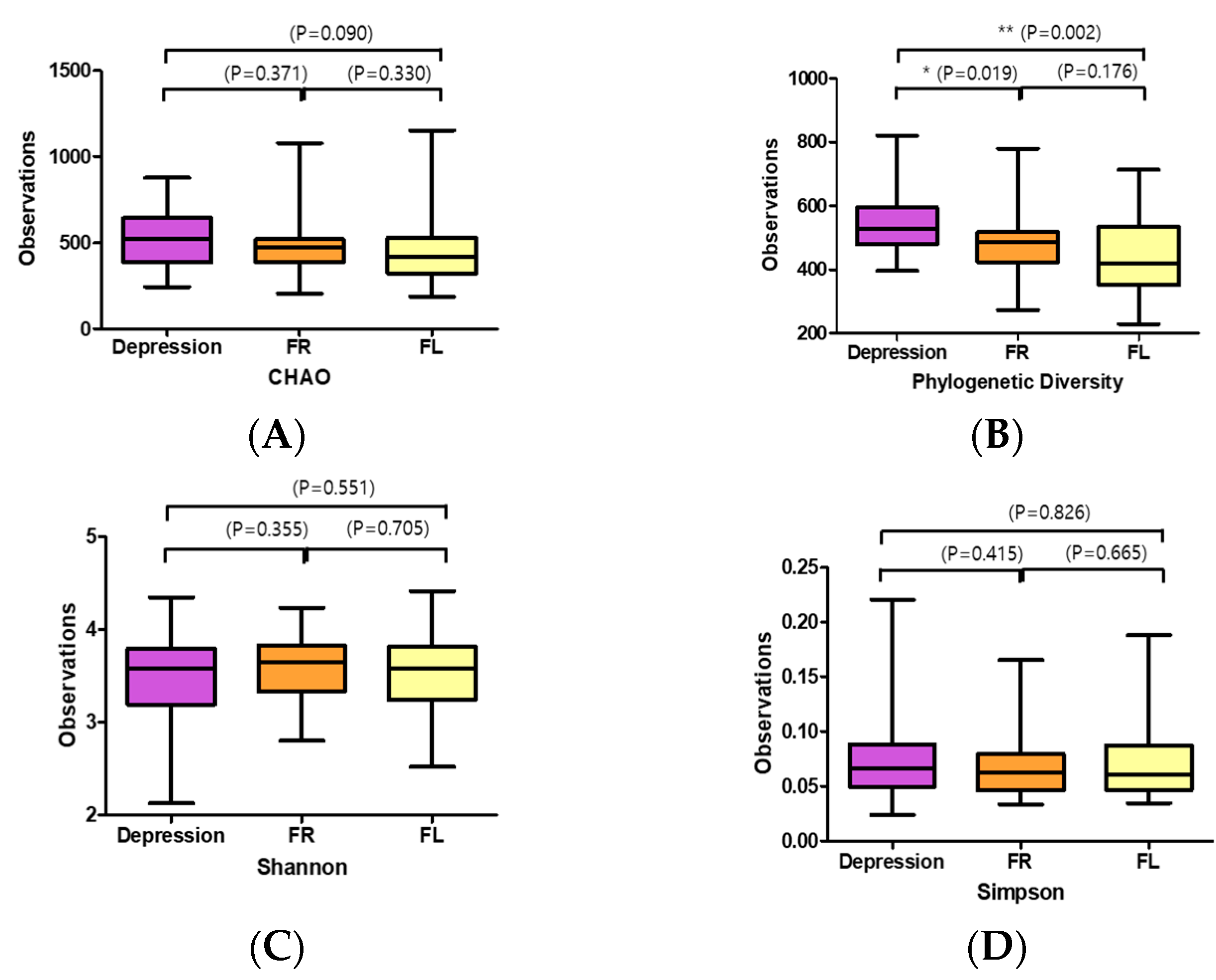
3.5. Sequencing Characteristics and Changes in Microbial Diversity in Depression Symptoms Group
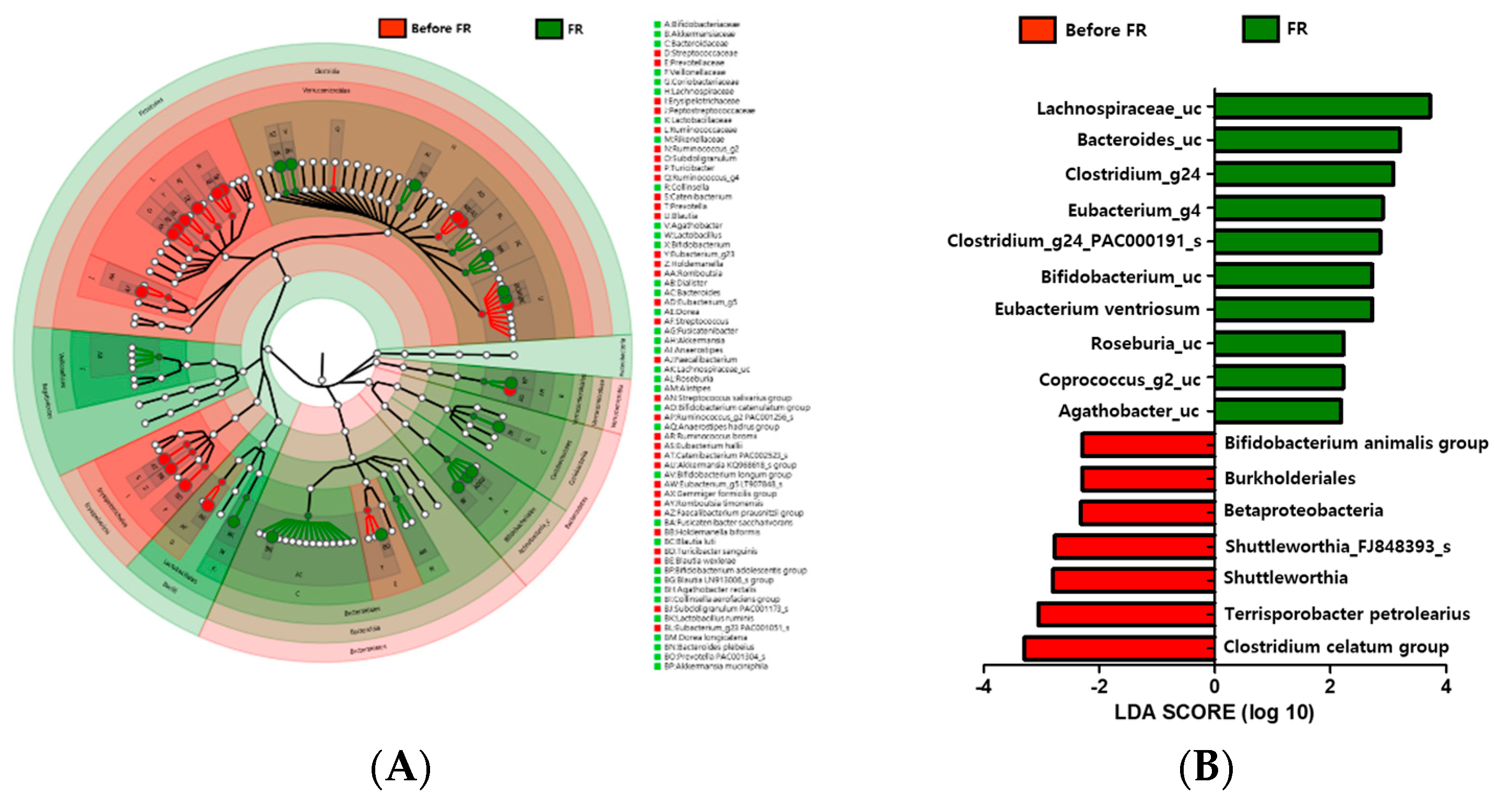
3.6. Changes in Microbiota Taxonomic Composition in the Before FR and FR Groups
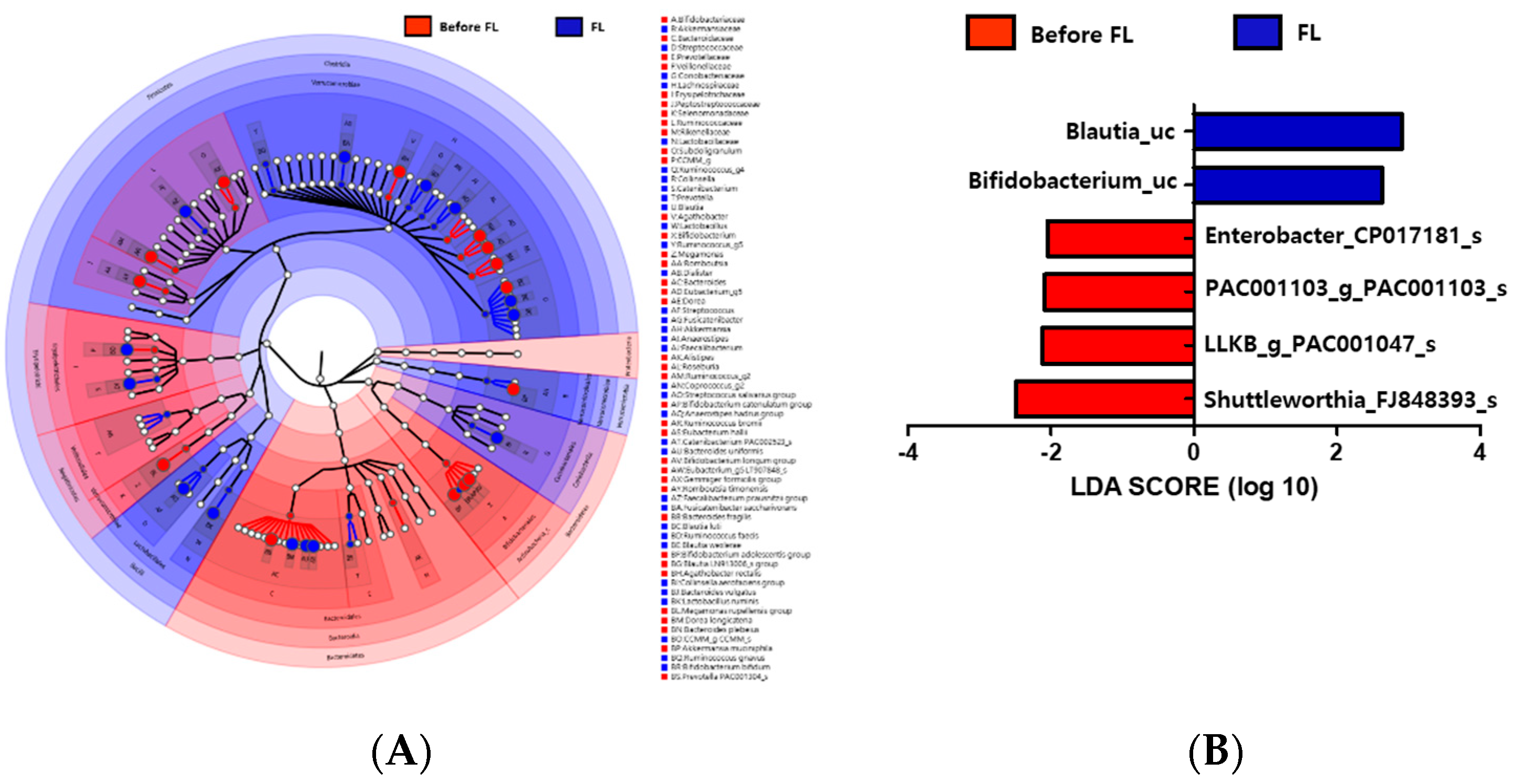
3.7. Changes in Microbiota Taxonomic Composition in the Before FL and FL Groups
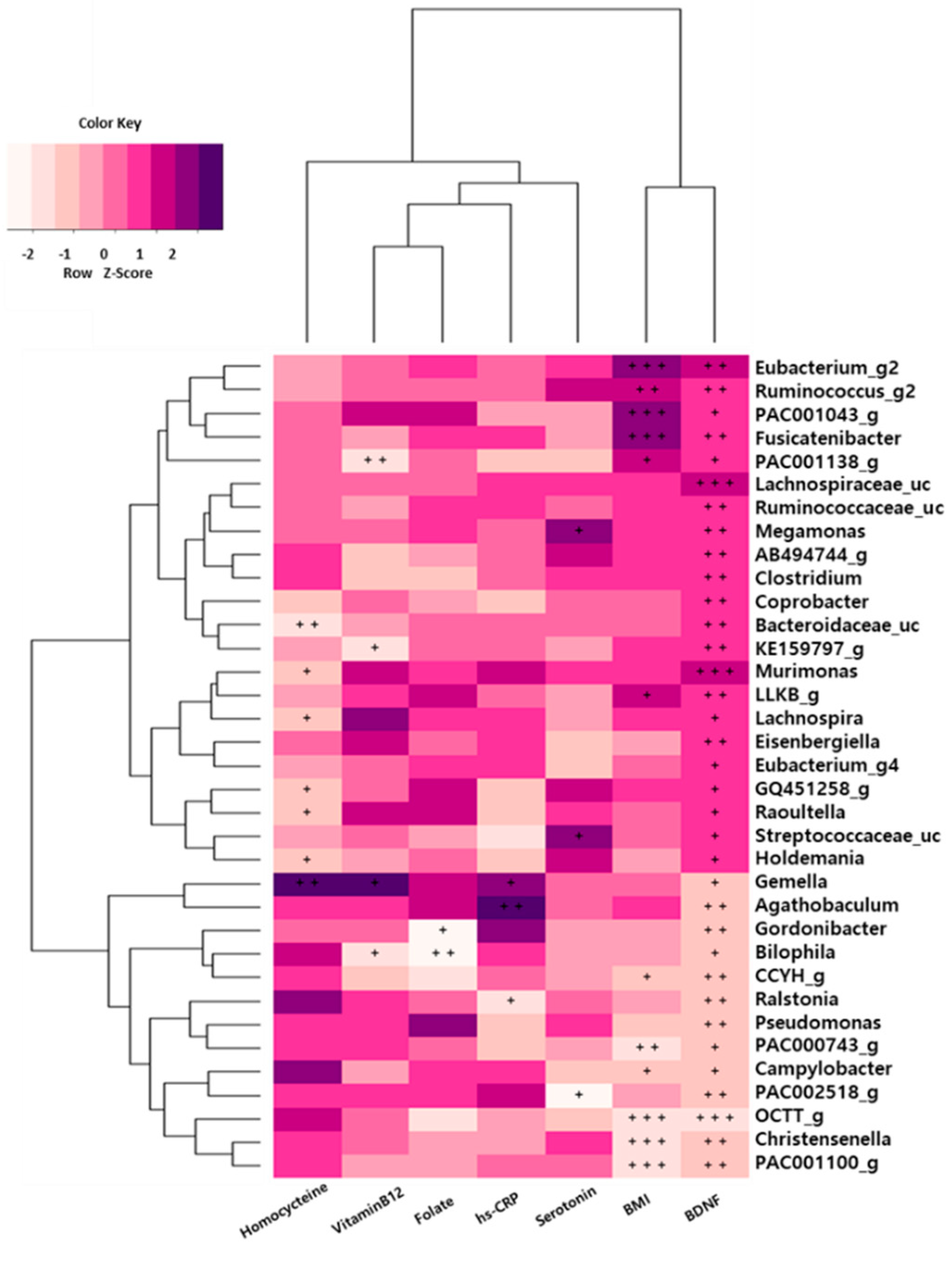
3.8. Association between Gut Microbiota and Depression
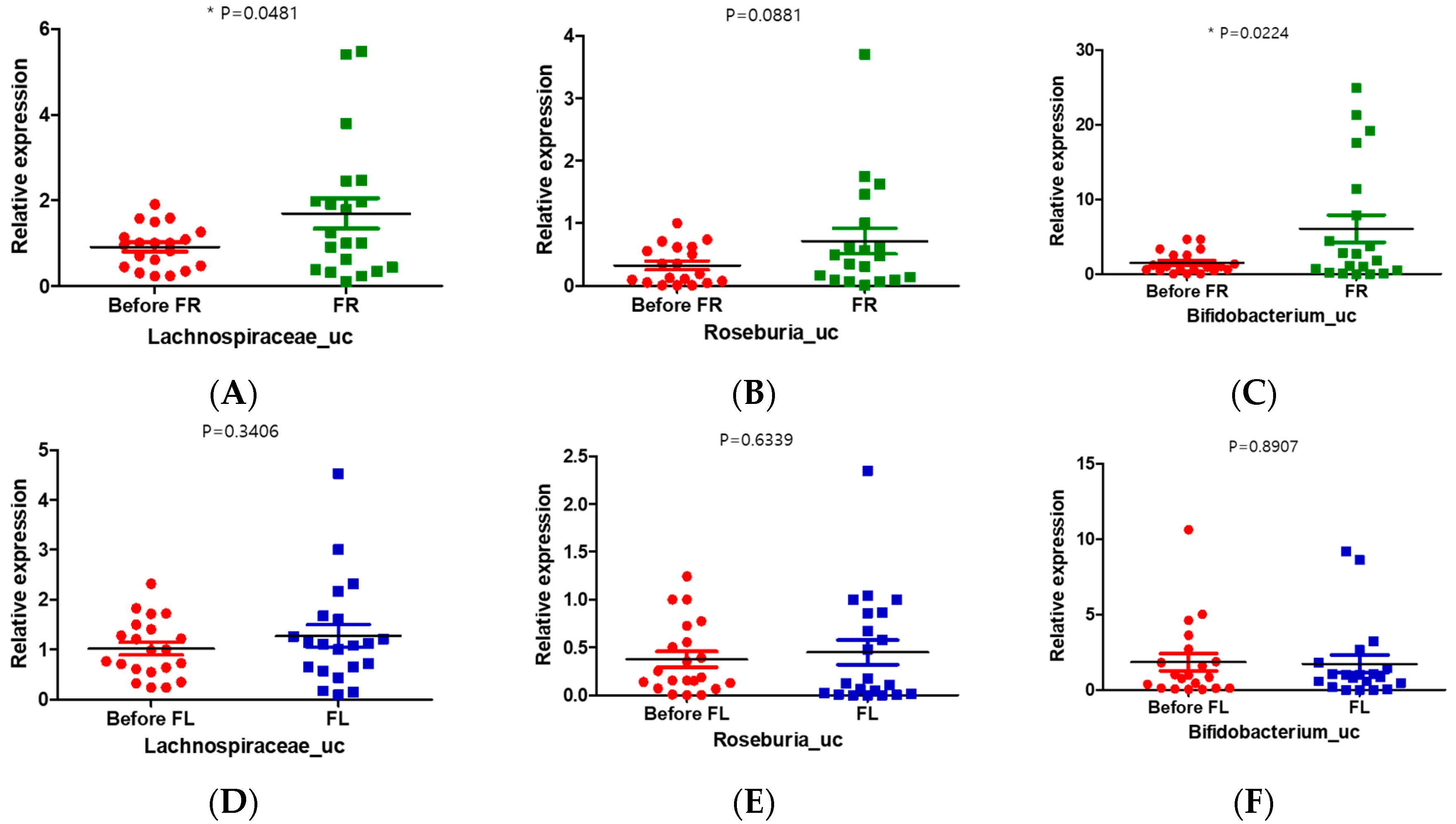
3.9. FR Increased the Relative Abundance of Lachnospiraceae_uc and Bifidobacterium_uc in Depression Symptoms Group
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Depression and Other Common Mental Disorders Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Gawlik, S.; Waldeier, L.; Müller, M.; Szabo, A.; Sohn, C.; Reck, C. Subclinical depressive symptoms during pregnancy and birth outcome—A pilot study in a healthy German sample. Arch. Women’s Ment. Health 2013, 16, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Solem, S.; Hagen, R.; Wang, C.E.A.; Hjemdal, O.; Waterloo, K.; Eisemann, M.; Halvorsen, M. Metacognitions and Mindful Attention Awareness in Depression: A Comparison of Currently Depressed, Previously Depressed and Never Depressed Individuals. Clin. Psychol. Psychother. 2017, 24, 94–102. [Google Scholar] [CrossRef]
- Akbaraly, T.N.; Brunner, E.J.; Ferrie, J.E.; Marmot, M.G.; Kivimaki, M.; Singh-Manoux, A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry 2009, 195, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.-R.; Wei, Y.-J.; Sun, L.; Zhang, J.-X.; Zhang, H.-G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hintikka, J.; Tolmunen, T.; Honkalampi, K.; Haatainen, K.; Koivumaa-Honkanen, H.; Tanskanen, A.; Viinamäki, H. Daily tea drinking is associated with a low level of depressive symptoms in the Finnish general population. Eur. J. Epidemiol. 2005, 20, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C. Omega-3 fatty acids and major depression a primer for the mental health professional. Lipids Health Dis. 2004, 3, 25. [Google Scholar] [CrossRef]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. Flavonoids and cognitive function a review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (−)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P.E. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef]
- Chang, S.-C.; Cassidy, A.; Willett, W.C.; Rimm, E.B.; O’Reilly, E.J.; Okereke, O.I. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 2016, 104, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Mihrshahi, S.; Dobson, A.J.; Mishra, G.D. Fruit and vegetable consumption and prevalence and incidence of depressive symptoms in mid-age women results from the Australian longitudinal study on women’s health. Eur. J. Clin. Nutr. 2014, 69, 585. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance a randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Firoozabadi, A.; Kolouri, S.; Zarshenas, M.M.; Salehi, A.; Mosavat, S.H.; Dastgheib, S.A. Efficacy of Nepeta Menthoides Boiss and Buhse Freeze-Dried Aqueous Extract on Anxiety of Patients with Depression A Double-Blind Randomized Controlled Clinical Trial. Iran. J. Med Sci. 2016, 41, S4. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry focus on depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.-J.; Poeggeler, B.; Omar, R.A.; Chain, D.G.; Frangione, B.; Ghiso, J.; Pappolla, M.A. Potent Neuroprotective Properties against the Alzheimer β-Amyloid by an Endogenous Melatonin-related Indole Structure, Indole-3-propionic Acid. J. Biol. Chem. 1999, 274, 21937–21942. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cabezas, R.; Davideau, J.-L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis a systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Shab-Bidar, S.; Speakman, J.R.; Parastui, K.; Daneshi-Maskooni, M.; Djafarian, K. Probiotics Reduce the Risk of Antibiotic-Associated Diarrhea in Adults (18–64 Years) but Not the Elderly (>65 Years). Nutr. Clin. Pract. 2016, 31, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Emge, J.R.; Huynh, K.; Miller, E.N.; Kaur, M.; Reardon, C.; Barrett, K.E.; Gareau, M.G. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am. J. Phys. Gastrointest. Liver Phys. 2016, 310, G989–G998. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes a link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against LPS-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale, a Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Bae, J.N.; Cho, M.J. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J. Psychosom. Res. 2004, 57, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Kim, K.H. Use of the Center for Epidemiologic Studies Depression (CES-D) Scale in Korea. J. Nerv. Ment. Dis. 1998, 186, 304–310. [Google Scholar] [CrossRef] [PubMed]
- WMA. WMA Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects; Finland World Medical Association: Helsinki, Finland, 1964. [Google Scholar]
- Yun, S.H.; Shim, J.S.; Kweon, S.H.; Oh, K.W. Development of a Food Frequency Questionnaire for the Korea National Health and Nutrition Examination Survey Data from the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV). Korean J. Nutr. 2013, 46, 186–196. [Google Scholar] [CrossRef]
- Jang, L.-G.; Choi, G.; Kim, S.-W.; Kim, B.-Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota an observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic a flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLOS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Lee, B.; Moon, T.; Yoon, S.; Weissman, T. DUDE-Seq Fast, flexible, and robust denoising for targeted amplicon sequencing. PLOS ONE 2017, 12, e0181463. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.W.; Miller, W. Optimal alignments in linear space. Bioinformatics 1988, 4, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Suzuki, M.T.; Taylor, L.T.; DeLong, E.F. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5’-nuclease assays. Appl. Environ. Microbiol. 2000, 66, 4605–4614. [Google Scholar] [CrossRef]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef]
- Henry, S.K.; Grant, M.M.; Cropsey, K.L. Determining the optimal clinical cutoff on the CES-D for depression in a community corrections sample. J. Affect. Disord. 2018, 234, 270–275. [Google Scholar] [CrossRef]
- Umegaki, Y.; Todo, N. Psychometric properties of the Japanese CES–D, SDS, and PHQ–9 depression scales in university students. Psychol. Assess. 2017, 29, 354–359. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood–brain barrier in vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Menze, E.T.; Tadros, M.G.; Abdel-Tawab, A.M.; Khalifa, A.E. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. NeuroToxicology 2012, 33, 1265–1275. [Google Scholar] [CrossRef]
- Kao, C.-F.; Liu, Y.-L.; Yu, Y.W.Y.; Yang, A.C.; Lin, E.; Kuo, P.-H.; Tsai, S.-J. Gene-based analysis of genes related to neurotrophic pathway suggests association of BDNF and VEGFA with antidepressant treatment-response in depressed patients. Sci. Rep. 2018, 8, 6983. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.-F.; Young, L.T. Increased hippocampal bdnf immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; et al. What’s Normal? Microbiomes in Human Milk and Infant Feces Are Related to Each Other but Vary Geographically: The INSPIRE Study. Front. Nutr. 2019, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Taverniti, V.; Ruas-Madiedo, P.; Duranti, S.; Guglielmetti, S.; Lugli, G.A.; Gioiosa, L.; Palanza, P.; Margolles, A.; van Sinderen, D.; et al. Bifidobacterium bifidum PRL2010 Modulates the Host Innate Immune Response. Appl. Environ. Microbiol. 2014, 80, 730–740. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Devi, R.S.; Mary, R.R.; Prabhavathi, D.; Vidya, R.; Mechenro, J.; Mahendri, N.V.; Pugazhendhi, S.; Ramakrishna, B.S. Effect of yoghurt containing Bifidobacterium lactis Bb12®on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers. Nutr. J. 2011, 10, 138. [Google Scholar] [CrossRef]
- Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Micro 2017, 8, 2345. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2010, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher Sydney, E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside—from diversity to function the case of Akkermansia. ISME J. 2012, 6, 1449. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zheng, P.; Liu, Y.-Y.; Zhong, X.-G.; Wang, H.-Y.; Guo, Y.-J.; Xie, P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Flint, H.J. Cultivable bacterial diversity from the human colon. Lett. Appl. Microbiol. 2007, 44, 343–350. [Google Scholar] [CrossRef]
- Vince, A.J.; McNeil, N.I.; Wager, J.D.; Wrong, O.M. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br. J. Nutr. 1990, 63, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, M.; Nankova, B.B.; Shah, P.; Patel, P.; Mally, P.V.; Mishra, R.; La Gamma, E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol. Brain Res. 2005, 142, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.K.; Schneider, J.S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010, 1354, 172–178. [Google Scholar] [CrossRef]
- Fuchikami, M.; Yamamoto, S.; Morinobu, S.; Okada, S.; Yamawaki, Y.; Yamawaki, S. The potential use of histone deacetylase inhibitors in the treatment of depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 64, 320–324. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
| Variables | Flavonoid-Rich Orange Juice (FR, n = 20) | p-Value † | Flavonoid-Low Orange Cordial (FL, n = 20) | p-Value † | Δ Group Comparison ¥ | ||
|---|---|---|---|---|---|---|---|
| Baseline | After Intervention | Baseline | After Intervention | ||||
| Mean ± SE | Mean ± SE | ||||||
| Age | 22.20 ± 2.608 | 21.45 ± 2.259 | 0.337 † | ||||
| Male | n = 8 (40%) | n = 8 (40%) | 1.000 | ||||
| Weight, kg | 66.28 ± 3.41 | 66.57 ± 3.40 | 0.382 † | 60.22 ± 2.32 | 59.98 ± 2.31 | 0.510 † | 0.672 |
| BMI, kg/m2 | 23.45 ± 0.87 | 23.62 ± 0.88 | 0.178 † | 21.74 ± 0.66 | 21.62 ± 0.63 | 0.387 † | 0.122 |
| Percent body fat, % | 27.72 ± 1.76 | 27.90 ± 1.85 | 0.609 † | 25.84 ± 2.08 | 24.73 ± 2.18 | 0.052 † | 0.050 |
| SBP, mmHg | 121.25 ± 2.98 | 123.05 ± 2.78 | 0.520 † | 121.20 ± 2.39 | 118.40 ± 3.55 | 0.307 † | 0.117 |
| DBP, mmHg | 74.80 ± 2.01 | 76.20 ± 1.63 | 0.522 † | 70.55 ± 2.24 | 72.60 ± 1.47 | 0.397 † | 0.063 |
| BDNF | 255.30 ± 40.78 | 322.08 ± 42.80 | 0.038 ‡ | 267.23 ± 45.00 | 287.45 ± 53.24 | 0.673‡ | 0.132 |
| Serotonin, ng/mL | 151.73 ± 22.76 | 187.66 ± 27.12 | 0.219 † | 122.62 ± 13.37 | 154.23 ± 20.69 | 0.102 † | 0.058 |
| Folate, ng/mL | 6.31 ± 0.69 | 7.47 ± 1.00 | 0.013 † | 6.39 ± 1.45 | 6.72 ± 3.41 | 0.536 † | 0.057 |
| hs-CRP, mg/L | 1.76 ± 0.56 | 0.81 ± 0.29 | 0.180 ‡ | 2.03 ± 0.89 | 0.41 ± 0.10 | 0.061‡ | 0.031 |
| Vitamin B12, pg/mL | 517.70 ± 30.57 | 507.75 ± 25.80 | 0.694 † | 550.45 ± 45.51 | 542.00 ± 38.89 | 0.768 † | 0.143 |
| CES-D score | 30.4 ± 7.97 | 15.15 ± 8.95 | <0.0001 † | 28.35 ± 6.49 | 17.85 ± 7.36 | 0.001 † | 0.889 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Choi, J.; Lee, H.-J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. https://doi.org/10.3390/nu12061815
Park M, Choi J, Lee H-J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients. 2020; 12(6):1815. https://doi.org/10.3390/nu12061815
Chicago/Turabian StylePark, Miey, Jihee Choi, and Hae-Jeung Lee. 2020. "Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study" Nutrients 12, no. 6: 1815. https://doi.org/10.3390/nu12061815
APA StylePark, M., Choi, J., & Lee, H.-J. (2020). Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients, 12(6), 1815. https://doi.org/10.3390/nu12061815






