How Important Is Eating Rate in the Physiological Response to Food Intake, Control of Body Weight, and Glycemia?
Abstract
1. Introduction
2. Studies Concerning Healthy Individuals
i. Manipulating Eating Rate
ii. Manipulating Food Texture
iii. Manipulating Masticatory Cycles
3. Studies Concerning Patients with Overweight/Obesity
i. Manipulating Eating Rate
ii. Manipulating Masticatory Cycles
4. Studies Concerning Subjects with Diabetes Mellitus
5. Devices Manipulating Eating Rate
i. Noninvasive Oral Devices
ii. Wearable Devices
6. Effect of Eating Rate on Glycemic Response
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Sato, S.; Ohira, T.; Maeda, K.; Noda, H.; Kubota, Y.; Nishimura, S.; Kitamura, A.; Kiyama, M.; Okada, T.; et al. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: Cross sectional survey. BMJ 2008, 337, a2002. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Murata, C.; Sekiya, A.; Wada, K.; Zhang, H.M.; Matsushita, K.; Sugiura, K.; Takefuji, S.; et al. Eating fast leads to obesity: Findings based on self-administered questionnaires among middle-aged japanese men and women. J. Epidemiol. 2006, 16, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Katagiri, A.; Tsuji, T.; Shimoda, T.; Amano, K. Self-Reported rate of eating correlates with body mass index in 18-y-old Japanese women. Int. J. Obes. 2003, 27, 1405–1410. [Google Scholar] [CrossRef]
- Takayama, S.; Akamine, Y.; Okabe, T.; Koya, Y.; Haraguchi, M.; Miyata, Y.; Sakai, T.; Sakura, H.; Sasaki, T. Rate of eating and body weight in patients with type 2 diabetes or hyperlipidaemia. J. Int. Med. Res. 2002, 30, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Hirakawa, Y.; Nakamura, U.; Kiyohara, Y.; Kitazono, T.; Ninomiya, T. Association between eating rate and obesity: A systematic review and meta-analysis. Int. J. Obes. 2015, 39, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, S.; Imatoh, T.; Miyazaki, M.; Babazono, A.; Momose, Y.; Baba, M.; Uryu, Y.; Une, H. Retrospective longitudinal study on the relationship between 8-year weight change and current eating speed. Appetite 2011, 57, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yamane, M.; Ekuni, D.; Mizutani, S.; Kataoka, K.; Sakumoto-Kataoka, M.; Kawabata, Y.; Omori, C.; Azuma, T.; Tomofuji, T.; Iwasaki, Y.; et al. Relationships between eating quickly and weight gain in japanese university students: A longitudinal study: Eating quickly and overweight. Obesity 2014, 22, 2262–2266. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Young, A.J.; Montain, S.J. Eating rate during a fixed-portion meal does not affect postprandial appetite and gut peptides or energy intake during a subsequent meal. Physiol. Behav. 2011, 102, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, N.; Mars, M.; Stafleu, A.; de Graaf, C. The effect of texture differences on satiation in 3 pairs of solid foods. Appetite 2010, 55, 490–497. [Google Scholar] [CrossRef]
- Llewellyn, C.H.; van Jaarsveld, C.H.; Boniface, D.; Carnell, S.; Wardle, J. Eating rate is a heritable phenotype related to weight in children. Am. J. Clin. Nutr. 2008, 88, 1560–1566. [Google Scholar] [CrossRef]
- Robinson, E.; Almiron-Roig, E.; Rutters, F.; de Graaf, C.; Forde, C.G.; Tudur Smith, C.; Nolan, S.J.; Jebb, S.A. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am. J. Clin. Nutr. 2014, 100, 123–151. [Google Scholar] [CrossRef]
- Andrade, A.M.; Greene, G.W.; Melanson, K.J. Eating slowly led to decreases in energy intake within meals in healthy women. J. Am. Diet. Assoc. 2008, 108, 1186–1191. [Google Scholar] [CrossRef]
- Kokkinos, A.; le Roux, C.W.; Alexiadou, K.; Tentolouris, N.; Vincent, R.P.; Kyriaki, D.; Perrea, D.; Ghatei, M.A.; Bloom, S.R.; Katsilambros, N. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and Glucagon-Like Peptide-1. J. Clin. Endocrinol. Metab. 2010, 95, 333–337. [Google Scholar] [CrossRef]
- Zandian, M.; Ioakimidis, I.; Bergh, C.; Brodin, U.; Södersten, P. Decelerated and linear eaters: Effect of eating rate on food intake and satiety. Physiol. Behav. 2009, 96, 270–275. [Google Scholar] [CrossRef]
- Wilkinson, L.L.; Ferriday, D.; Bosworth, M.L.; Godinot, N.; Martin, N.; Rogers, P.J.; Brunstrom, J.M. Keeping pace with your eating: Visual feedback affects eating rate in humans. PLoS ONE 2016, 11, e0147603. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Ponnalagu, S.; Bi, X.; Forde, C. Does basal metabolic rate drive eating rate? Physiol. Behav. 2018, 189, 74–77. [Google Scholar] [CrossRef]
- McCrickerd, K.; Forde, C.G. Sensory influences on food intake control: Moving beyond palatability: Sensory influences on food intake. Obes. Rev. 2016, 17, 18–29. [Google Scholar] [CrossRef]
- Hawton, K.; Ferriday, D.; Rogers, P.; Toner, P.; Brooks, J.; Holly, J.; Biernacka, K.; Hamilton-Shield, J.; Hinton, E. Slow down: Behavioural and physiological effects of reducing eating rate. Nutrients 2018, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; van Kuijk, N.; Thaler, T.; de Graaf, C.; Martin, N. Texture and savoury taste influences on food intake in a realistic hot lunch time meal. Appetite 2013, 60, 180–186. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Forde, C.G.; Cheng, Y.; Xu, H.; Martin, N.; de Graaf, C. Slow food: Sustained impact of harder foods on the reduction in energy intake over the course of the day. PLoS ONE 2014, 9, e93370. [Google Scholar] [CrossRef]
- Karl, J.P.; Young, A.J.; Rood, J.C.; Montain, S.J. Independent and combined effects of eating rate and energy density on energy intake, appetite, and gut hormones: Eating rate, energy density, and appetite. Obesity 2013, 21, E244–E252. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, N.; Mars, M.; de Wijk, R.A.; Westerterp-Plantenga, M.S.; de Graaf, C. The effect of viscosity on ad libitum food intake. Int. J. Obes. 2008, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, K.R.; Purhonen, A.-K.; Salmenkallio-Marttila, M.; Lähteenmäki, L.; Laaksonen, D.E.; Herzig, K.-H.; Uusitupa, M.I.J.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef]
- Viskaal-van Dongen, M.; Kok, F.J.; de Graaf, C. Eating rate of commonly consumed foods promotes food and energy intake. Appetite 2011, 56, 25–31. [Google Scholar] [CrossRef]
- Blundell, J.E.; MacDiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety and patterns of eating. J. Am. Diet. Assoc. 1997, 97, 63–69. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br. J. Nutr. 2013, 110, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R. Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite 1990, 15, 103–113. [Google Scholar] [CrossRef]
- The LifeLines Cohort Study; The ADIPOGen Consortium; The AGEN-BMI Working Group; The CARDIOGRAMplusC4D Consortium; The CKDGen Consortium; The GLGC; The ICBP; The MAGIC Investigators; The MuTHER Consortium; The MIGen Consortium; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ferster, C.B.; Nurnberger, J.I.; Levtit, E.B. The control of eating. Obes. Res. 1996, 4, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Koidis, F.; Brunger, L.; Gibbs, M.; Hampton, S. The effect of eating rate on satiety in healthy and overweight people—A pilot study. e-SPEN J. 2014, 9, e54–e58. [Google Scholar] [CrossRef]
- Shah, M.; Copeland, J.; Dart, L.; Adams-Huet, B.; James, A.; Rhea, D. Slower eating speed lowers energy intake in normal-weight but not overweight/obese subjects. J. Acad. Nutr. Diet. 2014, 114, 393–402. [Google Scholar] [CrossRef]
- Martin, C.K.; Anton, S.D.; Walden, H.; Arnett, C.; Greenway, F.L.; Williamson, D.A. Slower eating rate reduces the food intake of men, but not women: Implications for behavioral weight control. Behav. Res. Ther. 2007, 45, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Scisco, J.L.; Muth, E.R.; Dong, Y.; Hoover, A.W. Slowing bite-rate reduces energy intake: An application of the bite counter device. J. Am. Diet. Assoc. 2011, 111, 1231–1235. [Google Scholar] [CrossRef]
- Smit, H.J.; Kemsley, E.K.; Tapp, H.S.; Henry, C.J.K. Does prolonged chewing reduce food intake? Fletcherism Revisited. Appetite 2011, 57, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, T.A.; Kaplan, J.M.; Tomassini, A.; Stellar, E. Bite size, ingestion rate, and meal size in lean and obese women. Appetite 1993, 21, 131–145. [Google Scholar] [CrossRef]
- Parkes, E. Nutritional management of patients after bariatric surgery. Am. J. Med. Sci. 2006, 331, 207–213. [Google Scholar] [CrossRef]
- Canterini, C.-C.; Gaubil-Kaladjian, I.; Vatin, S.; Viard, A.; Wolak-Thierry, A.; Bertin, E. Rapid eating is linked to emotional eating in obese women relieving from bariatric surgery. Obes. Surg. 2018, 28, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kawai, K.; Yanagisawa, M.; Yokoyama, H.; Kuribayashi, N.; Sugimoto, H.; Oishi, M.; Wada, T.; Iwasaki, K.; Kanatsuka, A.; et al. Self-Reported rate of eating is significantly associated with body mass index in Japanese patients with type 2 diabetes. Japan Diabetes Clinical Data Management Study Group (JDDM26). Appetite 2012, 59, 252–255. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, T.; Kokkinos, A.; Liaskos, C.; Tentolouris, N.; Alexiadou, K.; Miras, A.D.; Mourouzis, I.; Perrea, D.; Pantos, C.; Katsilambros, N.; et al. The effect of slow spaced eating on hunger and satiety in overweight and obese patients with type 2 diabetes mellitus. BMJ Open Diab. Res. Care 2014, 2, e000013. [Google Scholar] [CrossRef] [PubMed]
- Toft-Nielsen, M.-B.; Madsbad, S.; Holst, J.J. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 86, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ranawana, D.V.; Tan, W.J.K.; Quek, Y.C.R.; Henry, C.J. The impact of eating methods on eating rate and glycemic response in healthy adults. Physiol. Behav. 2015, 139, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ranawana, V.; Clegg, M.E.; Shafat, A.; Henry, C.J. Postmastication digestion factors influence glycemic variability in humans. Nutr. Res. 2011, 31, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ranawana, V.; Leow, M.K.-S.; Henry, C.J.K. Mastication effects on the glycaemic index: Impact on variability and practical implications. Eur. J. Clin. Nutr. 2014, 68, 137–139. [Google Scholar] [CrossRef] [PubMed]
- McGee, T.L.; Grima, M.T.; Hewson, I.D.; Jones, K.M.; Duke, E.B.; Dixon, J.B. First australian experiences with an oral volume restriction device to change eating behaviors and assist with weight loss. Obesity 2012, 20, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Von Seck, P.; Sander, F.M.; Lanzendorf, L.; von Seck, S.; Schmidt-Lucke, A.; Zielonka, M.; Schmidt-Lucke, C. Persistent weight loss with a non-invasive novel medical device to change eating behaviour in obese individuals with high-risk cardiovascular risk profile. PLoS ONE 2017, 12, e0174528. [Google Scholar] [CrossRef] [PubMed]
- James, L.J.; Maher, T.; Biddle, J.; Broom, D.R. Eating with a smaller spoon decreases bite size, eating rate and Ad Libitum food intake in healthy young males. Br. J. Nutr. 2018, 120, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, S.; Frost, J.H.; Robinson, E.; Higgs, S.; Mars, M.; Hermans, R.C.J. Evaluation of a smart fork to decelerate eating rate. J. Acad. Nutr. Diet. 2016, 116, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, S.; Mars, M.; Higgs, S.; Frost, J.H.; Hermans, R.C.J. Effects of eating with an augmented fork with vibrotactile feedback on eating rate and body weight: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Zuoyi, Z.; Junhyeok, K.; Yumiko, S.; Teng, H.; Pourang, I. Applying a pneumatic interface to intervene with rapid eating behaviour. Stud. Health Technol. Inf. 2019, 257, 513–519. [Google Scholar]
- Zhang, R.; Amft, O. Monitoring chewing and eating in free-living using smart eyeglasses. IEEE J. Biomed. Health Inform. 2018, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, M.; Papapanagiotou, V.; Diou, C.; Zandian, M.; Nolstam, J.; Södersten, P.; Bergh, C. Control of eating behavior using a novel feedback system. JoVE 2018, 57432. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. The glucose triad and its role in comprehensive glycaemic control: Current status, future management: Comprehensive glycaemic control in T2D management. Int. J. Clin. Pract. 2010, 64, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Boutati, E.; Lambadiari, V.; Mitrou, P.; Maratou, E.; Brunel, P.; Raptis, S.A. Restoration of early insulin secretion after a meal in type 2 diabetes: Effects on lipid and glucose metabolism. Eur. J. Clin. Investig. 2004, 34, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.D.; Tessari, P.; Go, V.L.W.; Gerich, J.E. A-Glucosidase inhibition improves postprandial hyperglycemia and decreases insulin requirements in insulin-dependent diabetes mellitus. Metabolism 1985, 34, 261–265. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Hatziagellaki, E.; Alexopoulos, E.; Kordonouri, O.; Komesidou, V.; Ganotakis, M.; Raptis, S. Effects of A-Glucosidase inhibition on meal glucose tolerance and timing of insulin administration in patients with type 1 diabetes mellitus. Diabetes Care 1991, 14, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Tessari, P.; Gerich, J. Effects of a long-acting somatostatin analogue on postprandial hyperglycemia in insulin-dependent diabetes mellitus. Metabolism 1983, 32, 987–992. [Google Scholar] [CrossRef]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Wada, K.; Matsushita, K.; OuYang, P.; Hotta, Y.; Takefuji, S.; Mitsuhashi, H.; Sugiura, K.; et al. Eating fast leads to insulin resistance: Findings in middle-aged Japanese men and women. Prev. Med. 2008, 46, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P. Minimizing glycemic fluctuations in patients with type 2 diabetes: Approaches and importance. Diabetes Technol. Ther. 2017, 19, 498–506. [Google Scholar] [CrossRef] [PubMed]
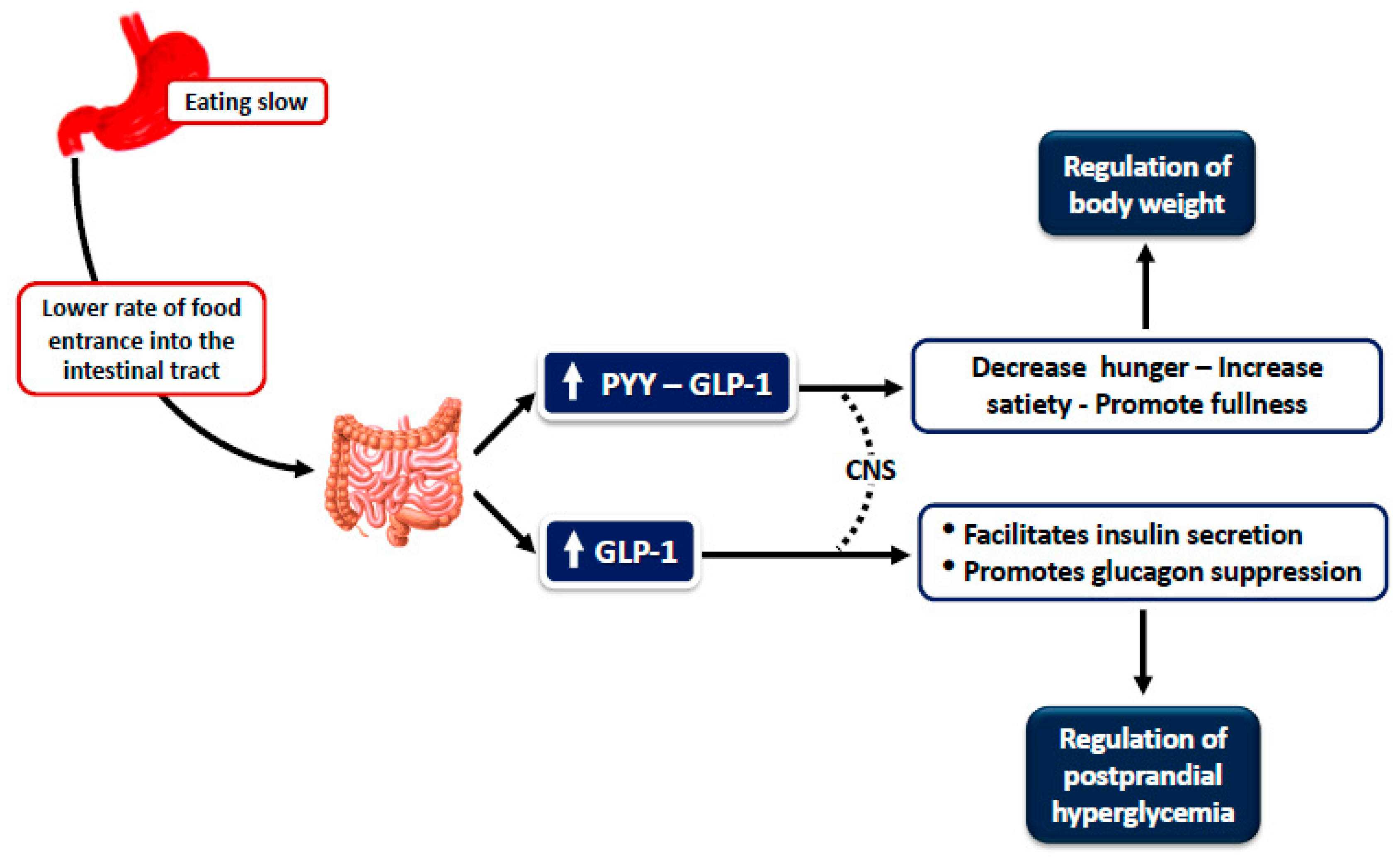

| First Author, Year, (Ref) | Participants | Study Design | Eating Rate Manipulation | Ad Libitum Meal after Standardized Meal | Hunger Measurements | Biochemical Profile and Hormones Response | Results |
|---|---|---|---|---|---|---|---|
| Zijlstra 2010 [11] | N = 106 | Ad libitum meals (seven) in a real-life setting (cinema) | Test meal: luncheon meat, vegetarian meat replacer and chewy candy | no | Type: VAS | Not measured | Fast condition |
| Healthy | Soft (fast condition) and hard (slow condition) version | Time: at the beginning and end of the movie | ER: 25 ± 13 g/min | ||||
| 45 males | Intake: 157 ± 125 g | ||||||
| 61 females | Hunger: 35.0 ± 27.0 mm | ||||||
| Aged 24 ± 7 years | Slow condition | ||||||
| BMI: 21 ± 1.7 kg/m2 | ER: 21 ± 10 g/min | ||||||
| Intake: 148 ± 121 g | |||||||
| Hunger: 35.0 ± 27.0 mm | |||||||
| No significant differences between fast and slow condition | |||||||
| Llewellyn 2008 [12] | N = 254 twin children | Ad libitum meal | 24 sandwich quarters and chopped fruit salad | no | no | Not measured | Significant differences between groups for ER |
| Overweight or obese: 68 | Heritability of ER | Overweight or obese | |||||
| Higher normal weight: 87 | Video | ER: 4.3 ± 0.16 bites/min | |||||
| Lower normal weight: 99 | Recording of ER at home | Higher normal weight | |||||
| Monozygotic: 126 | ER: 4.1 ± 0.14 bites/min | ||||||
| Dizygotic: 128 | Lower normal weight | ||||||
| Age: 11.2 ± 0.55 years | ER: 3.8 ± 0.14 bites/min | ||||||
| Heritability of ER: | |||||||
| Higher for MZ twin pair than for DZ twin pairs | |||||||
| Andrade 2008 [14] | N = 30 healthy females | Ad libitum meals (two) eaten fast and slow | Standardized breakfast (400 kcal) | No | Type: VAS | Not measured | Meal duration: approximately 21 min longer in slow condition |
| Age: 22.9 ± 7.1 years | Separate sessions | Test meal: ad libitum pasta (600 g) after 4h fast | Time: every 5 min up to 30 min and at 45 and 60 min | Fast condition | |||
| BMI: 22.1 ± 2.9 kg/m2 | Fast condition: with a large spoon, with no pause between bites | ER: 84.8 ± 36.32 kcal/min | |||||
| Slow condition: with a small spoon, between bites spoons were down and every bite was chewed 20–30 times | Energy intake: 645.7 ± 155.9 kcal | ||||||
| Slow condition | |||||||
| ER: 21.0 ± 7.2 kcal/min | |||||||
| Energy intake: 579.0 ± 154.7 kcal | |||||||
| Slow rates of ingestion led to significant decreases in energy intake | |||||||
| Kokkinos 2010 [15] | N = 17 healthy males | Fixed meals (two) within 5 min and 30 min | Test meal: ice cream 300 ML (675 kcal) | no | Type: VAS | 30-min meal AUCs for PYY and GLP-1 were significantly greater than 5-min meal | 30-min meal: higher fullness VAS rating immediately after the end of the meal |
| Age: 29.7 ± 1.2 years | Separate | Meal duration | Time: before the test meal and at 30, 60, 90, 120, 150, 180 and 210 min after the consumption | 30-min meal: PYY concentrations were higher at 90, 120, 150 min postprandially | No differences in hunger VAS ratings | ||
| BMI: 26.1 ± 0.9 kg/m2 | Sessions | 5 min meal: 2 portions 5 min apart | No differences for ghrelin glucose and insulin AUCs values | ||||
| 30-min meal: 7 portions 5 min apart | |||||||
| Zandian 2009 [16] | N = 47 healthy females | Ad libitum meals (five) | Test meal: rice, sliced chicken and vegetables (400 kj/100 g) | no | Type: VAS | Not measured | Between group comparison: Decelerated eaters ate significantly less food than linear eaters when the meal was short, interrupted and ER was increased |
| 17 decelerated eaters | Mandometer | control | Time: satiety ratings before the test meal and every minute after | Decelerated eaters reached a significant higher level of satiety compared to linear eaters only under the control condition | |||
| 30 linear eaters | Software records the amount of food consumed and the duration of the meal | short (40% less time of control time) | Desire to eat and hunger ratings were measured before and after the test meal | ||||
| BMI: 22.2 (20.2–24.3) kg/m2 | fast ER: +40% more food | ||||||
| Age: 21.2 (19.5–23.1) years | slow ER: −30% less food | ||||||
| Interrupted (1 min break every 60 g) | |||||||
| Wilkinson 2016 [17] | N = 80 healthy | Eight different test meals, one for every group | Tomato soup (39 kcal/100 g) | no | Type: VAS | Not measured | Participants who saw 300 ML but actually consumed 500 ML ate at a significantly faster rate than participants who saw 500 ML but consumed 300 ml |
| 50 females | Custard (77 kcal/100 g) | Time: at the beginning of the test meal, immediately after eating, 20, 40, 60 min after meal termination | |||||
| 30 males | Manipulation of visual information about the amount of food, i.e., saw 300 ML but consumed 500 ml | ||||||
| 8 groups of 10 participants | |||||||
| Age: 24.8 ± 8.7 years | |||||||
| BMI: 23.2 ± 3.8 kg/m2 | |||||||
| Henry 2018 [18] | N = 272 | Video recording of eating behavioral habits | Standardized breakfast: orange juice and 2 slices of white bread with kaya spread | Ad libitum buffet: 1000 g (189 kcal/100 g) | no | Not measured | Positive association between BMR and ER, that was independent of BMI |
| 91 males | BMR measurements | of olive vegetable fried rice in 15 min | Positive association between ER and FFM | ||||
| Age: 40.8 ± 14.3 years | |||||||
| BMI: 23.3 ± 2.7 kg/m2 | |||||||
| 181 females | |||||||
| Age: 38.7 ± 13.8 years | |||||||
| BMI:21.6 ± 3.3 kg/m2 | |||||||
| Hawton 2018 [20] | N = 21 healthy | Normal (6 min) and slow (24 min) rate groups | Test meal: macaroni and cheese (600 kcal) | Ad libitum snacks 3h postmeal: 500 kcal crisps and 500 kcal cookies | Type: VAS | PYY: increased more in the normal rate group | Slow rate group consumed a smaller quantity of the ad libitum meal |
| 11 males | fMRI 2-hours postmeal while undergoing a memory task concerning the meal | Normal rate: 2 pieces every 12 sec vs. slow rate 1 piece every 24 sec | Time: at the beginning of the test meal and every 30 min for 3h | Ghrelin: suppression was greater in the slow rate group | fMRI: the slower eating group reported more accurate portion size memory | ||
| 10 females | |||||||
| Normal rate group | |||||||
| Age: 23.4 ± 4.7 years | |||||||
| BMI: 21.8. ± 2.0 kg/m2 | |||||||
| Slow rate group | |||||||
| Age: 22.7 ± 3.3 years | |||||||
| BMI: 21.4 ± 1.7 kg/m2 | |||||||
| Forde 2013 [21] | N = 157 healthy | Four ad libitum different test meals that were modified in the texture of the meal components and the taste of the gravy | Meat, potato and vegetables (1250 kcal) | no | Type: VAS | Not measured | The ad libitum consumption in the savory mashed meal was significantly higher |
| 76 males | Fast condition: Savory mashed (n = 39) | Time: at the beginning and at the end of the meal | ER was increased in the mashed texture condition | ||||
| 81 females | Standard mashed (n = 37) | ||||||
| Age: 44.8 ± 5.3 years | Slow condition: | ||||||
| BMI: 22.6 ± 1.7 kg/m2 | Savory whole (n= 41) | ||||||
| Standard whole (n = 40) | |||||||
| Bolhuis 2014 [22] | N = 50 healthy 11 males | Two separate days for lunch and dinner on the same day | Ad libitum lunch: 4 (700 g) hamburgers and 600 g of rice salad | Ad libitum dinner chicken noodles (463 kJ/100 g) | Type: VAS | Not measured | Compared with softer foods, lunch with harder foods led to 16% lower intake |
| 39 females | Video recording for oral processing characteristics | Soft–hard manipulation was established by changing the type of bread, rice and boiled vs. raw vegetables | Women were served 800 g and men 1000 g | Time: before and after ad libitum intake of the lunch and dinner | ER of the lunch with the hard foods was ~32% lower | ||
| Age: 24 ± 2 years | Energy intake at dinner was not different after both test meals | ||||||
| BMI: 21 ± 2 kg/m2 | Oral processing data: the hard foods were consumed with smaller bites, longer oral duration per gram food and more chews per gram food | ||||||
| Karl 2013 [23] | N = 20 healthy | Ad libitum breakfast and lunch on the same day | Ad libitum breakfast: HED, (1.6 kcal/g ) and LED (1.2 kcal/g ) oatmeal consumed slowly (20 g/min) and quickly (80 g/min) | Ad libitum lunch 3 h after breakfast | Type: SLIM | Main effects of ED and ER on insulin, PYY, and GLP-1 AUC were observed, FR and HED being associated with larger AUC | Energy intake was higher during FR-HED |
| 12 males | Four separate sessions | Lasagna 1.4 kcal/g | Time: before breakfast, at 15, 30, 45, 60, 90, 120 and 180 min | No effects on active or total ghrelin AUC were documented | AUC of appetite ratings was not different between meals | ||
| 8 females | Mandometer: constant ER by following a preprogrammed eating curve on a screen | Total energy intake over both meals was higher during the FR-HED trial | |||||
| Age: 30 ± 11 years | |||||||
| BMI: 24 ± 2 kg/m2 | |||||||
| Zijlstra 2008 [24] | In real-life setting: | Study 1 | Standardization of satiety before ad libitum intake Preload: mini pizza (1130 kJ) | Study 1: ad libitum test meal of liquid chocolate milk, semiliquid chocolate custard and | Type: VAS | Not measured | Study 1: the intake of the liquid was respectively 14 and 30% higher compared to the semiliquid and semisolid product |
| N = 108 | ad libitum intake in a real-life setting (cinema) | One-sixth of daily energy estimated needs was provided | semisolid chocolate custard | Time: before and after ad libitum intake | Study 2: in the free ER/no effort condition, the intake of the liquid was 29% higher compared to the semiliquid | ||
| 36 males | Each subject participated in three sessions | Study 1: 7 subjects received 1 mini pizza, 78 received 1.5 mini pizzas and 23 received 2 mini pizzas | Study 2 | In the fixed ER/no effort condition, the intake of the liquid was 12% higher compared to the semiliquid | |||
| 72 females | Study 2 | Study 2: 4 subjects received 1 mini pizza, 37 received 1.5 mini pizzas and 8 received 2 mini pizza | Liquid chocolate milk and semisolid chocolate custard | If not controlled, the difference in intake between liquid and semisolid was comparable to the real-life setting | |||
| Age: 26 ± 7 years | ad libitum intake in laboratory setting: subjects returned for six sessions | Three conditions: Free ER, different effort | |||||
| BMI: 22.7 ± 2.4 kg/m2 | Test products: | Free ER, no effort | |||||
| In laboratory setting: | Different in viscosity and equal in ED, volume and macronutrient composition | Fixed ER, no effort | |||||
| N = 49 | |||||||
| 14 males | |||||||
| 35 females | |||||||
| Age: 24 ± 6 years | |||||||
| BMI: 22.2 ± 2.3 kg/m2 | |||||||
| Juvonen 2009 [25] | N = 20 | Two test meals with different viscosity | Isocaloric oat bran 300 ML | Ad libitum meal 3h later consisted of vegetable soup oat and rye breads, margarine, cheese, tomato, cucumber slices, noncaloric juice and tap water | Type: VAS | The beverage with low viscosity induced a greater postprandial increase in plasma glucose, insulin, cholecystokinin, GLP-1, and PYY and a greater decrease in postprandial ghrelin than the beverage with high-viscosity | Energy intake at the meal consumed ad libitum was not affected by the test beverages |
| 16 females | Lower viscosity produced by the modification of content of b-glucan | (1250 kJ) with low or high viscosity | Time: before the meal and at 15, 30, 45, 60, 90, 120 and 180 min | Low viscosity beverage induced a greater postprandial increase in satiety | |||
| 4 males | OGTT (75 g glucose) to ascertain normal glucose tolerance | ||||||
| Age: 22.6 ± 0.7 years | Paracetamol absorption test for gastric emptying | ||||||
| BMI: 21.6 ± 0.3 kg/m2 | |||||||
| Viskaal-van Dongen 2011 [26] | N = 37 | Each subject tested a total of 7 food items (2 of them were similar for all reference foods) in separate test sessions | Measuring ingestion time: 50 g of the food with no pausing between bites or sips and eating time was recorded | Measuring ad libitum food intake: the same food in a large preweighed amount until comfortably full | Type: VAS 9-point scale | Not measured | ER ranging from 4.2 ± 3.7 to 631 ± 507 g/min |
| 13 males | The sample consisted of 45 food items which were tested by at least 3 subjects and a maximum of 6 subjects | Time: before and after each session | ER was positively associated with energy intake and inversely associated with ED | ||||
| 24 females | Reference food tested 37 times | Carbohydrate, protein, and fiber content were inversely associated with ER in contrast to fat which showed no association | |||||
| Age: 23.3 ± 3.4 years | |||||||
| BMI: 21.7 ± 1.7 kg/m2 | |||||||
| Zhu 2013 [28] | N = 21 healthy males | Preliminary session to determine a suitable portion size for all participants | Test meal: pizza (490 kcal) into 24 portions of 3.8 × 2.5 cm | Ad libitum pasta meal 3h after the pizza (900 kcal) | Type: VAS | Plasma concentrations of glucose, insulin, GIP and CCK were higher and ghrelin was lower following the 40-chews meal | Increasing the number of masticatory cycles before swallowing increases satiety |
| Mean age: 24 years range: 18–36 years | Two test sessions with different chewing time | Session 1: 8 min (15 chews) | Time: before the test meal and at 15, 30, 45, 60, 90, 120 and 180 min | There was no difference in food intake at the subsequent ad libitum meal after 3 h | |||
| BMI: 24.8 kg/m2 range: 20.3–28.3 kg/m2 | Session 2: 20 min (40 chews) |
| First Author, Year, (Ref) | Participants | Study Design | Eating Rate Manipulation | Ad Libitum Meal after Standardized Meal | Hunger Measurements | Biochemical Profile and Hormones Response | Results |
|---|---|---|---|---|---|---|---|
| Koidis 2014 [32] | N = 14 | Standardized breakfast and 3 h later a test meal | Standardized breakfast: blueberry muffin and orange juice (425 kcal) | no | Type: VAS | Not measured | Overweight/obese individuals ate at a faster rate compared to the normal-weight group |
| 9 females | Two different ER for each group | Test meal: chicken salad sandwich, a yoghurt and a blackcurrant drink (610 kcal) | Time: before test meal and at 15, 30, 45, 60, 90, 120 and 180 min | ||||
| 5 males | Two separate sessions | Fast ER group: consumption in 8 ± 3 min | |||||
| Age: 22.1 ± 1.7 years | Slow ER group: consumption in 31 ± 10 min | ||||||
| 7 normal-weight group BMI: 20.3 ± 2 kg/m2 | |||||||
| 7 overweight or obese group BMI: 31.7 ± 6.6 kg/m2 | |||||||
| Karl 2011 [9] | N = 25 | Three test meals with different ER | Test meal: corned beef hash | Ad libitum meal 3h after test meal: lasagna | Type: SLIM | Postprandial glucose, insulin, PYY, and leptin were not affected by ER | Eating slowly delayed time to peak fullness, but did not alter peak fullness |
| 15 normal weight | Each volunteer received all three meals | Volunteers consumed 40% of their total energy expenditure | Time: before test meal and at 15, 30, 45, 60, 90, 120 and 180 min | ER altered the postprandial CCK and PP response, but no effects on AUC were observed | Ad libitum energy intake was not different between sessions | ||
| 8 males | Mandometer: | Meal duration | |||||
| 7 females | constant ER by following a preprogrammed eating curve on a screen | FM: 7 min | |||||
| 10 obese | MM: 14 min | ||||||
| 8 males | SM: 28 min | ||||||
| 2 females | |||||||
| Age: 30 ± 12 years | |||||||
| BMI: 27.3 ± 6.7 kg/m2 | |||||||
| Shah 2014 [33] | N = 70 | Ad libitum meal at two different speeds | Test meal: Vegetable pasta | no | Type: VAS | Not measured | During the slow compared to the fast condition: |
| 36 females | Two separate days | Females: 900 g (1.300 kcal) | Time: before test meal and at 5, 10, 15, 20, 25, 30, 45, 60 min | Energy intake was significantly lower in normal-weight group | |||
| 34 males | Males: 1.200 g (1.734 kcal) | ||||||
| 35 normal weight | Fast condition: with no pause between bites | ||||||
| Age: 33.3 ± 12.5 years | Slow condition: with pause between bites | ||||||
| BMI: 23.9 ± 2.6 kg/m2 | |||||||
| 35 Overweight or obese | |||||||
| Age: 44.1 ± 13 years | |||||||
| BMI: 31.3 ± 4.6 kg/m2 | |||||||
| Martin 2007 [34] | N=48 | First meal: | Test meal: popcorn chicken (1000 g) cut into standard bite size units 8 g | no | Type: VAS | Not measured | Reduced rate and combined rate meals resulted in less food intake compared to baseline for males, but not for females |
| 22 males | Acclimation meal to determine ER of each participant | Baseline: mimic acclimation rate | Time: each minute during the meal (desire to eat) | ||||
| 26 females | Ad libitum meal at three different ER conditions | Reduced rate: by 50% of acclimation meal | Before and after the meal (hunger, desire to eat, fullness, prospective food consumption, thirst) | ||||
| Age: 30.7 ± 10.2 years | Universal eating monitors to record food intake and generate cumulative food intake curves | Combined rate: acclimation rate at the first half and 50% reduced at the rest of the meal | |||||
| BMI: 30.1 ± 2.9 kg/m2 | |||||||
| Scisco 2011 [35] | N = 30 | Ad libitum test meal at three different speeds | Test meal: mini waffle 72 bite size pieces | no | Type: VAS | Not measured | Energy intake was less in the slow rate condition compared with the feedback condition |
| 23 females | Three separate sessions | Baseline condition | Time: before and after the test meal | ||||
| 7 males | Bite data were collected from an attached athletic wrist-band on the dominant wrist | Feedback: baseline with bite rate feedback | |||||
| Age: 19.7 ± 3.5 years BMI: 25.04 ± 6.49 kg/m2 | Slow bite rate: 50% slower from baseline | ||||||
| Smitt 2011 [36] | N = 11 | Three ad libitum test meals | Test meal: 500 g cooked pasta with pesto (820 kj/100 g) | no | Type: VAS | Not measured | Participants ate 12% less when chewing at 35 CPM compared to 10 CPM |
| 4 males | CPM were measured by | Session 1: Ad libitum chewing | Time: before and after the test meal | 35 CPM resulted in longer meal duration, but also faster chewing (chews/sec) | |||
| 7 females | EMG | Session 2: 10 CPM | |||||
| 6 normal weight | Session 3: 35 CPM | ||||||
| BMI: 22.0 ± 2.0 kg/m2 | |||||||
| 5 obese | |||||||
| BMI: 33.6 ±2.1 kg/m2 | |||||||
| Spiegel 1993 [37] | N = 18 females | Ad libitum test meal with 5 different bite size pieces | Test meal: three bite sizes of tuna or turkey (5 g, 10 g, 15 g pieces) and two bite sizes of bagel with cream cheese (6 g and 12 g pieces) | no | Type: VAS | Not measured | As bite size decreased from 15 g to 5 g, the average ingestion rate decreased from 19.4 ± 2.0 to 15.9 ± 2 g/min |
| 9 normal weight | Five separate sessions | Time: before and after the test meal | The initial ingestion rate was decreased from 30.0 ± 2.9 to 19.6 ± 1.7 g/min | ||||
| Age: 25.1 ± 8.6 years | Chewing was monitored through | ||||||
| BMI: 21.1 ± 1.6 kg/m2 | EMG | ||||||
| 9 obese | |||||||
| Age: 32.4 ± 10.1 years | |||||||
| BMI: 32.6 ± 5.8 kg/m2 |
| First Author, Year, (Ref) | Participants | Study Design | Eating Rate Manipulation | Ad Libitum Meal after Standardized Meal | Hunger Measurements | Biochemical Profile and Hormones Response | Results |
|---|---|---|---|---|---|---|---|
| Angelopoulos 2014 [42] | N = 20 overweight or obese with T2DM on metformin | Standard test meal at different rates | Test meal: 300 ML ice-cream (675 kcal) | no | Type: VAS | There were no differences in glucose, insulin, PYY, GLP-1 and ghrelin responses | The AUC for fullness was higher and the AUC for hunger was lower after the 30 min meal than after the 5 min meal |
| Age: 62.6 ± 1.8 years | Two separate sessions | Meal duration | Time: before the test meal and at 30, 60, 90, 120, 150 and 180 min after the consumption | ||||
| BMI: 30.6 ± 1.1 kg/m2 | 5 min meal: 2 equal portions, 5 min apart | ||||||
| 30 min meal: 7 equal portions, 5 min apart | |||||||
| Sun 2015 [44] | N=11 | Six test sessions | Reference: glucose 50 g | no | no | Eating with chopsticks resulted in decreased postprandial glucose response | Eating with chopsticks resulted in higher chewing rate, smaller bite size, smaller number of chews per mouthful and lowered ER |
| 7 males | Three for glucose reference and three for different eating methods | Test meal: white boiled rice (63.6 g prior to cooking) | |||||
| Age: 23.0 ± 0.3 years | Mastication parameters were measured by EMG | Three eating methods: chopsticks, spoon, fingers | |||||
| BMI: 21.8 ± 0.92 kg/m2 | |||||||
| 4 females | |||||||
| Age: 24.8 ± 1.5 years | |||||||
| BMI: 19.0 ± 0.7 kg/m2 | |||||||
| Ranawana 2011 [45] | N = 12 males | Sodium acetate labeled with 13C was used to measure gastric emptying and breath samples were obtained every 15 min from the commencement of the meal until 240 min afterward | Test meal within 15 min | no | no | The total IAUCs for glucose and insulin were greater in the test meal with the small particles than those with the large particles | The small particles had a significant shorter gastric emptying time for Tlat, Tlag, Thalf, but no for Tacs |
| Age: 27 ± 5 years | Basmati rice: large and small particles | ||||||
| BMI: 23.3 ± 0.6 kg/m2 | Participants were instructed to swallow the foods without chewing | ||||||
| Ranawana 2014 [46] | N = 15 | Five test sessions | Test meal: Jasmine rice within 15 min | no | no | The glucose was significantly lower when the rice was chewed 15 times than when it was chewed 30 times | |
| 8 males | Three to test a standard 50 g oral bolus of glucose | Session 1: 15 chews | |||||
| 7 females | Two test meals with rice | Session 2: 30 chews | |||||
| Age: 26 ± 6 years | Mastication parameters were measured by EMG | ||||||
| BMI: 20.5 ± 4 kg/m2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argyrakopoulou, G.; Simati, S.; Dimitriadis, G.; Kokkinos, A. How Important Is Eating Rate in the Physiological Response to Food Intake, Control of Body Weight, and Glycemia? Nutrients 2020, 12, 1734. https://doi.org/10.3390/nu12061734
Argyrakopoulou G, Simati S, Dimitriadis G, Kokkinos A. How Important Is Eating Rate in the Physiological Response to Food Intake, Control of Body Weight, and Glycemia? Nutrients. 2020; 12(6):1734. https://doi.org/10.3390/nu12061734
Chicago/Turabian StyleArgyrakopoulou, Georgia, Stamatia Simati, George Dimitriadis, and Alexander Kokkinos. 2020. "How Important Is Eating Rate in the Physiological Response to Food Intake, Control of Body Weight, and Glycemia?" Nutrients 12, no. 6: 1734. https://doi.org/10.3390/nu12061734
APA StyleArgyrakopoulou, G., Simati, S., Dimitriadis, G., & Kokkinos, A. (2020). How Important Is Eating Rate in the Physiological Response to Food Intake, Control of Body Weight, and Glycemia? Nutrients, 12(6), 1734. https://doi.org/10.3390/nu12061734






