Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide Are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Liver Ultrasonography
2.2. Med-Diet Questionnaire
2.3. Serum sNox2-dp
2.4. Serum LPS
2.5. Statistical Analysis
3. Results
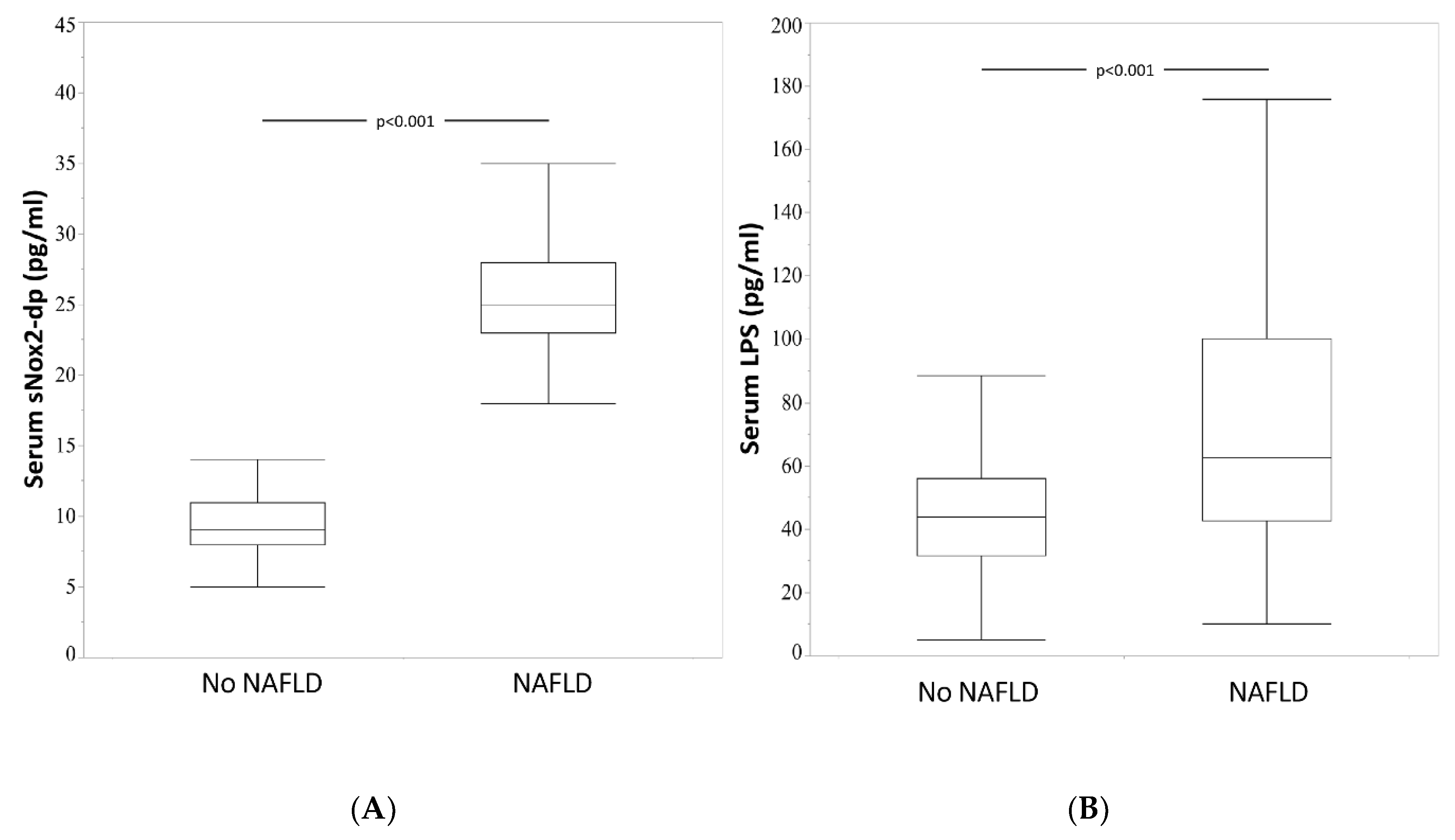
3.1. NAFLD Patients
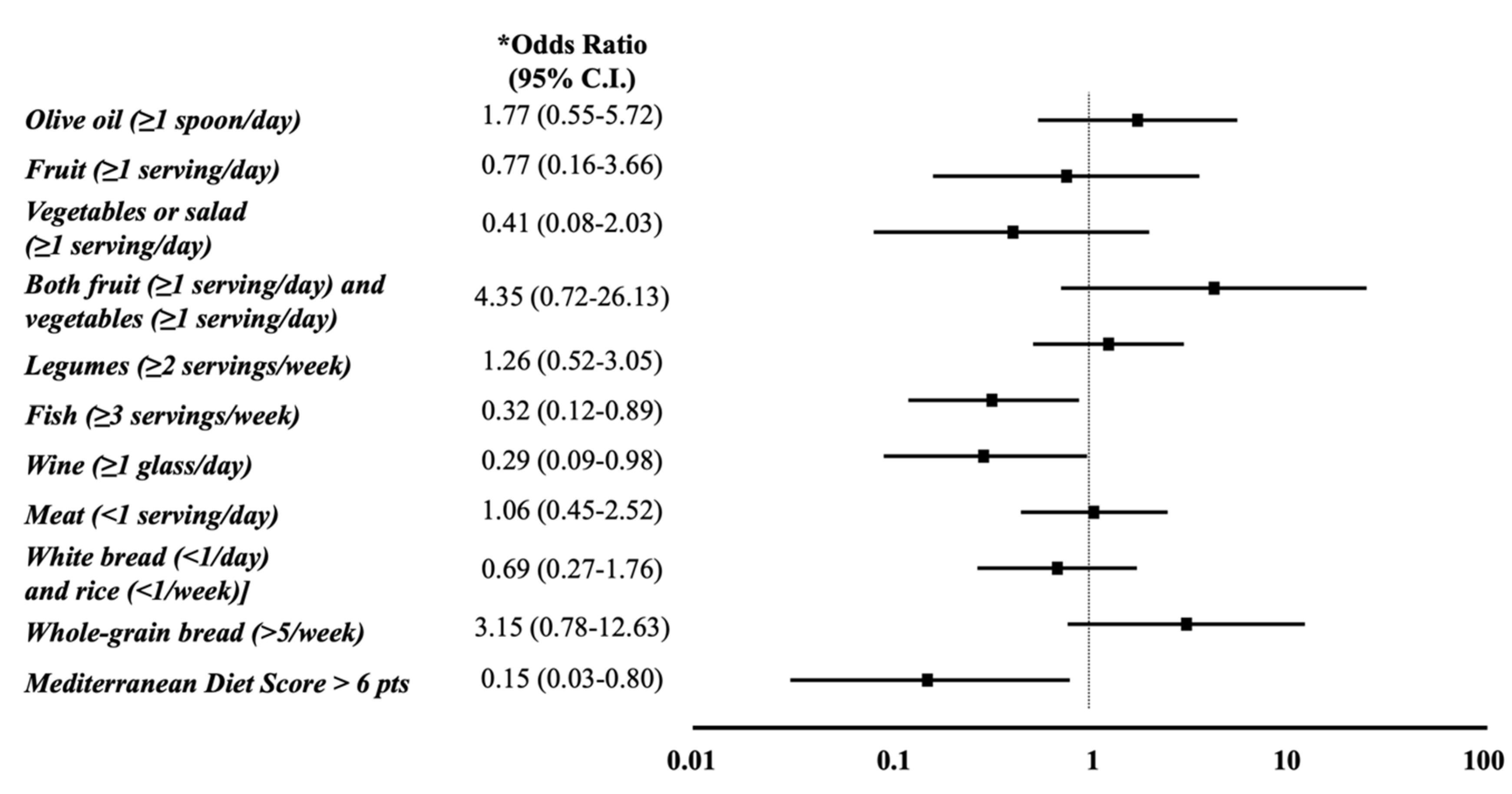
3.2. Mediterranean Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, L.; Del Ben, M.; Baratta, F.; Perri, L.; Albanese, F.; Pastori, D.; Violi, F.; Angelico, F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015, 7, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Pastori, D.; Baratta, F.; Overi, D.; Labbadia, G.; Polimeni, L.; Di Costanzo, A.; Pannitteri, G.; Carnevale, R.; Del Ben, M.; et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: A possible role for oxidative stress. Sci. Rep. 2017, 7, 15756. [Google Scholar] [CrossRef] [PubMed]
- Cave, A.C.; Brewer, A.C.; Narayanapanicker, A.; Ray, R.; Grieve, D.J.; Walker, S.; Shah, A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006, 8, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Sohda, T.; Iwata, K.; Kunimoto, H.; Fukunaga, A.; Kuno, S.; Yotsumoto, K.; Sakurai, K.; Iwashita, H.; Hirano, G.; et al. Levels of the oxidative stress marker γ-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J. Int. Med. Res. 2012, 40, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Pirgon, Ö.; Bilgin, H.; Çekmez, F.; Kurku, H.; Dündar, B.N. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Baratta, F.; Loffredo, L.; Pignatelli, P.; Violi, F.; Angelico, F. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; De Simone, C.; Del Vecchio Blanco, C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; De Simone, T.; Federico, A.; Terracciano, F.; Tuccillo, C.; Di Chicco, M.; Cartenì, M. Gut-liver axis: A new point of attack to treat chronic liver damage? Am. J. Gastroenterol. 2002, 97, 2144–2146. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Q.; Li, H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- de Faria Ghetti, F.; Oliveira, D.G.; de Oliveira, J.M.; de Castro Ferreira, L.E.V.V.; Cesar, D.E.; Moreira, A.P.B. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur. J. Nutr. 2018, 57, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased liver localization of lipopolysaccharides in human and experimental non-alcoholic fatty liver disease. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Novo, M.; Del Ben, M.; Farcomeni, A.; Angelico, F.; Violi, F. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: Effect of extra-virgin olive oil. Eur. J. Nutr. 2019, 58, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.H. Dietary patterns reflecting healthy food choices are associated with lower serum LPS activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef] [PubMed]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jeong, W.K.; Baik, S.K. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J. Gastroenterol. 2014, 20, 4300–4315. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Jain, N.; Rachapalli, V.; Cochlin, D.L.; Robinson, M. Non-invasive evaluation of liver cirrhosis using ultrasound. Clin. Radiol. 2009, 64, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Fernandez-Jarne, E.; Serrano-Martinez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Tanzilli, G.; Cangemi, R.; Vicario, T.; Catena, M.; Violi, F.; Pignatelli, P. Does Mediterranean Diet Reduce Cardiovascular Events and Oxidative Stress in Atrial Fibrillation? Antioxid. Redox Signal. 2015, 23, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Fernández-Jarne, E.; Serrano-Martínez, M.; Marti, A.; Martinez, J.A.; Martín-Moreno, J.M. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: An operational healthy dietary score. Eur. J. Nutr. 2002, 41, 153–160. [Google Scholar] [CrossRef] [PubMed]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Bartimoccia, S.; Carnevale, R.; Loffredo, L.; Pignatelli, P.; Violi, F.; Angelico, F. Serum Cytokeratin-18 Is Associated with NOX2-Generated Oxidative Stress in Patients with Nonalcoholic Fatty Liver. Int. J. Hepatol. 2014, 2014, 784985. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Pastori, D.; Carnevale, R.; Farcomeni, A.; Cangemi, R.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Saliola, M.; Lip, G.Y.; et al. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb. Haemost. 2015, 113, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Baratta, F.; Carnevale, R.; Cangemi, R.; Del Ben, M.; Bucci, T.; Polimeni, L.; Labbadia, G.; Nocella, C.; Scardella, L.; et al. Similar Reduction of Cholesterol-Adjusted Vitamin E Serum Levels in Simple Steatosis and Non-Alcoholic Steatohepatitis. Clin. Transl. Gastroenterol. 2015, 6, e113. [Google Scholar] [CrossRef] [PubMed]
- Paik, Y.H.; Iwaisako, K.; Seki, E.; Inokuchi, S.; Schnabl, B.; Osterreicher, C.H.; Kisseleva, T.; Brenner, D.A. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 2011, 53, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Moreno-Fernandez, M.E.; Giles, D.A.; Cappelletti, M.; Stankiewicz, T.E.; Chan, C.C.; Divanovic, S. Nicotinamide adenine dinucleotide phosphate (reduced) oxidase 2 modulates inflammatory vigor during nonalcoholic fatty liver disease progression in mice. Hepatol. Commun. 2018, 2, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Baratta, F.; Del Ben, M.; Angelico, F.; Sciarretta, S.; Bartimoccia, S.; Novo, M.; et al. Low-grade endotoxemia, gut permeability and platelet activation in patients with impaired fasting glucose. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Carnevale, R.; Nocella, C.; Novo, M.; Santulli, M.; Cammisotto, V.; Menichelli, D.; Pignatelli, P.; Violi, F. Gut-Derived Serum Lipopolysaccharide is Associated With Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Raparelli, V.; Basili, S.; Carnevale, R.; Napoleone, L.; Del Ben, M.; Nocella, C.; Bartimoccia, S.; Lucidi, C.; Talerico, G.; Riggio, O.; et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017, 65, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yang, Y.J.; Yang, Y.K.; Oh, S.Y.; Hong, Y.C.; Lee, E.K.; Kwon, O. Diet quality scores and oxidative stress in Korean adults. Eur. J. Clin. Nutr. 2011, 65, 1271–1278. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean diet and 10-year incidence (2002-2012) of diabetes: Correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes/Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moreno, J.; Garcia-Carpintero, S.; Gomez-Delgado, F.; Jimenez-Lucena, R.; Vals-Delgado, C.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Rangel-Zuñiga, O.A.; Yubero-Serrano, E.M.; Malagon, M.M.; et al. Endotoxemia is modulated by quantity and quality of dietary fat in older adults. Exp. Gerontol. 2018, 109, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Pignatelli, P.; Guglielmini, G.; Carnevale, R.; Mezzasoma, A.M.; Ghiselli, A.; Momi, S.; Violi, F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J. Nutr. 2008, 138, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Hellgren, L.I.; Petersen, M.; Basu, S.; Straarup, E.M.; Schnohr, P.; Sandström, B. A solid dietary fat containing fish oil redistributes lipoprotein subclasses without increasing oxidative stress in men. J. Nutr. 2004, 134, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.; Talajic, M.; Roy, D.; Nattel, S.; Lambert, J.; Nozza, A.; Jones, P.; Ramprasath, V.R.; O’Hara, G.; Kopecky, S.; et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J. Am. Coll. Cardiol. 2014, 64, 1441–1448. [Google Scholar] [CrossRef]
- Ottestad, I.; Vogt, G.; Retterstøl, K.; Myhrstad, M.C.; Haugen, J.E.; Nilsson, A.; Ravn-Haren, G.; Nordvi, B.; Brønner, K.W.; Andersen, L.F.; et al. Oxidised fish oil does not influence established markers of oxidative stress in healthy human subjects: A randomised controlled trial. Br. J. Nutr. 2012, 108, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Bandarra, N.M.; Kiely, M.; Thorsdottir, I.; Martínez, J.A. Impact of fish intake on oxidative stress when included into a moderate energy-restricted program to treat obesity. Eur. J. Nutr. 2007, 46, 460–467. [Google Scholar] [CrossRef] [PubMed]
| sNox2-dp Tertiles | |||||
|---|---|---|---|---|---|
| I (n = 72) | II (n = 67) | III (n = 54) | p# | p | |
| Age (years) | 53.7 ± 12.0 | 55.4 ± 10.9 | 48.9 ± 12.2 | 0.008 | 0.026 ° 0.395 § 0.002 † |
| Women | n = 29, 40.3% | n = 26, 38.8% | n = 26, 48.1% | 0.547 | 0.378 ° 0.859 § 0.302 † |
| Smokers | n = 17, 23.6% | n = 20, 29.9% | n = 14, 25.9% | 0.703 | 0.765 ° 0.406 § 0.633 † |
| BMI (kg/m2) ** | 31.2 ± 5.4 | 30.3 ± 4.1 | 29.4 ± 4.2 | 0.098 | 0.042 ° 0.273 § 0.235 † |
| Waist circumference (cm) * | 107.5 (101.0–116.0) | 106.0 (98.7–113.2) | 103.0 (97.0–108.5) | 0.072 | 0.027 ° 0.417 § 0.114 † |
| Diabetes | n = 28, 38.9% | n = 17, 25.4% | n = 16, 29.6% | 0.216 | 0.281 ° 0.089 § 0.601 † |
| Fasting blood glucose (mg/dL) * | 113.2 ± 45.1 | 105.1 ± 26.8 | 101.0 ± 30.9 | 0.146 | 0.090 ° 0.340 § 0.179 † |
| Statin use ***** | n = 37, 52.9% | n = 21, 32.3% | n = 12, 22.6% | 0.002 | 0.001 ° 0.016 § 0.245 † |
| Arterial Hypertension | n = 47, 65.3% | n = 45, 67.2% | n = 24, 44.4% | 0.021 | 0.020 ° 0.814 § 0.012 † |
| GGT (UI/L) **** | 23.0 (15.0–36.0) | 28.0 (18.0–42.5) | 39.0 (27.5–109.5) | <0.001 | <0.001 ° 0.092 § 0.001 † |
| AST (UI/L) | 21.0 (17.0–24.0) | 20.0 (16.0–30.0) | 34.5 (25.0–49.5) | <0.001 | <0.001 ° 0.853 § <0.001 † |
| ALT (UI/L) | 25.0 (19.3–35.8) | 24.0 (17.0–36.0) | 50.0 (33.8–91.8) | <0.001 | <0.001 ° 0.706 § <0.001 † |
| Platelets (n/mL) | 248.0 (197.0– 283.0) | 227.0 (186.0–278.0) | 235.0 (197.3–280.0) | 0.287 | 0.499 ° 0.110 § 0.451 † |
| APRI ≥ 0.7 | n = 2, 2.8% | n = 3, 4.5% | n = 10, 18.5% | 0.002 | 0.004 ° 0.591 § 0.013 † |
| LPS (pg/mL) | 50.6 (41.4–81.2) | 52.0 (39.3–90.0) | 85.3 (49.8–136.3) | 0.002 | 0.002 ° 0.683 § 0.002 † |
| p | Odds Ratio | 95% C.I. for Odds Ratio Lower-Upper | ||
|---|---|---|---|---|
| Age (continuous) | 0.227 | 0.98 | 0.94 | 1.01 |
| Women | 0.973 | 1.01 | 0.45 | 2.29 |
| Statin use | 0.062 | 0.41 | 0.16 | 1.05 |
| Smokers | 0.608 | 0.79 | 0.32 | 1.94 |
| Good Adherence to Mediterranean Diet (Med-Diet score > 6) | 0.026 | 0.14 | 0.03 | 0.79 |
| Highest LPS tertile (>27 pg/mL) | <0.001 | 4.71 | 2.11 | 10.51 |
| APRI>0.7 | 0.005 | 6.96 | 1.80 | 26.98 |
| Diabetes | 0.215 | 1.81 | 0.71 | 4.63 |
| Arterial Hypertension | 0.114 | 0.51 | 0.22 | 1.17 |
| High Waist Circumference * | 0.220 | 1.91 | 0.68 | 5.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, F.; Pastori, D.; Bartimoccia, S.; Cammisotto, V.; Cocomello, N.; Colantoni, A.; Nocella, C.; Carnevale, R.; Ferro, D.; Angelico, F.; et al. Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide Are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 1732. https://doi.org/10.3390/nu12061732
Baratta F, Pastori D, Bartimoccia S, Cammisotto V, Cocomello N, Colantoni A, Nocella C, Carnevale R, Ferro D, Angelico F, et al. Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide Are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2020; 12(6):1732. https://doi.org/10.3390/nu12061732
Chicago/Turabian StyleBaratta, Francesco, Daniele Pastori, Simona Bartimoccia, Vittoria Cammisotto, Nicholas Cocomello, Alessandra Colantoni, Cristina Nocella, Roberto Carnevale, Domenico Ferro, Francesco Angelico, and et al. 2020. "Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide Are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease" Nutrients 12, no. 6: 1732. https://doi.org/10.3390/nu12061732
APA StyleBaratta, F., Pastori, D., Bartimoccia, S., Cammisotto, V., Cocomello, N., Colantoni, A., Nocella, C., Carnevale, R., Ferro, D., Angelico, F., Violi, F., & Del Ben, M. (2020). Poor Adherence to Mediterranean Diet and Serum Lipopolysaccharide Are Associated with Oxidative Stress in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients, 12(6), 1732. https://doi.org/10.3390/nu12061732










