The Effect of Fructose Feeding on Intestinal Triacylglycerol Production and De Novo Fatty Acid Synthesis in Humans
Abstract
1. Introduction
2. Materials and Methods
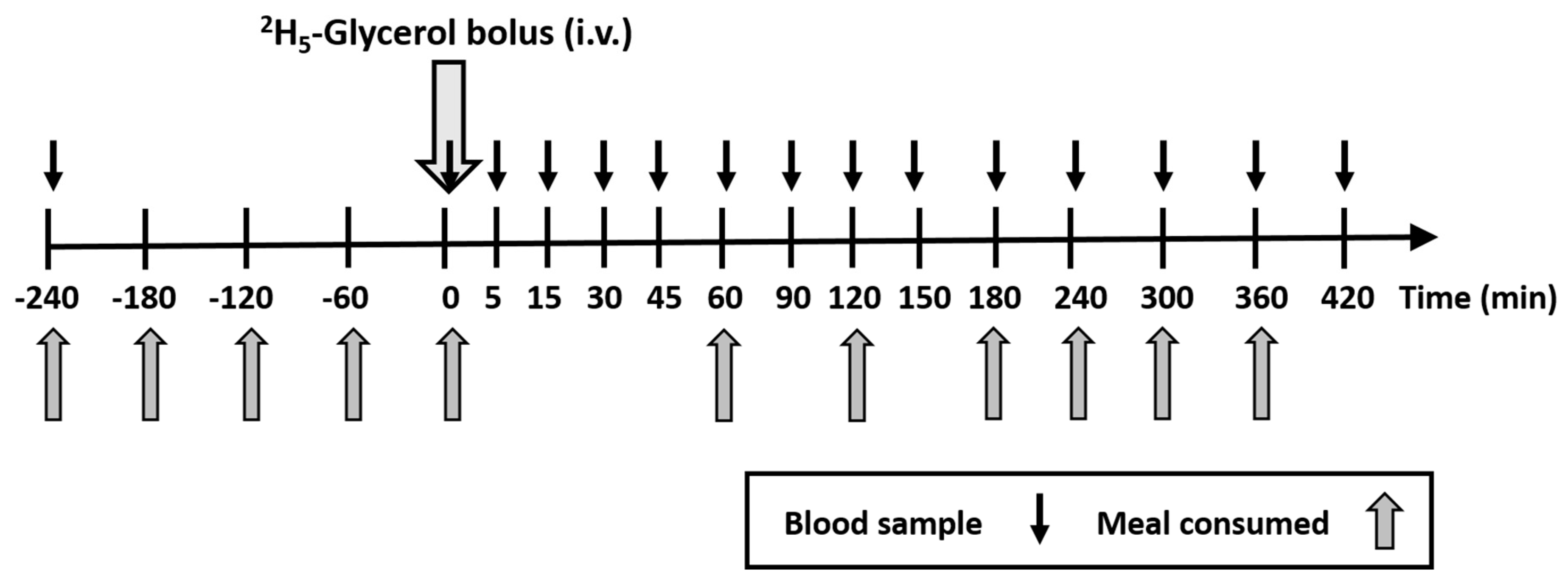
2.1. Experimental Design
2.2. Laboratory Analyses
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
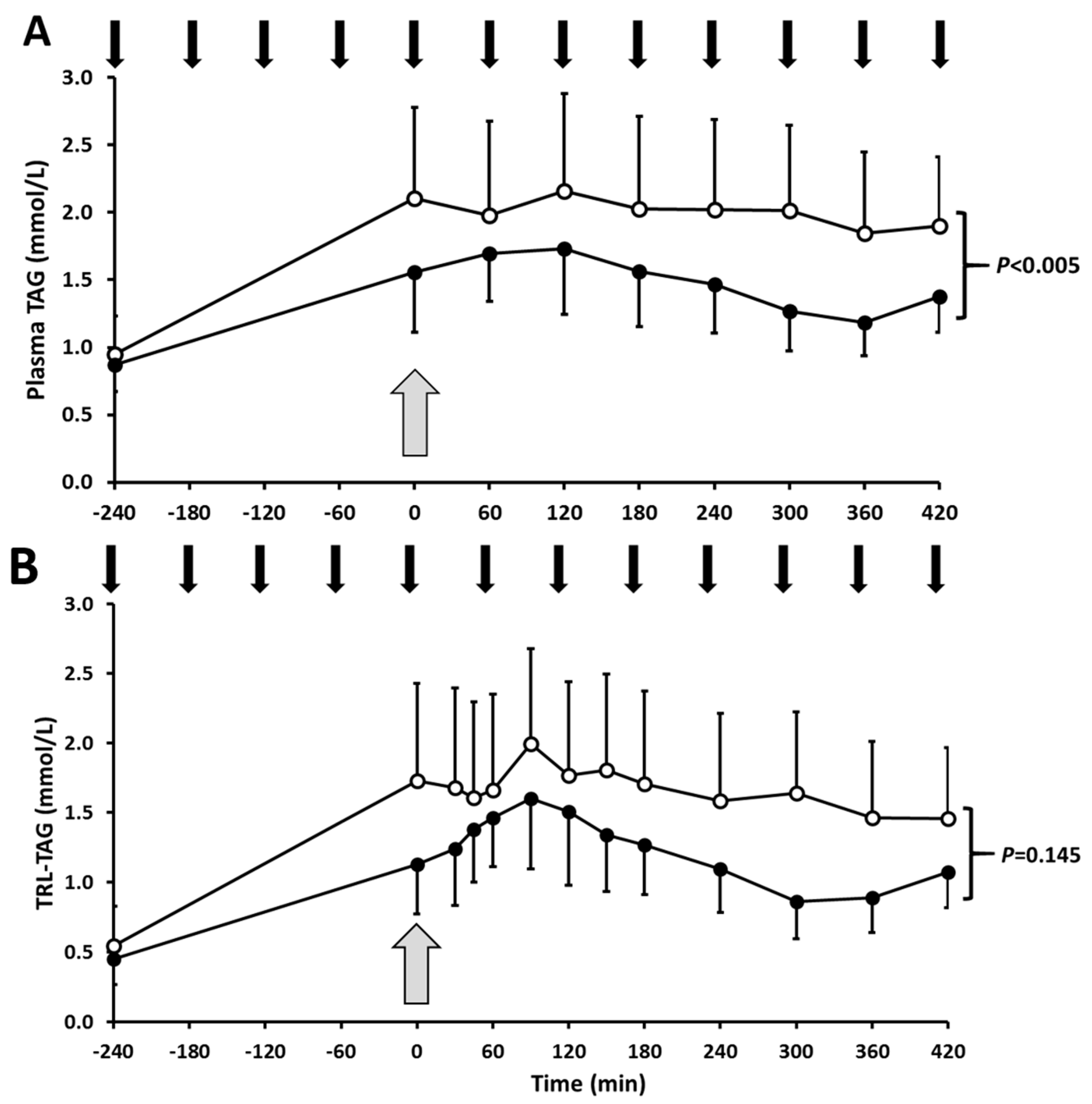
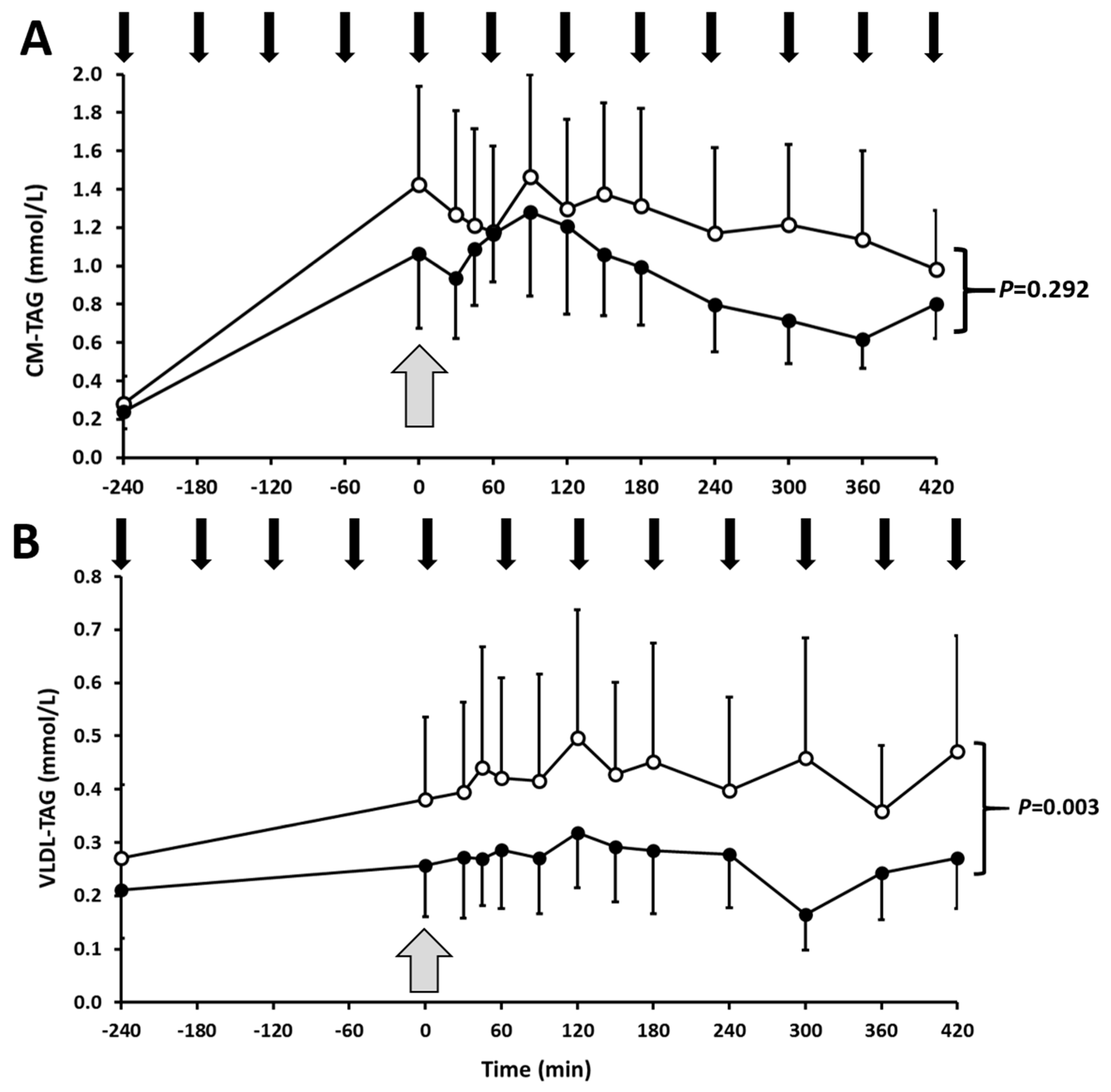
3.2. Postprandial Lipoprotein Kinetics
3.3. De Novo Lipogenesis
3.4. Other Postprandial Measurements
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eberly, L.E.; Stamler, J.; Neaton, J.D. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch. Intern. Med. 2003, 163, 1077–1083. [Google Scholar] [CrossRef]
- Bantle, J.P. Is fructose the optimal low glycemic index sweetener? Nestle Nutr. Workshop Series Clin. Perform. Programme 2006, 11, 83–95. [Google Scholar]
- Silbernagel, G.; Machann, J.; Unmuth, S.; Schick, F.; Stefan, N.; Haring, H.U.; Fritsche, A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: An exploratory trial. Br. J. Nutr. 2011, 106, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ngo Sock, E.T.; Le, K.A.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br. J. Nutr. 2010, 103, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Bremer, A.A.; Medici, V.; Nakajima, K.; Ito, Y.; Nakano, T.; Chen, G.; Fong, T.H.; Lee, V.; Menorca, R.I.; et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, ldl-cholesterol, and apolipoprotein-b in young men and women. J. Clin. Endocrinol. Metab. 2011, 96, E1596–E1605. [Google Scholar] [CrossRef]
- Teff, K.L.; Grudziak, J.; Townsend, R.R.; Dunn, T.N.; Grant, R.W.; Adams, S.H.; Keim, N.L.; Cummings, B.P.; Stanhope, K.L.; Havel, P.J. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: Influence of insulin resistance on plasma triglyceride responses. J. Clin. Endocrinol. Metab. 2009, 94, 1562–1569. [Google Scholar] [CrossRef]
- Beysen, C.; Ruddy, M.; Stoch, A.; Mixson, L.; Rosko, K.; Riiff, T.; Turner, S.M.; Hellerstein, M.K.; Murphy, E.J. Dose-dependent quantitative effects of acute fructose administration on hepatic de novo lipogenesis in healthy humans. Am. J. Physiology. Endocrinol. Metab. 2018, 315, E126–E132. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Soderlund, S.; Bogl, L.H.; Hakkarainen, A.; Matikainen, N.; Pietilainen, K.H.; Rasanen, S.; Lundbom, N.; Bjornson, E.; Eliasson, B.; et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017, 282, 187–201. [Google Scholar] [CrossRef]
- Haidari, M.; Leung, N.; Mahbub, F.; Uffelman, K.D.; Kohen-Avramoglu, R.; Lewis, G.F.; Adeli, K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and apob48-containing lipoprotein overproduction. J. Biol. Chem. 2002, 277, 31646–31655. [Google Scholar]
- Al-Jawadi, A.; Patel, C.R.; Shiarella, R.J.; Romelus, E.; Auvinen, M.; Guardia, J.; Pearce, S.C.; Kishida, K.; Yu, S.; Gao, N.; et al. Cell-type–specific, ketohexokinase-dependent induction by fructose of lipogenic gene expression in mouse small intestine. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shojaee-Moradie, F.; Ma, Y.; Lou, S.; Hovorka, R.; Umpleby, A.M. Prandial hypertriglyceridemia in metabolic syndrome is due to an overproduction of both chylomicron and vldl triacylglycerol. Diabetes 2013, 62, 4063–4069. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Stolinski, M.; Shojaee-Moradie, F.; Umpleby, A.M. Measurement of endogenous and exogenous triacylglycerol kinetics in the fed and fasted states. Biochem. Soc. Trans. 2007, 35, 482–483. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Adiels, M.; Larsson, T.; Sutton, P.; Taskinen, M.R.; Boren, J.; Fielding, B.A. Optimization of n-methyl-n-[tert-butyldimethylsilyl]trifluoroacetamide as a derivatization agent for determining isotopic enrichment of glycerol in very-low density lipoproteins. Rapid Commun. Mass Spectrom. 2010, 24, 586–592. [Google Scholar] [CrossRef]
- Sun, F.; Stolinski, M.; Shojaee-Moradie, F.; Lou, S.; Ma, Y.; Hovorka, R.; Umpleby, A.M. A novel method for measuring intestinal and hepatic triacylglycerol kinetics. Am. J. Physiology. Endocrinol. Metab. 2013, 305, E1041–E1047. [Google Scholar] [CrossRef]
- Pearson, T.C.; Guthrie, D.L.; Simpson, J.; Chinn, S.; Barosi, G.; Ferrant, A.; Lewis, S.M.; Najean, Y. Interpretation of measured red cell mass and plasma volume in adults: Expert panel on radionuclides of the international council for standardization in haematology. Br. J. Haematol. 1995, 89, 748–756. [Google Scholar] [CrossRef]
- Diraison, F.; Pachiaudi, C.; Beylot, M. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: Determination of the average number of deuterium atoms incorporated. Metab. Clin. Exp. 1996, 45, 817–821. [Google Scholar] [CrossRef]
- Surowska, A.; De Giorgi, S.; Theytaz, F.; Campos, V.; Hodson, L.; Stefanoni, N.; Rey, V.; Schneiter, P.; Laville, M.; Giusti, V.; et al. Effects of roux-en-y gastric bypass surgery on postprandial fructose metabolism. Obesity (Silver Springmd.) 2016, 24, 589–596. [Google Scholar] [CrossRef]
- Theytaz, F.; de Giorgi, S.; Hodson, L.; Stefanoni, N.; Rey, V.; Schneiter, P.; Giusti, V.; Tappy, L. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients 2014, 6, 2632–2649. [Google Scholar] [CrossRef]
- Abraha, A.; Humphreys, S.M.; Clark, M.L.; Matthews, D.R.; Frayn, K.N. Acute effect of fructose on postprandial lipaemia in diabetic and non-diabetic subjects. Br. J. Nutr. 1998, 80, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Dash, S.; Morgantini, C.; Lewis, G.F. Novel role of enteral monosaccharides in intestinal lipoprotein production in healthy humans. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1056–1062. [Google Scholar] [CrossRef]
- Jackson, K.G.; Robertson, M.D.; Fielding, B.A.; Frayn, K.N.; Williams, C.M. Measurement of apolipoprotein b-48 in the svedberg flotation rate (s(f)) > 400, s(f) 60–400 and s(f) 20–60 lipoprotein fractions reveals novel findings with respect to the effects of dietary fatty acids on triacylglycerol-rich lipoproteins in postmenopausal women. Clin. Sci. (Lond. Engl. 1979) 2002, 103, 227–237. [Google Scholar]
- Eckel, R.H.; Fujimoto, W.Y.; Brunzell, J.D. Insulin regulation of lipoprotein lipase in cultured 3t3-l1 cells. Biochem. Biophys. Res. Commun. 1978, 84, 1069–1075. [Google Scholar] [CrossRef]
- Sadur, C.N.; Eckel, R.H. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J. Clin. Investig. 1982, 69, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.M.; Caccavello, R.; Palii, S.P.; Pullinger, C.R.; Kane, J.; Mulligan, K.; Gugliucci, A.; Schwarz, J.-M. Separation of postprandial lipoproteins: Improved purification of chylomicrons using an apob100 immunoaffinity method. J. Lipid Res. 2019, 61, 455–463. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Parker, T.S.; Levine, D.M.; Hellerstein, M.K. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J. Clin. Endocrinol. Metab. 2011, 96, 861–868. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31 (Suppl. 3), 38–45. [Google Scholar]
- Umpleby, A.M.; Shojaee-Moradie, F.; Fielding, B.; Li, X.; Marino, A.; Alsini, N.; Isherwood, C.; Jackson, N.; Ahmad, A.; Stolinski, M.; et al. Impact of liver fat on the differential partitioning of hepatic triacylglycerol into vldl subclasses on high and low sugar diets. Clin. Sci. (Lond. Engl. 1979) 2017, 131, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Linfoot, P.; Dare, D.; Aghajanian, K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003, 77, 43–50. [Google Scholar] [CrossRef] [PubMed]
| High-Fructose | Low-Fructose | p-Value | |
|---|---|---|---|
| Fasting glucose (mmol/L) | 4.63 ± 0.26 | 4.76 ± 0.12 | 0.495 |
| Fasting insulin (pmol/L) | 65.6 ± 12.4 | 85.4 ± 20.0 | 0.063 |
| Fasting NEFA (mmol/L) | 0.69 ± 0.17 | 0.81 ± 0.13 | 0.492 |
| Fasting apoB48 (mg/L) | 1.2 ± 0.5 | 1.1 ± 0.5 | 0.860 |
| Fasting apoB100 (mg/L) | 18.1 ± 5.4 | 19.7 ± 7.5 | 0.812 |
| Fasting Hepatic % DNL | 2.26 ± 0.65 | 1.30 ± 0.51 | 0.269 |
| Fasting Intestinal % DNL | 1.75 ± 0.46 | 1.27 ± 0.42 | 0.284 |
| Postprandial glucose (mmol/L) | 5.21 ± 0.09 | 5.67 ± 0.23 | <0.005 |
| Postprandial insulin (pmol/L) | 113.0 ± 16.5 | 171.5 ± 31.5 | <0.005 |
| Postprandial NEFA (mmol/L) | 0.38 ± 0.06 | 0.35 ± 0.05 | 0.013 |
| Postprandial apoB48 (mg/L) | 5.48 ± 2.11 | 3.35 ± 0.79 | 0.039 |
| Postprandial apoB100 (mg/L) | 23.89 ± 4.66 | 19.19 ± 6.26 | 0.013 |
| Hepatic postprandial % DNL | 4.36 ± 1.85 | 2.21± 1.36 | 0.308 |
| Intestinal postprandial % DNL | 2.33 ± 1.05 | 1.08 ± 0.24 | 0.100 |
| High-Fructose | Low-Fructose | p-Value | |
|---|---|---|---|
| CM-TAG PR (mg/kg/d) | 152.9 ± 76.6 | 375.93 ± 68.8 | 0.046 |
| CM-TAG FCR (pools/d) | 7.44 ± 4.01 | 21.69 ± 7.60 | 0.073 |
| CM-TAG PS (mg) | 2976 ± 1060 | 2247 ± 653 | 0.367 |
| Intestinal DNL (mg/d) | 216 ± 78 | 318 ± 68 | 0.225 |
| VLDL-TAG PR (mg/kg/d) | 81.03 ± 19.57 | 68.43 ± 12.18 | 0.641 |
| VLDL-TAG FCR (pools/d) | 10.35 ± 3.50 | 12.21 ± 2.78 | 0.254 |
| VLDL-TAG PS (mg) | 1042 ± 460 | 634 ± 217 | 0.072 |
| Hepatic DNL (mg/d) | 476 ± 310 | 165 ± 64 | 0.138 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steenson, S.; Shojaee-Moradie, F.; B. Whyte, M.; G. Jackson, K.; Lovegrove, J.A.; A. Fielding, B.; Umpleby, A.M. The Effect of Fructose Feeding on Intestinal Triacylglycerol Production and De Novo Fatty Acid Synthesis in Humans. Nutrients 2020, 12, 1781. https://doi.org/10.3390/nu12061781
Steenson S, Shojaee-Moradie F, B. Whyte M, G. Jackson K, Lovegrove JA, A. Fielding B, Umpleby AM. The Effect of Fructose Feeding on Intestinal Triacylglycerol Production and De Novo Fatty Acid Synthesis in Humans. Nutrients. 2020; 12(6):1781. https://doi.org/10.3390/nu12061781
Chicago/Turabian StyleSteenson, Simon, Fariba Shojaee-Moradie, Martin B. Whyte, Kim G. Jackson, Julie A. Lovegrove, Barbara A. Fielding, and A. Margot Umpleby. 2020. "The Effect of Fructose Feeding on Intestinal Triacylglycerol Production and De Novo Fatty Acid Synthesis in Humans" Nutrients 12, no. 6: 1781. https://doi.org/10.3390/nu12061781
APA StyleSteenson, S., Shojaee-Moradie, F., B. Whyte, M., G. Jackson, K., Lovegrove, J. A., A. Fielding, B., & Umpleby, A. M. (2020). The Effect of Fructose Feeding on Intestinal Triacylglycerol Production and De Novo Fatty Acid Synthesis in Humans. Nutrients, 12(6), 1781. https://doi.org/10.3390/nu12061781





