A Comparison of Vitamin and Lutein Concentrations in Breast Milk from Four Asian Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Materials
2.3. Water-Soluble Vitamin Analysis
2.3.1. Sample Preparation
2.3.2. LC-MS/MS Conditions
2.4. Fat-Soluble Vitamins and Lutein Analysis
2.4.1. Samples Preparation
2.4.2. HPLC Conditions
2.5. Statistical Analysis
3. Results
3.1. Variation of Vitamin Concentrations in Human Milk between Countries
3.1.1. Water-Soluble Vitamins
3.1.2. Fat-Soluble Vitamins
3.2. Correlation between Vitamins and Lutein
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, K.M.; Nazar, A.M. Breastfeeding, the immune response, and long-term health. J. Am. Osteopat. Assoc. 2006, 106, 203–207. [Google Scholar]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Walfisch, A.; Sermer, C.; Cressman, A.; Koren, G. Breast milk and cognitive development—The role of confounders: A systematic review. BMJ Open 2013, 3, e003259. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Dolinsky, M.; Matos, A.; Chagas, C.; Ramalho, A. Vitamin A concentration in human milk and its relationship with liver reserve formation and compliance with the recommended daily intake of vitamin A in pre-term and term infants in exclusive breastfeeding. Arch. Gynecol. Obstet. 2014, 291, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Maiya, S.; Sullivan, I.; Allgrove, J.; Yates, R.; Malone, M.; Brain, C.; Archer, N.; Mok, Q.; Daubeney, P.; Tulloh, R.; et al. Hypocalcaemia and vitamin D deficiency: An important, but preventable, cause of life-threatening infant heart failure. Heart 2008, 94, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Mactier, H.; Weaver, L.T. Vitamin A and preterm infants: What we know, what we don’t know, and what we need to know. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F103–F108. [Google Scholar] [CrossRef]
- Strobel, M.; Tinz, J.; Biesalski, H.-K. The importance of β-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur. J. Nutr. 2007, 46, 1–20. [Google Scholar] [CrossRef]
- Grilo, E.C.; Medeiros, W.F.; Silva, A.G.A.; Gurgel, C.S.S.; Ramalho, H.M.M.; Dimenstein, R. Maternal supplementation with a megadose of vitamin A reduces colostrum level of α-tocopherol: A randomised controlled trial. J. Hum. Nutr. Diet. 2016, 29, 652–661. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Wildman, R.E. Advanced Human Nutrition, 2nd ed.; Jones & Bartlett Publishers: Burlington, MA, USA, 2013. [Google Scholar]
- Cannell, J.J.; Hollis, B.W.; Zasloff, M.; Heaney, R.P. Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 2007, 9, 107–118. [Google Scholar] [CrossRef]
- Pettifor, J.M. Vitamin D deficiency and nutritional rickets in children. In Vitamin D, 3rd ed.; Elsevier: London, UK, 2011; pp. 1107–1128. [Google Scholar]
- Kennedy, D.O. B Vitamins and the brain: Mechanisms, dose and efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Vitamin b-12 in human milk: A systematic review. Adv. Nutr. 2018, 9, 358S–366S. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. B Vitamins in Breast Milk: Relative Importance of Maternal Status and Intake, and Effects on Infant Status and Function. Adv. Nutr. 2012, 3, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Sherry, C.L.; Oliver, J.S.; Renzi-Hammond, L.M.; Marriage, B.J. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J. Nutr. 2014, 144, 1256–1263. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Adair, L.S.; Bentley, M.E.; Flax, V.L.; Jamieson, D.J.; Ellington, S.R.; Tegha, G.; Chasela, C.S.; Kamwendo, D.; et al. Thiamin and riboflavin in human milk: Effects of lipid-based nutrient supplementation and stage of lactation on vitamer secretion and contributions to total vitamin content. PLoS ONE 2016, 11, e0149479. [Google Scholar] [CrossRef]
- Caulfield, L.E.; Richard, S.A.; Rivera, J.A.; Musgrove, P.; Black, R.E. Stunting, wasting, and micronutrient deficiency disorders. In Disease Control Priorities in Developing Countries, 2nd ed.; The International Bank for Reconstruction and Development/The World Bank; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Kasalová, E.; Aufartová, J.; Krcmova, L.K.; Solichová, D.; Solich, P. Recent trends in the analysis of vitamin D and its metabolites in milk—A review. Food Chem. 2015, 171, 177–190. [Google Scholar] [CrossRef]
- da Silva, A.L.C.; da Ribeiro, K.D.S.; de Melo, L.R.M.; Bezerra, D.F.; de Queiroz, J.L.C.; Lima, M.S.R.; Pires, J.F.; Bezerra, D.S.; Osório, M.M.; Dimenstein, R. Vitamin E in human milk and its relation to the nutritional requirement of the term newborn. Rev. Paul. Pediatr. 2017, 35, 158–164. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Szlagatys-Sidorkiewicz, A.; Zagierski, M. Concentrations of alpha- and gamma-tocopherols in human breast milk during the first months of lactation and in infant formulas. Matern. Child. Nutr. 2012, 9, 473–482. [Google Scholar] [CrossRef]
- Ren, X.; Yang, Z.; Shao, B.; Yin, S.A.; Yang, X. B-vitamin levels in human milk among different lactation stages and areas in china. PLoS ONE 2015, 10, e0133285. [Google Scholar] [CrossRef] [PubMed]
- Page, R.; Robichaud, A.; Arbuckle, T.E.; Fraser, W.D.; Macfarlane, A.J. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am. J. Clin. Nutr. 2017, 105, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Miccheli, A.; Tomai, P.; Baldassarre, M.E.; Curini, R.; Fernandez, V.P. Liquid chromatography–tandem mass spectrometry method for the determination of vitamin K homologues in human milk after overnight cold saponification. J. Food Compos. Anal. 2016, 47, 21–30. [Google Scholar] [CrossRef]
- Haug, M.; Laubach, C.; Burke, M.; Harzer, G. Vitamin e in human milk from mothers of preterm and term infants. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin concentrations in human milk vary with time within feed, circadian rhythm, and single-dose supplementation. J. Nutr. 2017, 147, 603–611. [Google Scholar] [CrossRef]
- Xu, C.Z.; Wang, H.F.; Yang, J.Y.; Wang, J.H.; Duan, Z.Y.; Wang, C.; Liu, J.X.; Lao, Y. Effects of feeding lutein on production performance, antioxidative status, and milk quality of high-yielding dairy cows. J. Dairy Sci. 2014, 97, 7144–7150. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ranard, K.M.; Neuringer, M.; Johnson, E.E.; Renner, L.; Kuchan, M.J.; Pereira, S.L.; Johnson, E.J.; Erdman, J.W., Jr. Lutein is differentially deposited across brain regions following formula or breast feeding of infant rhesus macaques. J. Nutr. 2018, 148, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.J.W., Jr.; Smith, J.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and brain function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef]
- Giampietri, M.; Lorenzoni, F.; Moscuzza, F.; Boldrini, A.; Ghirri, P. Lutein and neurodevelopment in preterm infants. Front. Neurosci. 2016, 10, 4034. [Google Scholar] [CrossRef]
- Lipkie, T.E.; Morrow, A.L.; Jouni, Z.E.; McMahon, R.J.; Ferruzzi, M.G. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS ONE 2015, 10, e0127729. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Castellazzi, A.M.; Pietri, A.; Roggi, C.; Turconi, G. Lutein concentration in human milk during early lactation and its relationship with dietary lutein intake. Public Health Nutr. 2009, 12, 1878–1884. [Google Scholar] [CrossRef]
- Sakurai, T.; Furukawa, M.; Asoh, M.; Kanno, T.; Kojima, T.; Yonekubo, A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J. Nutr. Sci. Vitaminol. 2005, 51, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Redeuil, K.M.; Giménez, E.C.; Vinyes-Pares, G.; Zhao, A.; He, T.; Yang, X.; Zheng, Y.; Zhang, Y.; Wang, P.; et al. Regional, socioeconomic, and dietary factors influencing B-vitamins in human milk of urban Chinese lactating women at different lactation stages. BMC Nutr. 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.P.; Srinivasan, K.; Thomas, T.; Samuel, T.; Rajendran, R.; Muthayya, S.; Finkelstein, J.L.; Lukose, A.; Fawzi, W.; Allen, L.H.; et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J. Nutr. 2014, 144, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kamao, M.; Tsugawa, N.; Suhara, Y.; Wada, A.; Mori, T.; Murata, K.; Nishino, R.; Ukita, T.; Uenishi, K.; Tanaka, K.; et al. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2007, 859, 192–200. [Google Scholar] [CrossRef]
- Kim, H.; Jung, B.-M.; Lee, B.-N.; Kim, Y.-J.; Jung, J.A.; Chang, N. Retinol, α-tocopherol, and selected minerals in breast milk of lactating women with full-term infants in South Korea. Nutr. Res. Pr. 2017, 11, 64–69. [Google Scholar] [CrossRef]
- De Lira, L.Q.; Lima, M.S.R.; De Medeiros, J.M.S.; Da Silva, I.F.; Dimenstein, R. Correlation of vitamin A nutritional status on alpha-tocopherol in the colostrum of lactating women. Matern. Child. Nutr. 2011, 9, 31–40. [Google Scholar] [CrossRef]
- Mello-Neto, J.; Rondo, P.H.C.; Oshiiwa, M.; Morgano, M.A.; Zacari, C.Z.; Domingues, S. The influence of maternal factors on the concentration of vitamin A in mature breast milk. Clin. Nutr. 2009, 28, 178–181. [Google Scholar] [CrossRef]
- Oberhelman, S.S.; Meekins, M.E.; Fischer, P.R.; Lee, B.R.; Singh, R.J.; Cha, S.S.; Gardner, B.M.; Pettifor, J.M.; Croghan, I.T.; Thacher, T.D. Maternal vitamin D supplementation to improve the vitamin D status of breast-fed infants: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1378–1387. [Google Scholar] [CrossRef]
- Streym, S.; Højskov, C.S.; Møller, U.K.; Heickendorff, L.; Vestergaard, P.; Mosekilde, L.; Rejnmark, L. Vitamin D content in human breast milk: A 9-mo follow-up study. Am. J. Clin. Nutr. 2015, 103, 107–114. [Google Scholar] [CrossRef]
- Kojima, T.; Asoh, M.; Yamawaki, N.; Kanno, T.; Hasegawa, H.; Yonekubo, A. Vitamin K concentrations in the maternal milk of Japanese women. Acta Paediatr. 2004, 93, 457–463. [Google Scholar]
- Greer, F.R. Are breast-fed infants vitamin K deficient? In Bioactive Components of Human Milk; Springer: New York, NY, USA, 2001; pp. 391–395. [Google Scholar]
- Haroon, Y.; Shearer, M.J.; Rahim, S.; Gunn, W.G.; McEnery, G.; Barkhan, P. The content of phylloquinone (vitamin k1) in human milk, cows’ milk and infant formula foods determined by high-performance liquid chromatography. J. Nutr. 1982, 112, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Canfield, L.M.; Hopkinson, J.M.; Lima, A.F.; Silva, B.; Garza, C. Vitamin K in colostrum and mature human milk over the lactation period—a cross-sectional study. Am. J. Clin. Nutr. 1991, 53, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Marshall, S.; Cherry, J.; Suttie, J.W. Vitamin K status of lactating mothers, human milk, and breast-feeding infants. Pediatrics 1991, 88, 751–756. [Google Scholar] [PubMed]
- Jackson, J.G.; Zimmer, J.P. Lutein and zeaxanthin in human milk independently and significantly differ among women from Japan, Mexico, and the United Kingdom. Nutr. Res. 2007, 27, 449–453. [Google Scholar] [CrossRef]
- Xue, Y.; Giménez, E.C.; Redeuil, K.M.; Lévèques, A.; Actis-Goretta, L.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Concentrations of carotenoids and tocopherols in breast milk from urban chinese mothers and their associations with maternal characteristics: A cross-sectional study. Nutrients 2017, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization/World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Rome, Italy, 2005. [Google Scholar]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80, 1752S–1758S. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Nelson, S.E.; Jeter, J.M. Vitamin D supplementation of breastfed infants: A randomized dose–response trial. Pediatr. Res. 2014, 76, 177–183. [Google Scholar] [CrossRef]

| CHINA | KOREA | PAKISTAN | VIETNAM | |
|---|---|---|---|---|
| No. Sample | 111 | 155 | 97 | 92 |
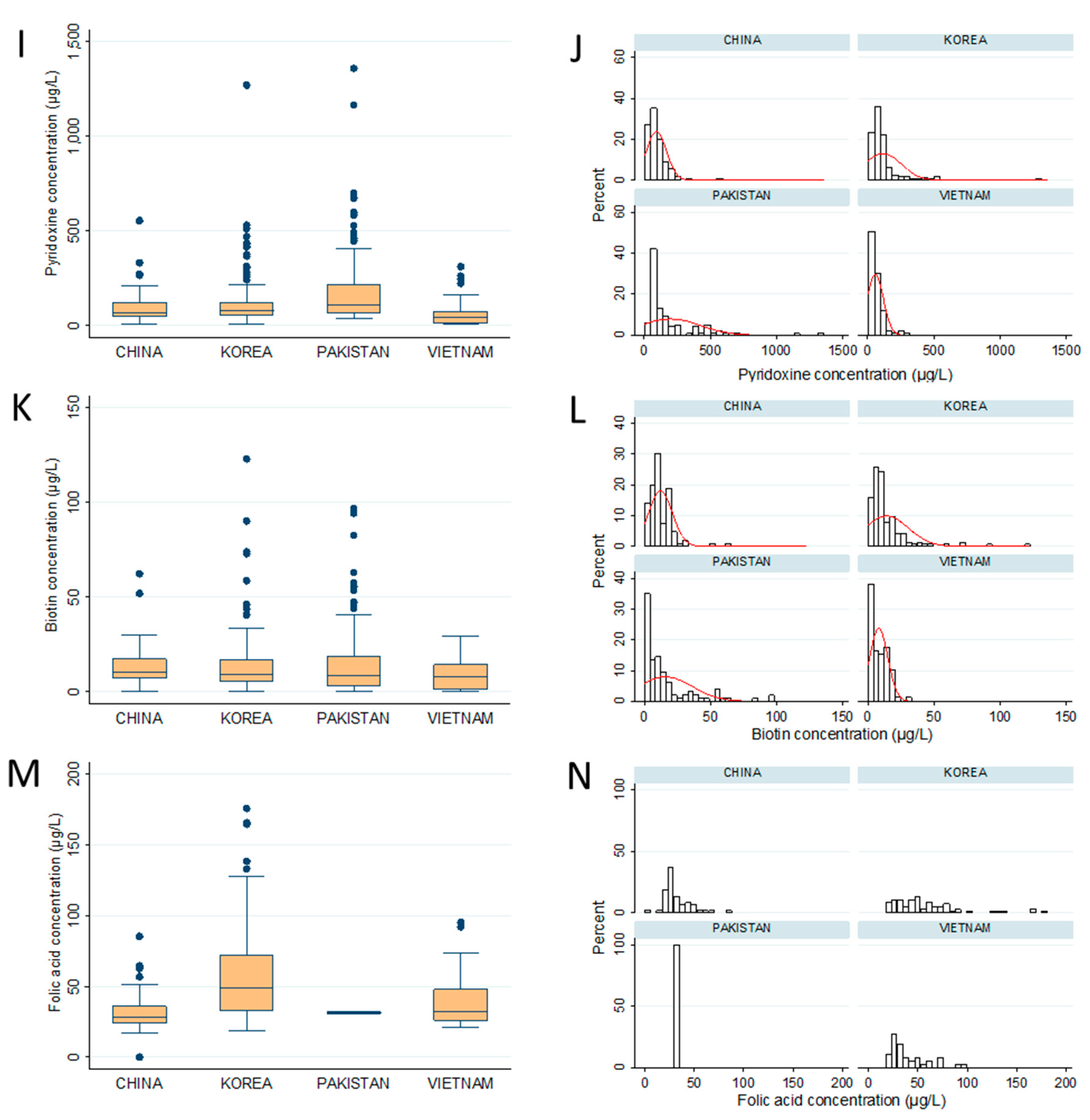
| Thiamin (B1) | 68.1 ± 51.2 a,b | 89.3 ± 74.3 b | 66.5 ± 93.7 a,b | 56.5 ± 61.5 a |
| Riboflavin (B2) | 25.3 ± 53.5 a (82.9%) | 36.1 ± 82.8 a,b (72.9%) | 58.0 ± 43.3 b (100%) | 19.9 ± 34.7 a (77.8%) |
| Niacin (B3) i | 396.7 ± 233.3 a | 393.7 ± 278.3 a | 523.9 ± 485.2 a,b | 553.8 ± 440.2 b |
| Pantothenic acid (B5) | 1924.0 ± 2047.4 a,b | 2571.2 ± 2932.2 b | 2557.4 ± 2576.5 b | 1266.1 ± 1242.7 a |
| Pyridoxine (B6) ii | 92.4 ± 75.8 a,b | 115.1 ± 137.3 b | 196.7 ± 225.3 c | 56.4 ± 60.4 a |
| Biotin (B7) | 12.1 ± 9.0 a,b (95.5%) | 14.0 ± 16.5 b (92.9%) | 15.8 ± 20.4 b (100%) | 8.1 ± 6.9 a (87.8%) |
| Folic acid (B9) | 32.3 ± 13.1 b (53.2%) | 58.6 ± 35.5 a (43.9%) | 31.5 ± 1.3 a (2%) | 40.2 ± 19.8 b (41.1%) |
| Cyanocobalamin (B12) | 4.9± 1.1 (13.5%) | 5.0± 1.4 (7.1%) | 2.7 ± 4 (25.8%) | 8.1 ± 4.9 (2.2%) |
| CHINA | KOREA | PAKISTAN | VIETNAM | |
|---|---|---|---|---|
| No. Sample | 137 | 254 | 97 | 92 |
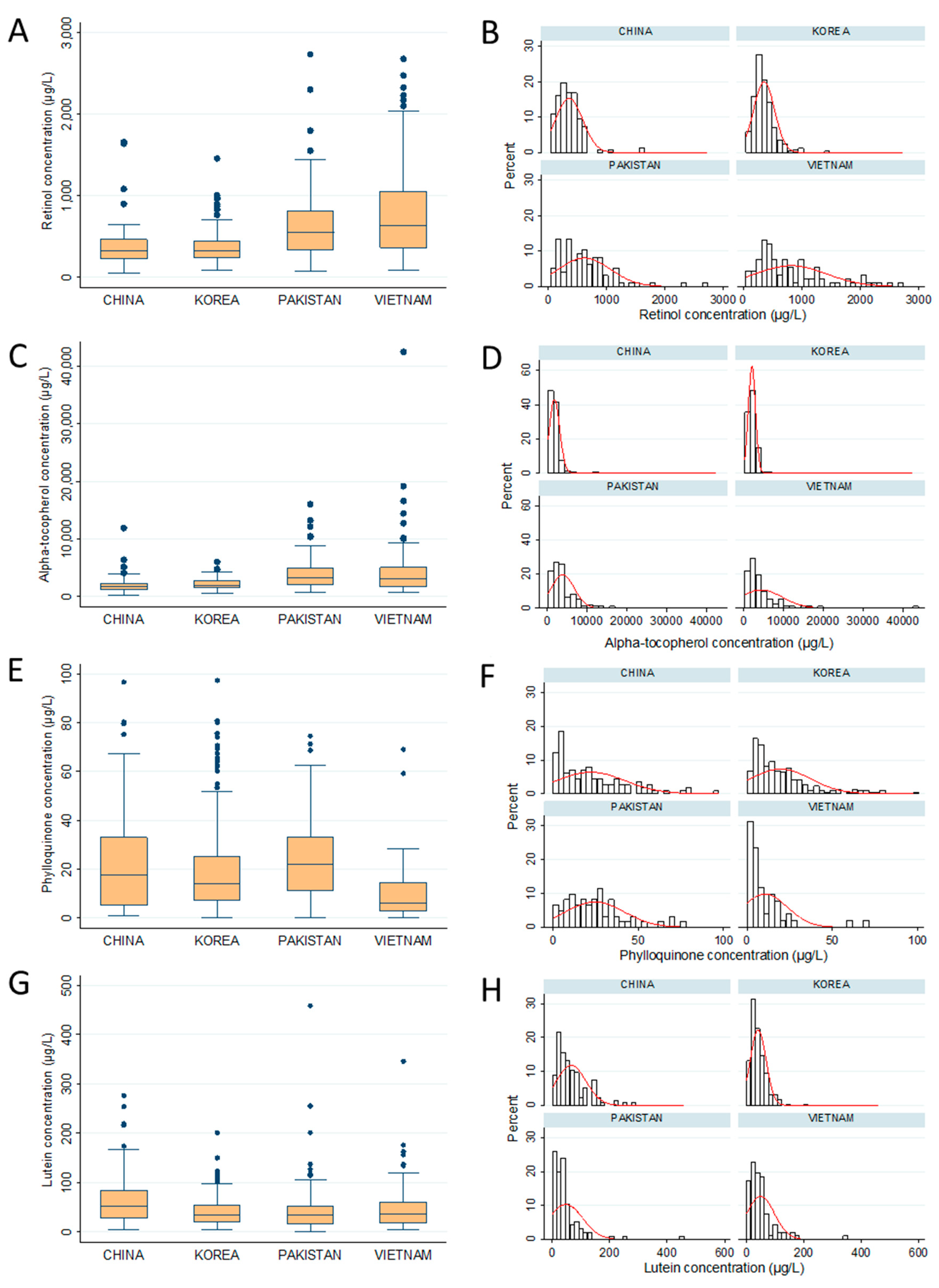
| Retinol (Vit A) | 364.5 ± 232.7 a | 356.5 ± 180.1 a | 622.1 ± 447.3 b | 813.6 ± 609.0 c |
| Tocopherol (Vit E) | 1907.5 ± 1309.1 a | 2140.1 ± 896.0 a | 3943.0 ± 2874.5 b | 4413.4 ± 5274.2 b |
| Phylloquinone (Vit K) | 19.3 ± 16.7 a,b (68.6%) | 18.9 ± 18.5 a,b (69.7%) | 25.7 ± 17.2 b (62.9%) | 12.9 ± 13.9 a (43.5%) |
| Lutein | 66.1 ± 51.6 b | 41.3 ± 27.4 a | 47.5 ± 58.9 a | 49.7 ± 47.9 a,b |
| B1 | B2 | B3 | B5 | B6 | B7 | B9 | B12 | Retinol | E | K | Lutein | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 1.000 | |||||||||||
| B2 | 0.074 | 1.000 | ||||||||||
| B3 | 0.318 | 0.083 | 1.000 | |||||||||
| B5 | 0.342 | 0.181 | 0.351 | 1.000 | ||||||||
| B6 | 0.450 | 0.362 | 0.208 | 0.464 | 1.000 | |||||||
| B7 | 0.137 | 0.399 | 0.118 | 0.228 | 0.207 | 1.000 | ||||||
| B9 | 0.171 | 0.115 | 0.106 | 0.340 | 0.165 | 0.253 | 1.000 | |||||
| B12 | 0.102 | 0.098 | 0.009 | 0.029 | 0.007 | 0.005 | −0.005 | 1.000 | ||||
| Retinol | −0.164 | 0.012 | 0.056 | −0.068 | −0.076 | −0.079 | −0.091 | −0.016 | 1.000 | |||
| E | −0.168 | 0.042 | −0.063 | −0.109 | −0.076 | −0.022 | −0.089 | −0.010 | 0.497 | 1.000 | ||
| K | 0.009 | 0.080 | −0.026 | −0.034 | −0.002 | 0.067 | −0.013 | −0.049 | −0.065 | −0.025 | 1.000 | |
| Lutein | −0.118 | −0.039 | −0.032 | −0.055 | −0.084 | −0.033 | −0.076 | −0.095 | 0.351 | 0.388 | 0.081 | 1.000 |
| Vitamin | Country | Analysis Method | n | Median | Mean | SD | Ref. |
|---|---|---|---|---|---|---|---|
| Thiamin (Vit. B1) | |||||||
| China | UPLC-MS/MS | 6419 | 5.0–40.7 | [24] | |||
| Japan | HPLC-FID | 691 | 123 | 32 | [35] | ||
| China | HPLC-MS/MS | 443 | 31.3–62.8 | [36] | |||
| Malawian | HPLC-FLD | 177 | 10.5–40.9 | [19] | |||
| Bangladesh | HPLC-FLD | 18 | 116 | [28] | |||
| Riboflavin (Vit. B2) | |||||||
| China | UPLC-MS/MS | 6419 | 29.3–40.6 | [24] | |||
| China | HPLC-MS/MS | 443 | 119–208 | [36] | |||
| Malawian | ULPC-MS/MS | 177 | 6.3–7.3 | [19] | |||
| Bangladesh | HPLC-MS/MS | 18 | 24 | [28] | |||
| Niacin (Vit. B3) | |||||||
| China | UPLC-MS/MS | 6419 | 470.7–687.0 | [24] | |||
| Japan | HPLC-UV | 619 | 329 | 204 | [35] | ||
| China | HPLC-MS/MS | 443 | 1940–3000 | [36] | |||
| Bangladesh | HPLC-MS/MS | 18 | 219 | [28] | |||
| Pantothenic acid (Vit. B5) | |||||||
| China | UPLC-MS/MS | 6419 | 1770.9–2626.8 | [24] | |||
| Japan | Microbiological assay | 619 | 2700 | 900 | [35] | ||
| China | HPLC-MS/MS | 443 | 1790–2910 | [36] | |||
| Pyridoxine (Vit. B6) | |||||||
| China | UPLC-MS/MS | 6419 | 4.6–80.7 | [24] | |||
| Japan | Microbiological assay | 619 | 57 | 25 | [35] | ||
| China | HPLC-MS/MS | 443 | 63.4–102.0 | [36] | |||
| Bangladesh | HPLC-MS/MS | 18 | 81 | [28] | |||
| Biotin (Vit. B7) | |||||||
| Japan | Microbiological | 619 | 5.0 | 2.3 | [35] | ||
| China | HPLC-MS/MS | 443 | 4.6–6.1 | [36] | |||
| Folic acid (Vit. B9) | |||||||
| Japan | HPLC-FID | 619 | 62 | 29 | [35] | ||
| China | HPLC-MS/MS | 443 | 7.3–24.4 | [36] | |||
| Canada | HPLC-MS/MS | 160 | 20.7 | 0.7 | [25] | ||
| Cyanocobalamin (Vit. B12) | |||||||
| Japan | Microbiological assay | 619 | 0.4 | 0.2 | [35] | ||
| Bangladesh | Quantitative immuno-analyzer | 18 | 0.175 | [28] | |||
| India | Competitive protein binding immunoassay | 326 | 0.9–1.8 | [37] | |||
| Retinol (Vit. A) | |||||||
| Japan | HPLC-FID | 82 | 455 | 264 | [38] | ||
| Bangladesh | HPLC | 18 | 391 | [28] | |||
| Korea | HPLC-UV | 334 | 395.8 | 196.4 | [39] | ||
| Brazil | HPLC-UV | 103 | 624.6 | 229.2 | [40] | ||
| Brazil | HPLC-UV | 136 | 483.3 | 197.3 | [41] | ||
| Tocopherol (Vit. E) | |||||||
| Japan | HPLC-FID | 619 | 3250 | 1650 | [35] | ||
| Japan | HPLC | 82 | 5087 | 5042 | [38] | ||
| Bangladesh | HPLC | 18 | 4400 | [28] | |||
| Korea | HPLC-UV | 334 | 230 | 130 | [39] | ||
| Brazil | HPLC-UV | 103 | 11,241.5 | 5513 | [40] | ||
| Cholecalciferol (Vit. D3) | |||||||
| Japanese | HPLC-UV | 114 | 0.08 | [35] | |||
| Japanese | LC-MS/MS | 88 | 0.088 | [38] | |||
| USA | LC-MS/MS | 40 | 0.008–0.04 | [42] | |||
| Denmark | LC-MS/MS (LOD: 0.2 nmol) | 120 | 0.11–0.57 (27–46% < LOD) | [43] | |||
| Phylloquinone (Vit. K) | |||||||
| Japan | HPLC-MS/MS | 82 | 3.7 | 2.2 | [38] | ||
| Japan | HPLC-FID | 4.3 | 2.9 | [44] | |||
| HPLC-FID | 0.9–1.2 | [45] | |||||
| UK | HPLC-MS/MS | 29 | 2.1–140 | [46] | |||
| 15 | 2.87–3.39 | [47] | |||||
| 1.1–130 | [48] | ||||||
| Lutein | |||||||
| Mexico | HPLC-UV | 20 | 27.3 | 11.8 | [49] | ||
| Japan | HPLC-UV | 20 | 29.1 | 21.3 | [49] | ||
| UK | HPLC-UV | 20 | 12.4 | 7.8 | [49] | ||
| China | HPLC-UV | 20 | 93.1 | [33] | |||
| USA | HPLC-UV | 20 | 41.7 | [33] | |||
| Mexico | HPLC-UV | 20 | 39.2 | [33] | |||
| Italy | HPLC-UV | 15 | 62.6 | 28.4 | [34] | ||
| China | HPLC-UV | 509 | 22–58 | [50] | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.T.T.; Kim, J.; Lee, H.; Won, S.; Kim, Y.; Jung, J.A.; Li, D.; To, X.H.M.; Huynh, K.T.N.; Le, T.V.; et al. A Comparison of Vitamin and Lutein Concentrations in Breast Milk from Four Asian Countries. Nutrients 2020, 12, 1794. https://doi.org/10.3390/nu12061794
Nguyen MTT, Kim J, Lee H, Won S, Kim Y, Jung JA, Li D, To XHM, Huynh KTN, Le TV, et al. A Comparison of Vitamin and Lutein Concentrations in Breast Milk from Four Asian Countries. Nutrients. 2020; 12(6):1794. https://doi.org/10.3390/nu12061794
Chicago/Turabian StyleNguyen, My Tuyen Thi, Jieun Kim, Hyunjun Lee, Soyoon Won, Yongki Kim, Ji A. Jung, Dan Li, Xuan Hong Mai To, Khanh Trang Nguyen Huynh, Thanh Van Le, and et al. 2020. "A Comparison of Vitamin and Lutein Concentrations in Breast Milk from Four Asian Countries" Nutrients 12, no. 6: 1794. https://doi.org/10.3390/nu12061794
APA StyleNguyen, M. T. T., Kim, J., Lee, H., Won, S., Kim, Y., Jung, J. A., Li, D., To, X. H. M., Huynh, K. T. N., Le, T. V., Israr, B., An, H. J., & Kim, J. (2020). A Comparison of Vitamin and Lutein Concentrations in Breast Milk from Four Asian Countries. Nutrients, 12(6), 1794. https://doi.org/10.3390/nu12061794







