Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Registration
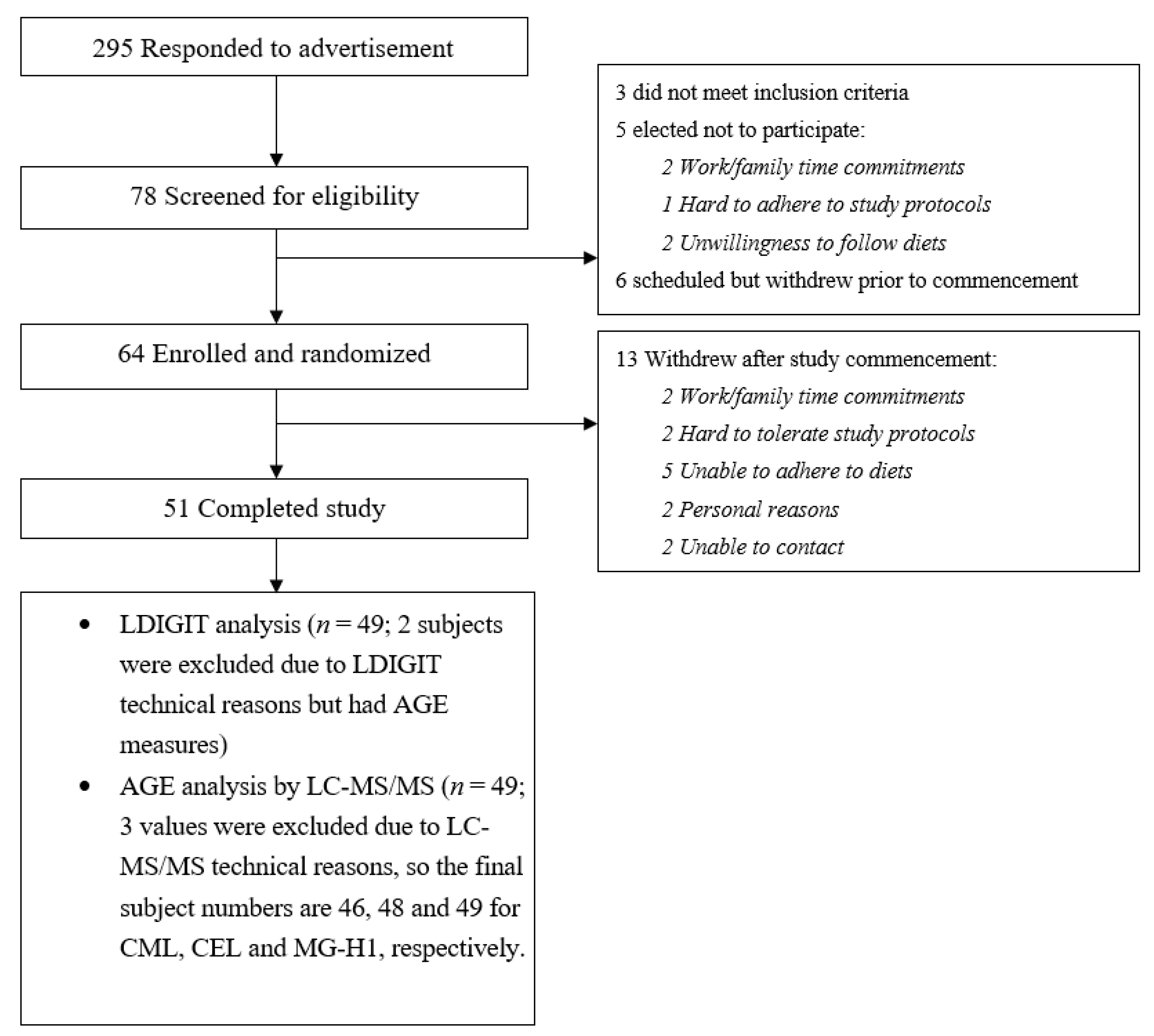
2.2. Study Participants
2.3. Dietary Intervention
2.4. Assessments of Insulin Sensitivity
2.5. Biochemical Analysis
2.6. LC-MS/MS
2.6.1. Materials
2.6.2. Blood Sample Preparation
2.6.3. LC-MS/MS
2.7. Statistical Analyses
3. Results
3.1. The Effect of Two Diets on AGEs Levels
3.2. Association between AGEs and Dietary Intake
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.E.; Dordevic, A.L.; Tan, S.M.; Ryan, L.; Coughlan, M.T. Dietary Advanced Glycation End Products and Risk Factors for Chronic Disease: A Systematic Review of Randomised Controlled Trials. Nutrients 2016, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; Currenti, W.; Urbano, F.; Scicali, R.; Piro, S.; Purrello, F.; Rabuazzo, A.M. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Westerink, J.; Scheijen, J.; van der Graaf, Y.; Stehouwer, C.D.A.; Schalkwijk, C.G. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease and Mortality in Individuals With Type 2 Diabetes. Diabetes Care 2018, 41, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Ko, H.A.; Chu, K.H.; Shieh, T.M.; Chi, T.C.; Chen, H.I.; Chang, W.C.; Chang, S.S. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxidative Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [PubMed]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal Metabolism and Aging-Related Disease: Moving from Correlation toward Causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. New York Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e912. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Taché, S.; Petit, C.R.; Van der Meer, R.; Corpet, D.E. Meat and cancer: Haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis 2003, 24, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Vulcain, E.; Goupy, P.; Caris-Veyrat, C.; Dangles, O. Inhibition of the metmyoglobin-induced peroxidation of linoleic acid by dietary antioxidants: Action in the aqueous vs. lipid phase. Free Radic. Res. 2005, 39, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor” when red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lim, B.O.; Park, G.B.; Joo, S.T. Effects of various fiber additions on lipid digestion during in vitro digestion of beef patties. J. Food Sci. 2009, 74, C653–C657. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Zhang, Y.; Zerlin, A.; Li, L.; Gao, K.; Lee, R.P.; Karp, H.; Thames, G.; Bowerman, S.; et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am. J. Clin. Nutr. 2010, 91, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Collinson, A. Red meat, dietary heme iron, and risk of type 2 diabetes: The involvement of advanced lipoxidation endproducts. Adv. Nutr. 2013, 4, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; de Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef] [PubMed]
- Birlouez-Aragon, I.; Saavedra, G.; Tessier, F.J.; Galinier, A.; Ait-Ameur, L.; Lacoste, F.; Niamba, C.N.; Alt, N.; Somoza, V.; Lecerf, J.M. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutr. 2010, 91, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Consumption of red and processed meat and refined grains for 4weeks decreases insulin sensitivity in insulin-resistant adults: A randomized crossover study. Metab. Clin. Exp. 2017, 68, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Effects of Two Different Dietary Patterns on Inflammatory Markers, Advanced Glycation End Products and Lipids in Subjects without Type 2 Diabetes: A Randomised Crossover Study. Nutrients 2017, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- de Courten, B.; de Courten, M.P.; Soldatos, G.; Dougherty, S.L.; Straznicky, N.; Schlaich, M.; Sourris, K.C.; Chand, V.; Scheijen, J.L.; Kingwell, B.A.; et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: A double-blind, randomized, crossover trial. Am. J. Clin. Nutr. 2016, 103, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, C.A.; Cathcart, S.; Clausen, J.; Shapiro, D.; Dominiczak, M.H. Factors in human serum interfere with the measurement of advanced glycation endproducts. Cell. Mol. Biol. 1998, 44, 1069–1079. [Google Scholar] [PubMed]
- Shibayama, R.; Araki, N.; Nagai, R.; Horiuchi, S. Autoantibody against N(epsilon)-(carboxymethyl)lysine: An advanced glycation end product of the Maillard reaction. Diabetes 1999, 48, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Differential Effects of Red Meat/Refined Grain Diet and Dairy/Chicken/Nuts/Whole Grain Diet on Glucose, Insulin and Triglyceride in a Randomized Crossover Study. Nutrients 2016, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; McCullough, C.L.; Almond, T.; Jaunay, E.L.; Donnellan, L.; Dhillon, V.S.; Fenech, M. Dietary sugars and related endogenous advanced glycation end-products increase chromosomal DNA damage in WIL2-NS cells, measured using cytokinesis-block micronucleus cytome assay. Mutagenesis 2020. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; Glenn, J.V.; Powell, L.A.; Stitt, A.W.; Ames, J.M. Upregulation of oxidative stress markers in human microvascular endothelial cells by complexes of serum albumin and digestion products of glycated casein. J. Biochem. Mol. Toxicol. 2009, 23, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.; Keogh, J.B.; Price, N.J.; Clifton, P.M. Effects of Weight Loss on Advanced Glycation End Products in Subjects with and without Diabetes: A Preliminary Report. Int. J. Environ. Res. Public Health 2017, 14, 1553. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Zamora, R. Interplay between the maillard reaction and lipid peroxidation in biochemical systems. Ann. New York Acad. Sci. 2005, 1043, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. Ser. ABiol. Sci. Med Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ang, A.; Talegawkar, S.; Crasto, C.; Dalal, M.; Jardack, P.; Traber, M.G.; Ferrucci, L.; Arab, L. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: The Energetics Study. Eur. J. Clin. Nutr. 2012, 66, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Bak, M.J.; Andersen, J.M.; Monosik, R.; Giraudi-Futin, A.C.; Holst, J.J.; Nielsen, J.; Lauritzen, L.; Larsen, L.H.; Bugel, S.; et al. Effect of dietary advanced glycation end products on postprandial appetite, inflammation, and endothelial activation in healthy overweight individuals. Eur. J. Nutr. 2014, 53, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.; Hanssen, N.M.J.; van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Association between habitual dietary and lifestyle behaviours and skin autofluorescence (SAF), a marker of tissue accumulation of advanced glycation endproducts (AGEs), in healthy adults. Eur. J. Nutr. 2018, 57, 2209–2216. [Google Scholar] [CrossRef]
- Teerlink, T.; Barto, R.; Ten Brink, H.J.; Schalkwijk, C.G. Measurement of Nepsilon-(carboxymethyl)lysine and Nepsilon-(carboxyethyl)lysine in human plasma protein by stable-isotope-dilution tandem mass spectrometry. Clin. Chem. 2004, 50, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
| All Subjects 1 | Subjects Who Completed LDIGIT (n = 47) | |||
|---|---|---|---|---|
| Insulin-Sensitive Group (n = 23) | Insulin-Resistant Group (n = 24) | p | ||
| Sex (M/F) | 15/34 | 9/14 | 6/18 | 0.3 † |
| Age (y) | 35.5 ± 15.6 | 36.0 ± 15.7 | 36.1 ± 16 | 0.98 |
| NGT (n) | 16 | 15 | 7 | 0.3 |
| IFG/IGT (n) | 33 | 8 | 17 | |
| Baseline fasting glucose (mmol/L) | 5.5 ± 0.7 | 5.3 ± 0.8 | 5.6 ± 0.6 | 0.09 |
| Baseline 2 h glucose (mmol/L) | 7.29 ± 1.6 | 7.05 ± 1.5 | 7.27 ± 1.6 | 0.2 |
| HOMA-IR (HWD) | 0.37, 0.43 | 0.3, 0.46 | 0.38, 0.47 | 0.13 ‡ |
| HOMA-IR (HMD) | 0.52, 0.67 | 0.22, 0.56 | 0.67, 0.86 | 0.01 ‡ |
| LDIGIT (120-150min) insulin (HWD pmol/L) 2 | 34.2, 28.9 | 120.7, 154 | < 0.001 # | |
| LDIGIT (120-150min) insulin (HMD pmol/L) 2 | 28.7, 20.1 | 152.8, 200 | < 0.001# | |
| Baseline weight (kg) | 79.7 ± 21.36 | 70.5 ± 14.9 | 85.4 ± 22.1 | 0.01 |
| BMI (kg/m2) | 27.7 ± 6.9 | 24.6 ± 4.6 | 29.2 ± 5.9 | 0.005 |
| Baseline SBP (mmHg) | 112.5 ± 10.8 3 | 110.7 ± 9.3 4 | 114.7 ± 12.6 5 | 0.3 |
| Baseline DBP (mmHg) | 70.6 ± 9.8 3 | 69.0 ± 9.9 4 | 72.6 ± 9.9 5 | 0.2 |
| Total Fat Mass (kg) | 28.9 ± 15.7 | 20.7 ± 11.5 | 33.8± 13.3 | 0.001 |
| Total Lean Mass (kg) | 46.9 ± 11.5 | 46.0 ± 11.4 | 47.8 ± 12.3 | 0.6 |
| Total Fat Mass (%) | 36.5 ± 12.7 | 30.2 ± 13.1 | 40.7 ± 8.6 | 0.002 |
| Metabolic Parameters | HMD | HWD | p |
|---|---|---|---|
| Fasting glucose (mmol/L) | 5.27, 0.76 | 5.3, 0.55 | 0.9 |
| Fasting insulin (µMmol/mL) | 2.15, 1.97 | 2.17, 1.15 | 0.07 |
| HOMA-IR | 0.52, 0.67 | 0.37, 0.43 | 0.28 |
| TG (mmol/L) | 0.92, 0.74 | 0.77, 0.65 | 0.033 |
| HDL-C (mmol/L) | 1.39 ± 0.43 | 1.37 ± 0.42 | 0.4 |
| Total cholesterol (mmol/L) | 4.7 ± 0.96 | 4.6 ± 1.0 | 0.038 |
| hs-CRP (mg/L) | 0.79, 2.93 | 0.58, 2.24 | 0.79 |
| IL-6 (pg/ml) | 7.9, 11.2 | 7.7, 11 | 0.5 |
| PAI-1 (ng/ml) | 159, 83 | 121, 51 | <0.001 |
| CML (nmol/mL) 1 | 4.05 ± 1.44 | 3.85 ± 1.41 | 0.3 |
| CEL (nmol/mL) 2 | 1.367, 0.78 | 1.096, 0.65 | 0.01 |
| MG-H1 (nmol/mL) | 6.47 ± 3.46 | 6.83 ± 2.83 | 0.25 |
| Insulin-Sensitive Group | Insulin-Resistant Group | |||||||
|---|---|---|---|---|---|---|---|---|
| HMD | HWD | N | p | HMD | HWD | N | p | |
| CML (nmol/mL) | 4.34 ± 1.55 | 4.25 ± 1.4 | 21 | 0.79 | 3.91 ± 1.34 | 3.59 ± 1.37 | 23 | 0.2 |
| CEL (nmol/mL) | 1.32, 0.78 | 1.29, 1.059 | 22 | 0.7 | 1.50, 0.85 | 1.13, 0.57 | 24 | 0.01 |
| MG-H1 (nmol/mL) | 6.09 ± 2.37 | 6.52 ±2.18 | 23 | 0.36 | 7.01 ± 4.32 | 7.05 ± 3.46 | 24 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Keogh, J.B.; Deo, P.; Clifton, P.M. Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study. Nutrients 2020, 12, 1767. https://doi.org/10.3390/nu12061767
Kim Y, Keogh JB, Deo P, Clifton PM. Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study. Nutrients. 2020; 12(6):1767. https://doi.org/10.3390/nu12061767
Chicago/Turabian StyleKim, Yoona, Jennifer B. Keogh, Permal Deo, and Peter M. Clifton. 2020. "Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study" Nutrients 12, no. 6: 1767. https://doi.org/10.3390/nu12061767
APA StyleKim, Y., Keogh, J. B., Deo, P., & Clifton, P. M. (2020). Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study. Nutrients, 12(6), 1767. https://doi.org/10.3390/nu12061767







