Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Lines
2.3. Cell Proliferation MTT Assay
2.4. Cell Cycle Analysis
2.5. AO/PI Assay (Acridine Orange/Propidium Iodide Double Staining Assay)
2.6. Annexin V/PI Assay
2.7. Caspase 3/7, 8 and 9 Measurements
2.8. Measurement of Multi-Parameter Cytotoxicity Using Confocal Microscope
2.9. Protein Expression Analysis (Proteomics)
2.10. Detection of NF-κB Activity
2.11. Measurement of Reactive Oxygen Species (ROS) Generation (DNA Damaging)
2.12. Statistical Analysis
3. Results
3.1. Cardamonin Inhibits Cell Proliferation of HepG2 Cells
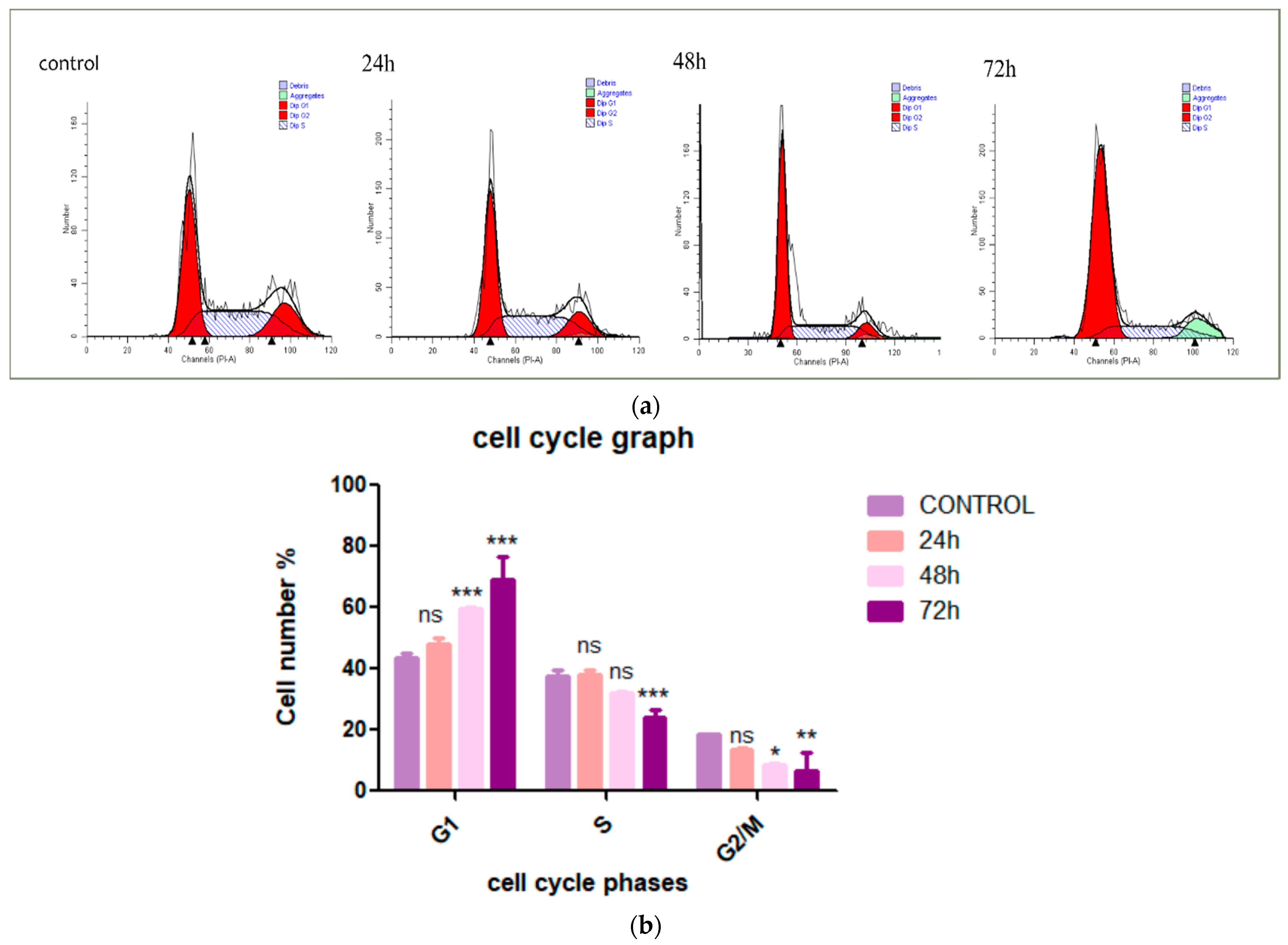
3.2. Cardamonin Induces HepG2 Cells Arrest at G1 Phase
3.3. Cardamonin Alters HepG2 Cell Morphology
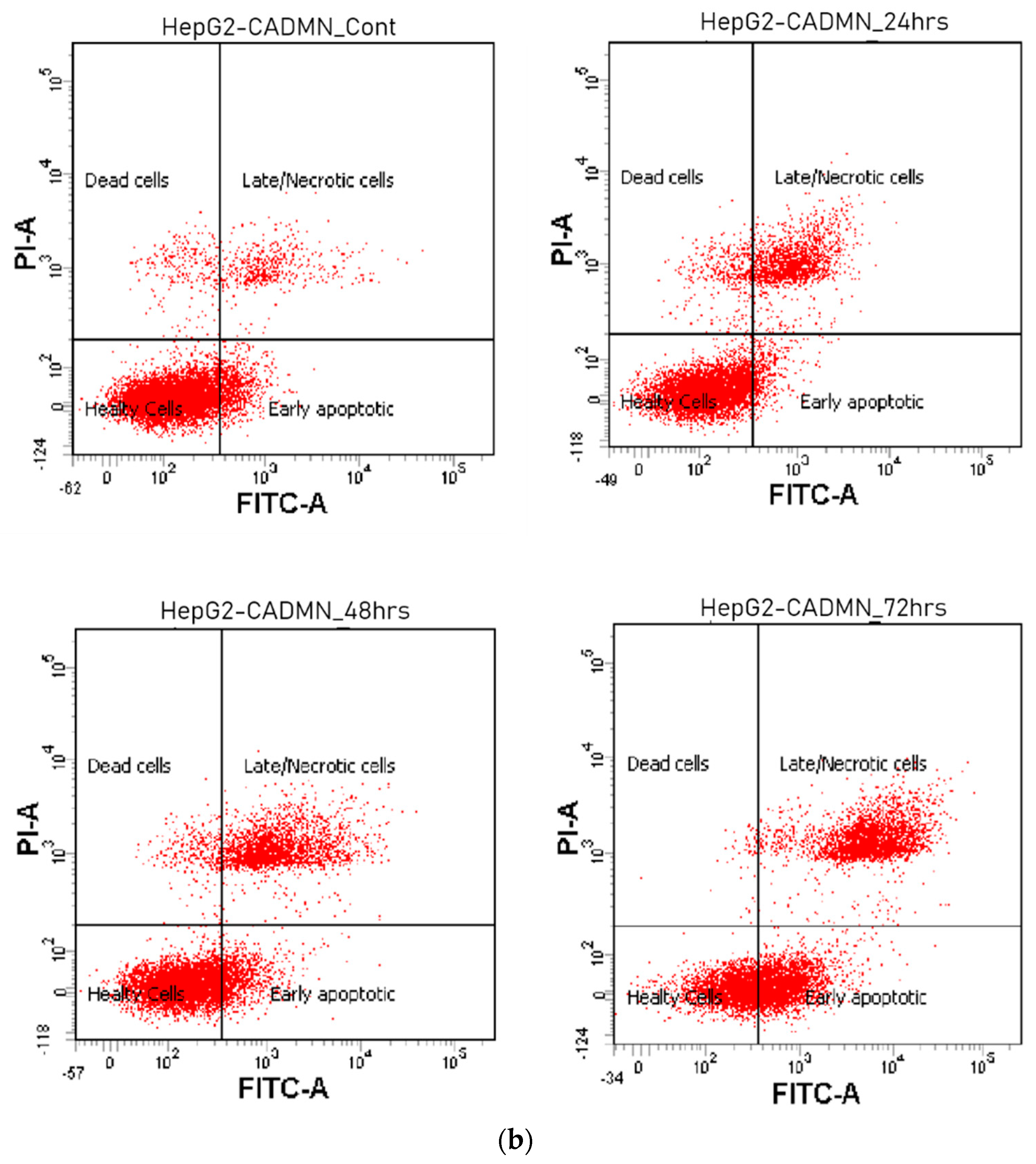
3.4. Cardamonin Induces Apoptosis in HepG2 Cells
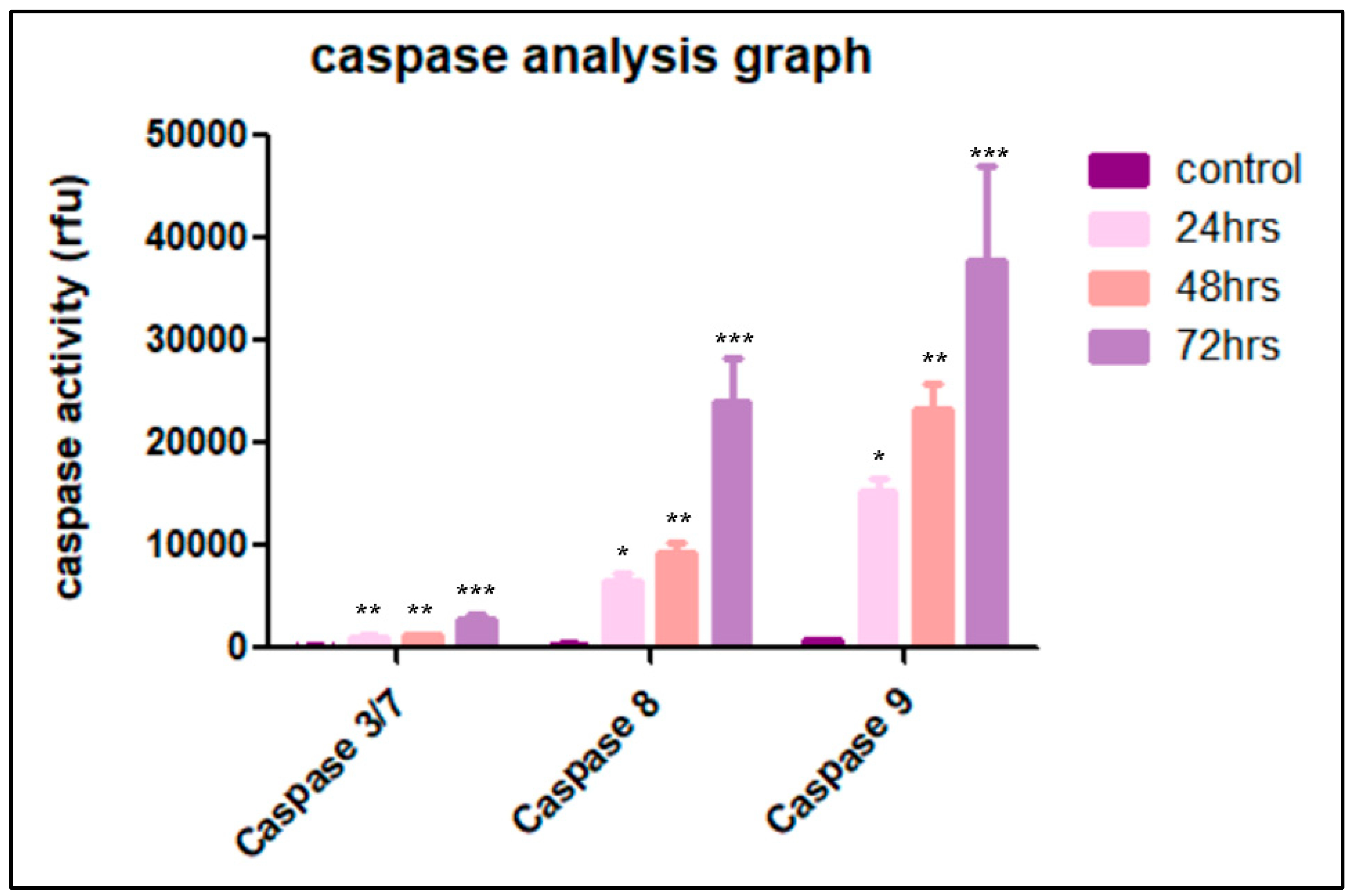
3.5. Cardamonin Induces Caspase-3/7, -8 and -9 Activities
3.6. Multiparameter Cytotoxicity Analysis
3.6.1. Cardamonin Induces Alterations in MOMP and Cell Membrane Permeability
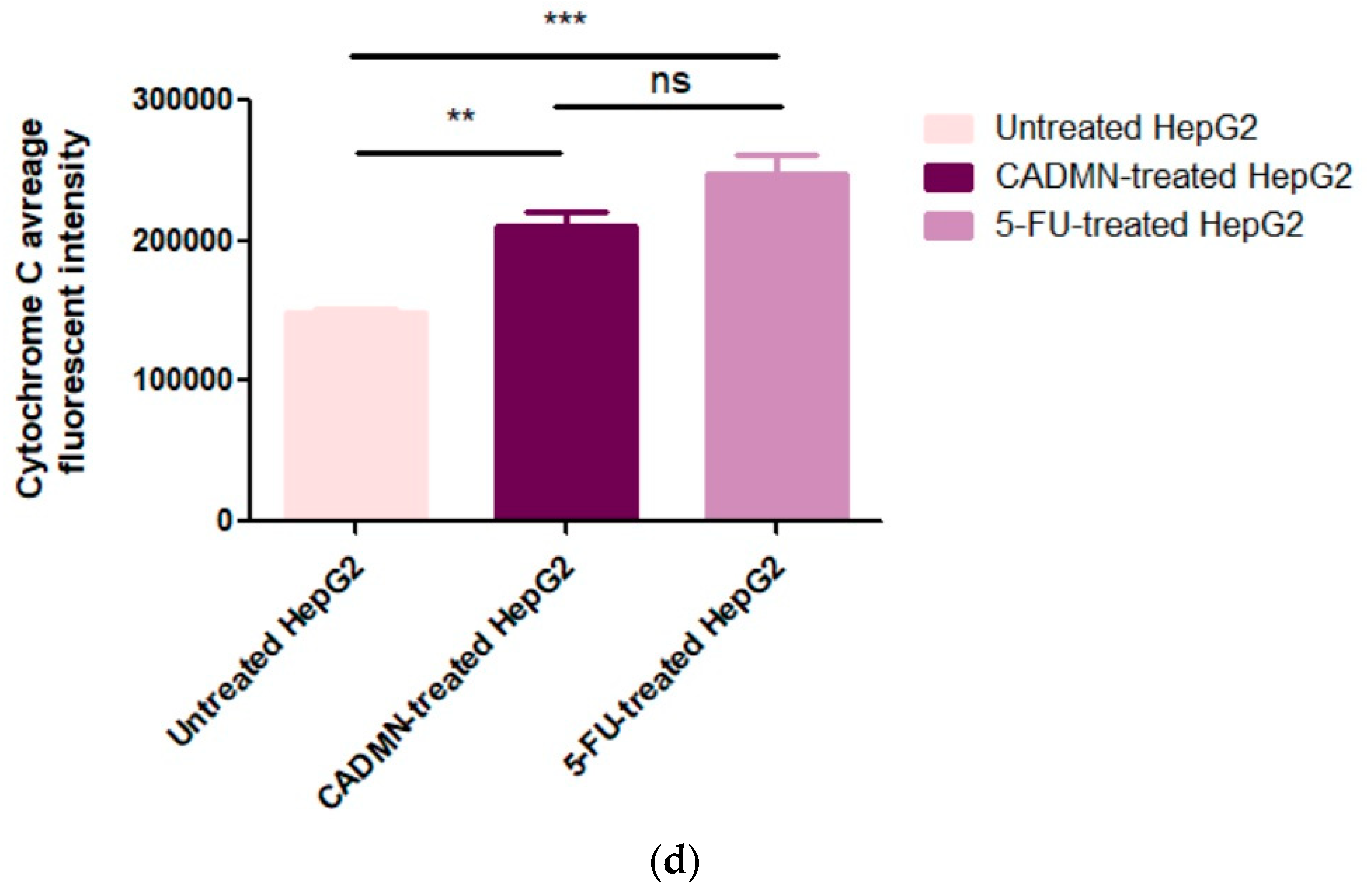
3.6.2. Cardamonin Induces Release of Cytochrome C to Cytosol and Nuclear Condensation
3.7. Protein Expression Analysis (Proteomics)
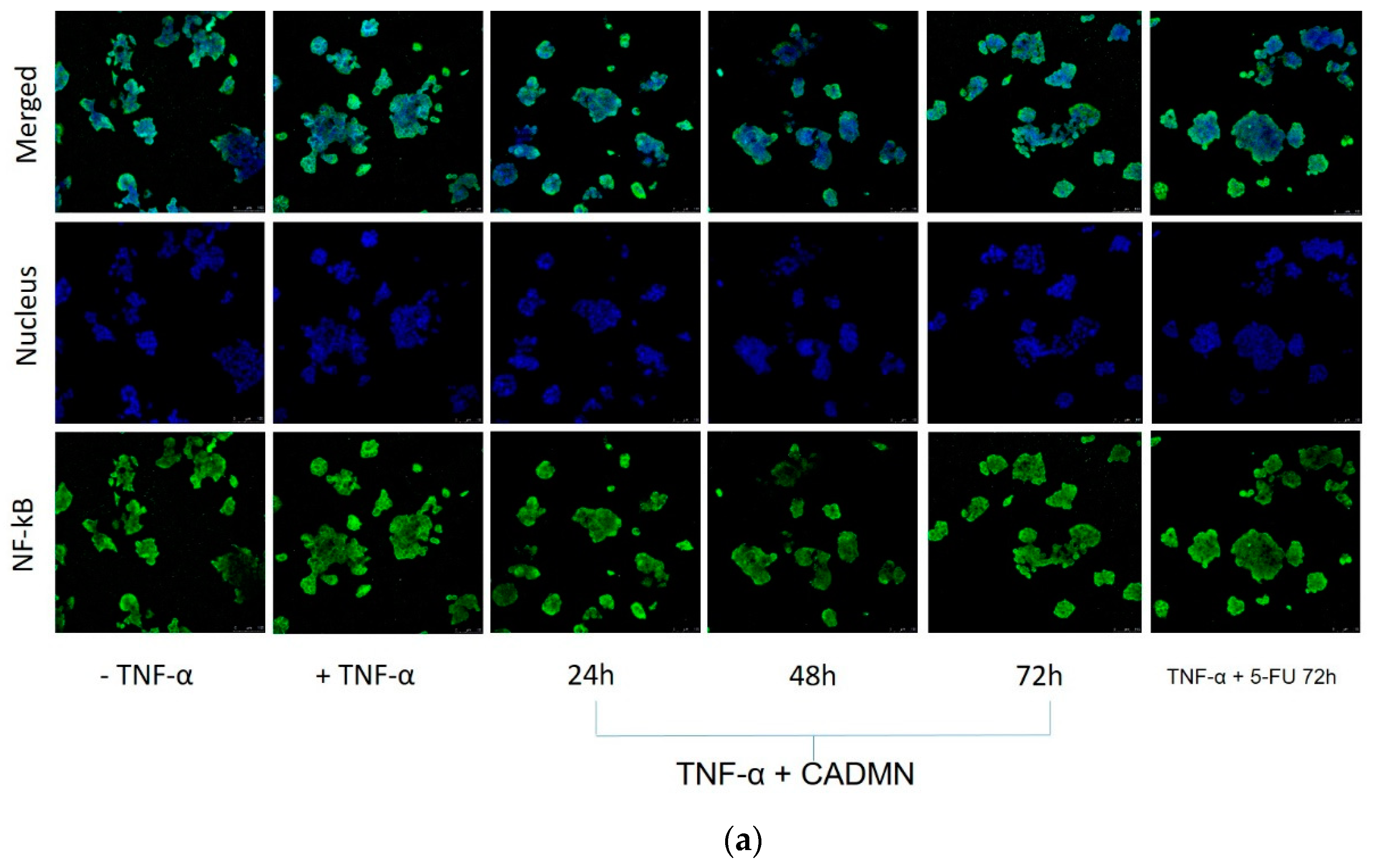
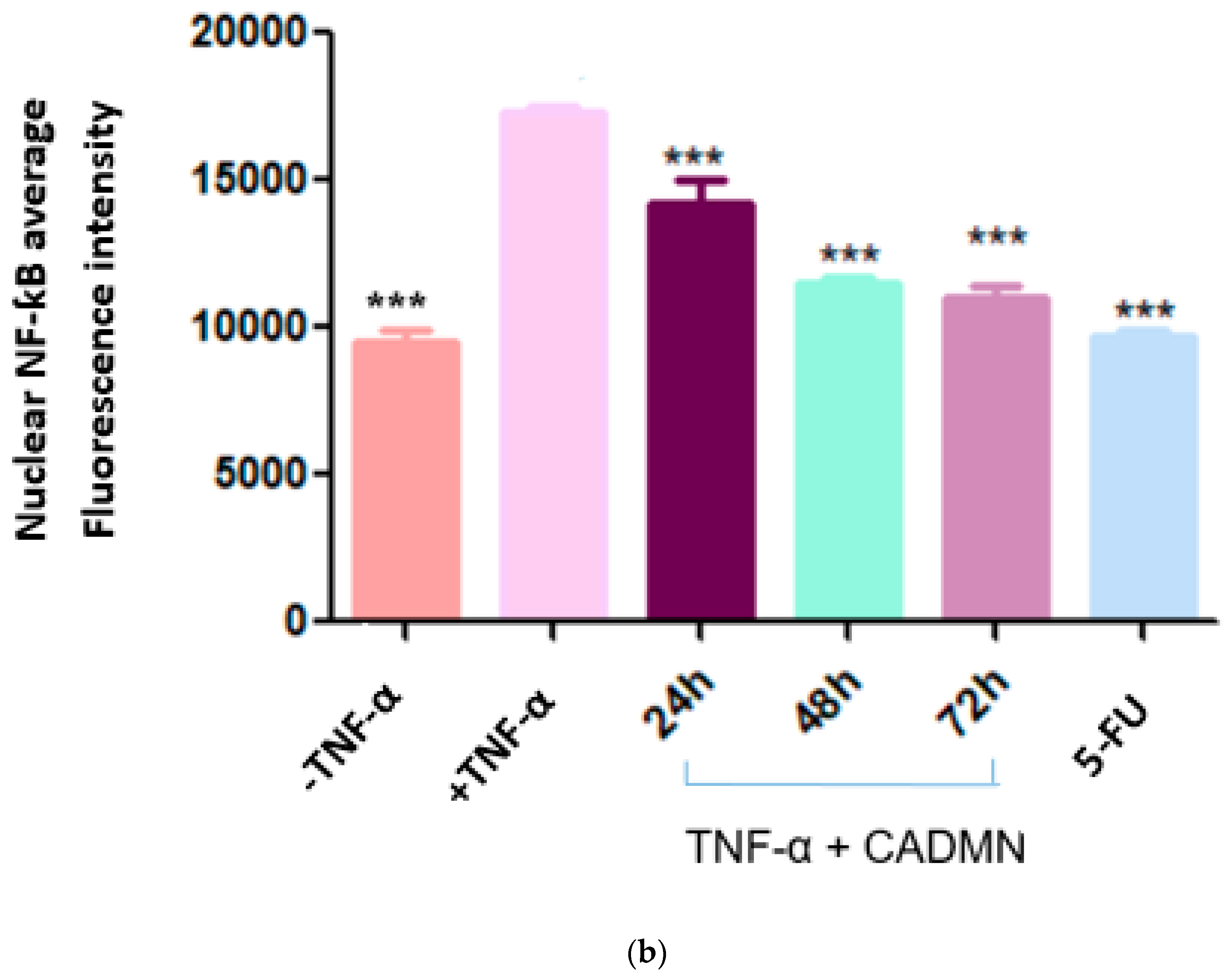
3.8. NF-κB Translocation Suppressed by Cardamonin
3.9. Cardamonin Induces High Level of ROS
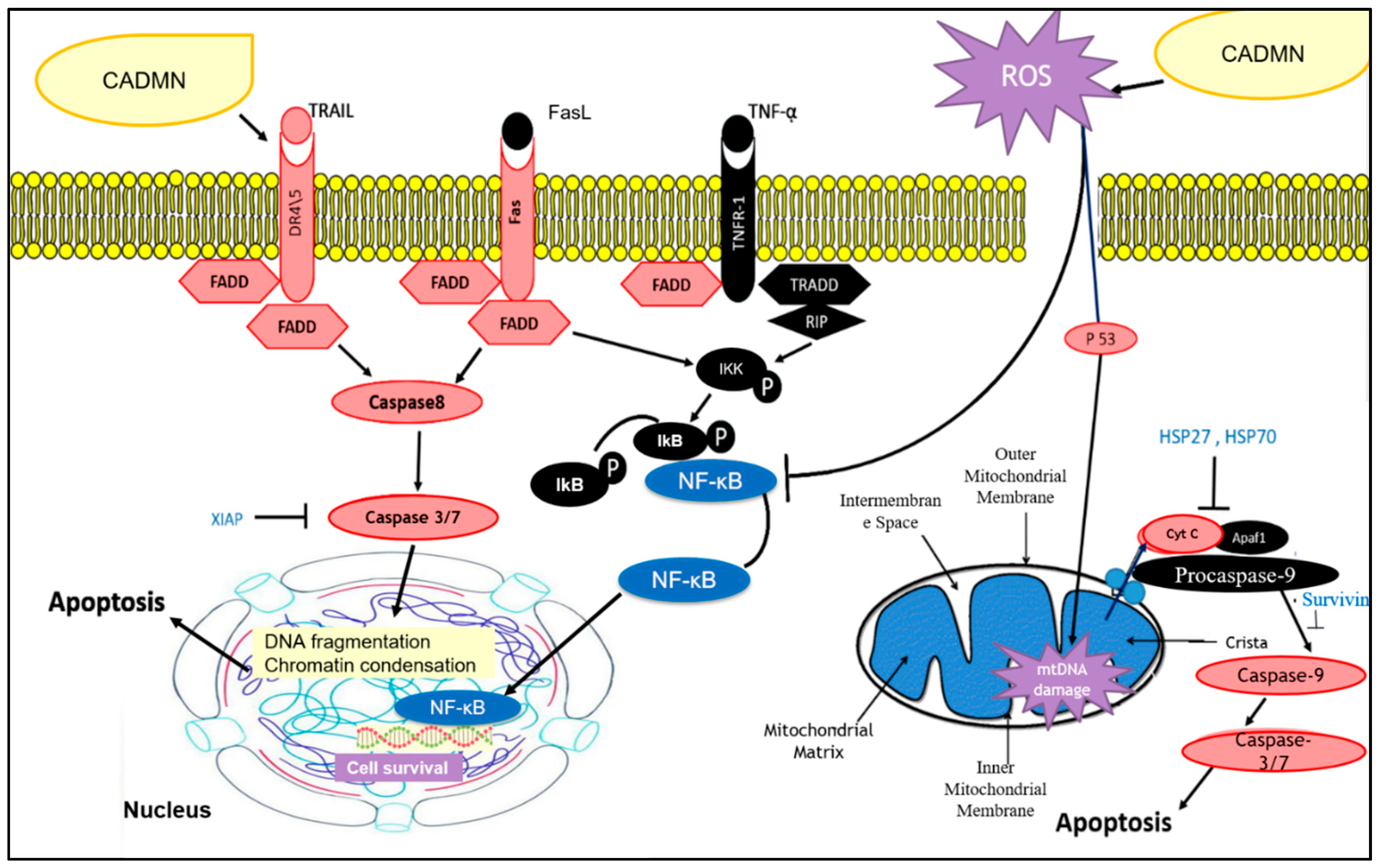
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galle, P.R.; Alejandro, F.; Josep, M.L.; Mazzaferro, V.; Piscaglia, F.; Schirmacher, J.-L.R.P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, D.; Lei, Z.; Wu, J. Inhibition of Tumor Growth of Human Hepatocellular Carcinoma Hepg2 Cells in a Nude Mouse Xenograft Model by the Total Flavonoids from Arachniodes Exilis. Evid. Based Complement. Altern. Med. 2017, 53, 10563. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Walter A, R.J.; Gomes, D.B.; Zanchet, B.; Schönell, A.P.; Diel, K.A.P.; Banzato, T.P.; Ruiz, A.L.T.G.; Carvalho, J.E.; Neppel, A.; Barison, A. Antiproliferative Effects of Pinostrobin and 5, 6-Dehydrokavain Isolated from Leaves of Alpinia Zerumbet. Rev. Bras. De Farmacogn. 2017, 27, 592–598. [Google Scholar]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An Overview on Cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Break, M.K.B. Semi-Synthesis of Novel Cardamonin Analogues and Identification of a Highly Active Cu (Ii)-Cardamonin Complex That Inhibits Migration and Induces Apoptosis Via Inhibition of Mtor Expression. Ph.D. Thesis, UNMC Malaysia Campus, University of Nottingham, Selangor, Malaysia, 2018. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Relja, B.; Meder, F.; Wilhelm, K.; Henrich, D.; Marzi, I.; Lehnert, M. Simvastatin Inhibits Cell Growth and Induces Apoptosis and G0/G1 Cell Cycle Arrest in Hepatic Cancer Cells. Int. J. Mol. Med. 2010, 26, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Paydar, M.; Rouhollahi, E.; Karimian, H. Annona Muricata Leaves Induced Apoptosis in A549 Cells through Mitochondrial-Mediated Pathway and Involvement of Nf-κb. BMC Complement. Altern. Med. 2014, 14, 299. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H.; Bae, S.-H.; Leem, S.-H. Induction of Apoptosis in Human Breast Cancer Cells by a Pulsed Atmospheric Pressure Plasma Jet. Appl. Phys. Lett. 2010, 97, 023702. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Koosha, S.; Mohamed, Z.; Sinniah, A.; Alshawsh, M.A. Investigation into the Molecular Mechanisms Underlying the Anti-Proliferative and Anti-Tumorigenesis Activities of Diosmetin against Hct-116 Human Colorectal Cancer. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arbab, I.A.; Looi, C.Y.; Abdul, A.B.; Cheah, F.K.; Wong, W.F.; Sukari, M.A.; Abdullah, R.; Mohan, S.; Syam, S.; Arya, A. Dentatin Induces Apoptosis in Prostate Cancer Cells Via Bcl-2, Bcl-Xl, Survivin Downregulation, Caspase-9,-3/7 Activation, and Nf-κb Inhibition. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pavelescu, L.A. On Reactive Oxygen Species Measurement in Living Systems. J. Med. Life 2015, 8, 38. [Google Scholar] [PubMed]
- Topcul, M.; Cetin, I. Endpoint of Cancer Treatment: Targeted Therapies. Asian Pac. J. Cancer Prev. 2014, 15, 4395–4403. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef]
- Duan, Y.-M.; Jin, Y.; Guo, M.-L.; Duan, L.-X.; Wang, J.-G. Differentially Expressed Genes of Hepg2 Cells Treated with Gecko Polypeptide Mixture. J. Cancer 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lau, L.-F.; Dai, X.; Li, B. In Vitro and in Vivo Anticancer Activity of Gimatecan against Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2016, 17, 4853. [Google Scholar]
- Parvesh, S.; Anand, A.; Kumar, V. Recent Developments in Biological Activities of Chalcones: A Mini Review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar]
- Jaiswal, S.; Shukla, M.; Sharma, A.; Rangaraj, N.; Vaghasiya, K.; Malik, M.Y.; Lal, J. Preclinical Pharmacokinetics and Adme Characterization of a Novel Anticancer Chalcone, Cardamonin. Drug Test. Anal. 2017, 9, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Sen, L.; Lin, C.; Cheng, X.; Hua, H.; Xiang, T.; Huang, Y.; Huang, X. Cardamonin Reduces Chemotherapy Resistance of Colon Cancer Cells via the Tsp50/Nf-κb Pathway in Vitro. Oncol. Lett. 2018, 15, 9641–9646. [Google Scholar]
- Li, Y.; Qin, Y.; Yang, C.; Zhang, H.; Li, Y.; Wu, B.; Huang, J.; Zhou, X.; Huang, B.; Yang, K. Cardamonin Induces Ros-Mediated G2/M Phase Arrest and Apoptosis through Inhibition of Nf-κb Pathway in Nasopharyngeal Carcinoma. Cell Death Dis. 2017, 8, e3024. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.-G.; Song, Z.-B.; Sun, L.-G.; Bao, Y.-L.; Yu, C.-L.; Wu, Y.; Li, Y.-X. Cardamonin Inhibited Cell Viability and Tumorigenesis Partially through Blockade of Testes-Specific Protease 50-Mediated Nuclear Factor-Kappab Signaling Pathway Activation. Int. J. Biochem. Cell Biol. 2016, 73, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.-Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Rangappa, K.S.; Sethi, G. Cardamonin Represses Proliferation, Invasion, and Causes Apoptosis through the Modulation of Signal Transducer and Activator of Transcription 3 Pathway in Prostate Cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Meng, L.; Ji, B.; Yang, D. Synergistic Antitumor Effect of Sorafenib in Combination with Atm Inhibitor in Hepatocellular Carcinoma Cells. Int. J. Med. Sci. 2017, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Vladimir, B.; Lewis, C.S.; Smith, C.D. Antitumor Activity of Sphingosine Kinase 2 Inhibitor Abc294640 and Sorafenib in Hepatocellular Carcinoma Xenografts. Cancer Biol. Ther. 2011, 11, 524–534. [Google Scholar]
- Pascoal, A.C.R.F.; Ehrenfried, C.A.; Lopez, B.G.-C.; De Araujo, T.M.; Pascoal, V.; Gilioli, R.; Anhê, G.F.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Stefanello, M.É.A. Antiproliferative Activity and Induction of Apoptosis in Pc-3 Cells by the Chalcone Cardamonin from Campomanesia Adamantium (Myrtaceae) in a Bioactivity-Guided Study. Molecules 2014, 19, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Sadia, A.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.P.; Daglia, M.; Atanasov, A.G. Dietary Phytochemicals in Colorectal Cancer Prevention and Treatment: A Focus on the Molecular Mechanisms Involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar]
- Park, S.; Gwak, J.; Han, S.J.; Oh, S. Cardamonin Suppresses the Proliferation of Colon Cancer Cells by Promoting Β-Catenin Degradation. Biol. Pharm. Bull. 2013, 36, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Ansar, K.; Ahmadi, Y.; Yousefi, B. Multiple Functions of P21 in Cell Cycle, Apoptosis and Transcriptional Regulation after DNA Damage. DNA Repair 2016, 2, 63–71. [Google Scholar]
- Maureen, R.-D.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar]
- Carmen, G.; Schmitt, E.; Candé, C.; Vahsen, N.; Parcellier, A.; Kroemer, G. Hsp27 and Hsp70: Potentially Oncogenic Apoptosis Inhibitors. Cell Cycle 2003, 2, 578–583. [Google Scholar]
- Mads, G.-H.; Meier, P. Iaps: From Caspase Inhibitors to Modulators of Nf-κb, Inflammation and Cancer. Nat. Rev. Cancer 2010, 10, 561. [Google Scholar]
- Chu, C.; Lou, T. Hypoxia Inducible Factors in Hepatocellular Carcinoma. Oncotarget 2017, 8, 46691. [Google Scholar]
- Liu, Y.; Shao, E.; Zhang, Z.; Yang, D.; Li, G.; Cao, H.; Huang, H. A Novel Indolizine Derivative Induces Apoptosis through Mitochondria P53 Pathway in Hepg2 Cells. Front. Pharmacol. 2019, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- AE, G.; Van der Wall, E. The Role of Hypoxia Inducible Factor 1 (Hif-1) in Hypoxia Induced Apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014. [Google Scholar]
- Kong, W.; Li, C.; Qi, Q.; Shen, J.; Chang, K. Cardamonin Induces G2/M Arrest and Apoptosis Via Activation of the Jnk–Foxo3a Pathway in Breast Cancer Cells. Cell Biol. Int. 2019, 44, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bardhan, K.; Yang, D.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Liles, G.B.; Lee, J.R.; Liu, K. Nf-κb Directly Regulates Fas Transcription to Modulate Fas-Mediated Apoptosis and Tumor Suppression. J. Biol. Chem. 2012, 287, 25530–25540. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.; Aparna, J.S.; Paul, A.M.; Lankadasari, M.B.; Mohammed, S.; Binu, V.S.; Santhoshkumar, T.R.; Reshmi, G.; Harikumar, K.B. Cardamonin Inhibits Colonic Neoplasia through Modulation of Microrna Expression. Sci. Rep. 2017, 7, 1–16. [Google Scholar]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and Nf-κb Signaling. Cell Res. 2011, 21, 103. [Google Scholar] [CrossRef] [PubMed]
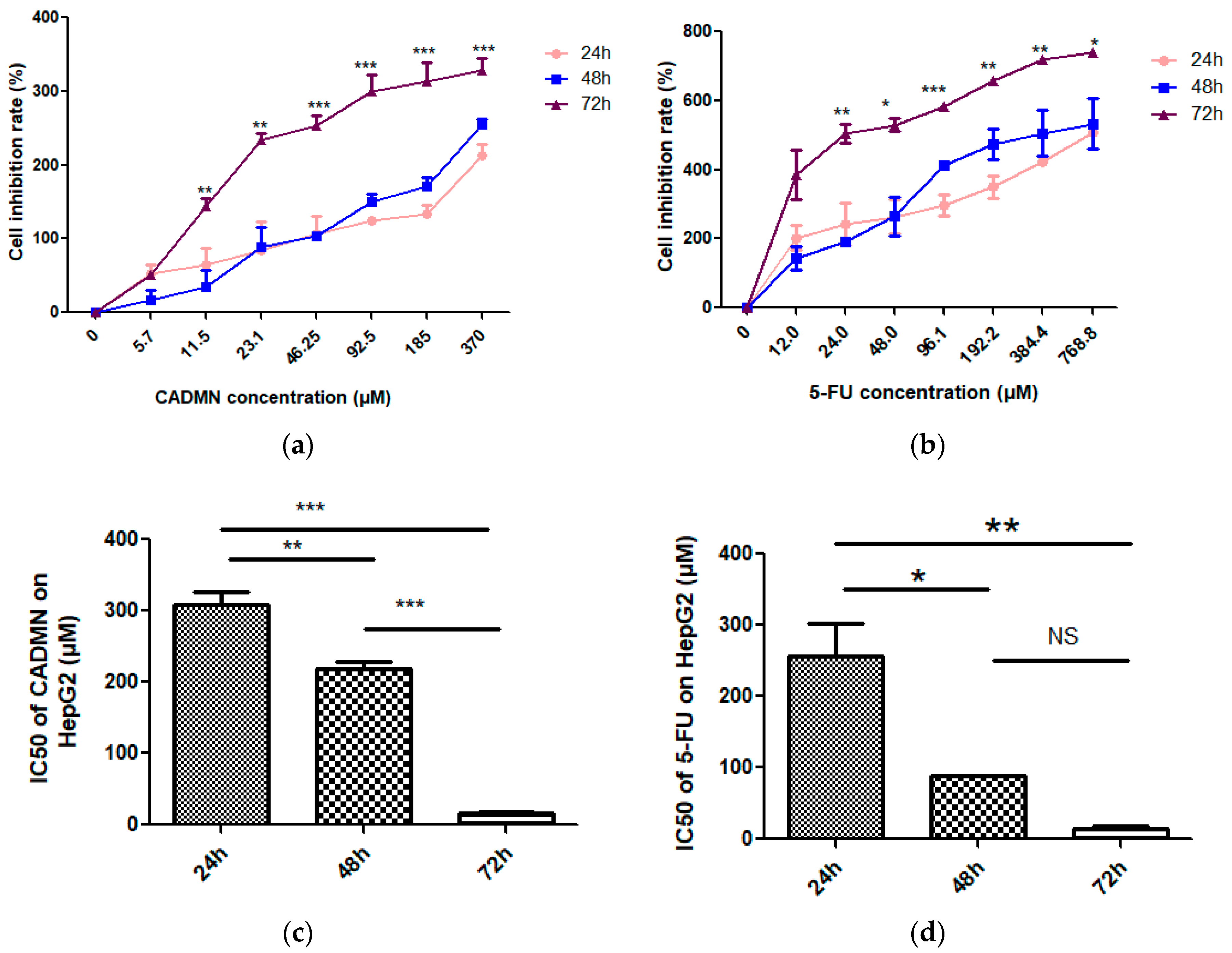





| IC50 (μM) | 72 h | S.I. |
|---|---|---|
| CADMN | 225.7 ± 15.53 | 13.2 |
| 5-FU | 11.53 ± 3.075 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badroon, N.A.; Abdul Majid, N.; Alshawsh, M.A. Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells. Nutrients 2020, 12, 1757. https://doi.org/10.3390/nu12061757
Badroon NA, Abdul Majid N, Alshawsh MA. Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells. Nutrients. 2020; 12(6):1757. https://doi.org/10.3390/nu12061757
Chicago/Turabian StyleBadroon, Nassrin A., Nazia Abdul Majid, and Mohammed A. Alshawsh. 2020. "Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells" Nutrients 12, no. 6: 1757. https://doi.org/10.3390/nu12061757
APA StyleBadroon, N. A., Abdul Majid, N., & Alshawsh, M. A. (2020). Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells. Nutrients, 12(6), 1757. https://doi.org/10.3390/nu12061757






