The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review
Abstract
1. Introduction
Biological Plausibility of Magnesium for Brain and Psychiatric Disorders
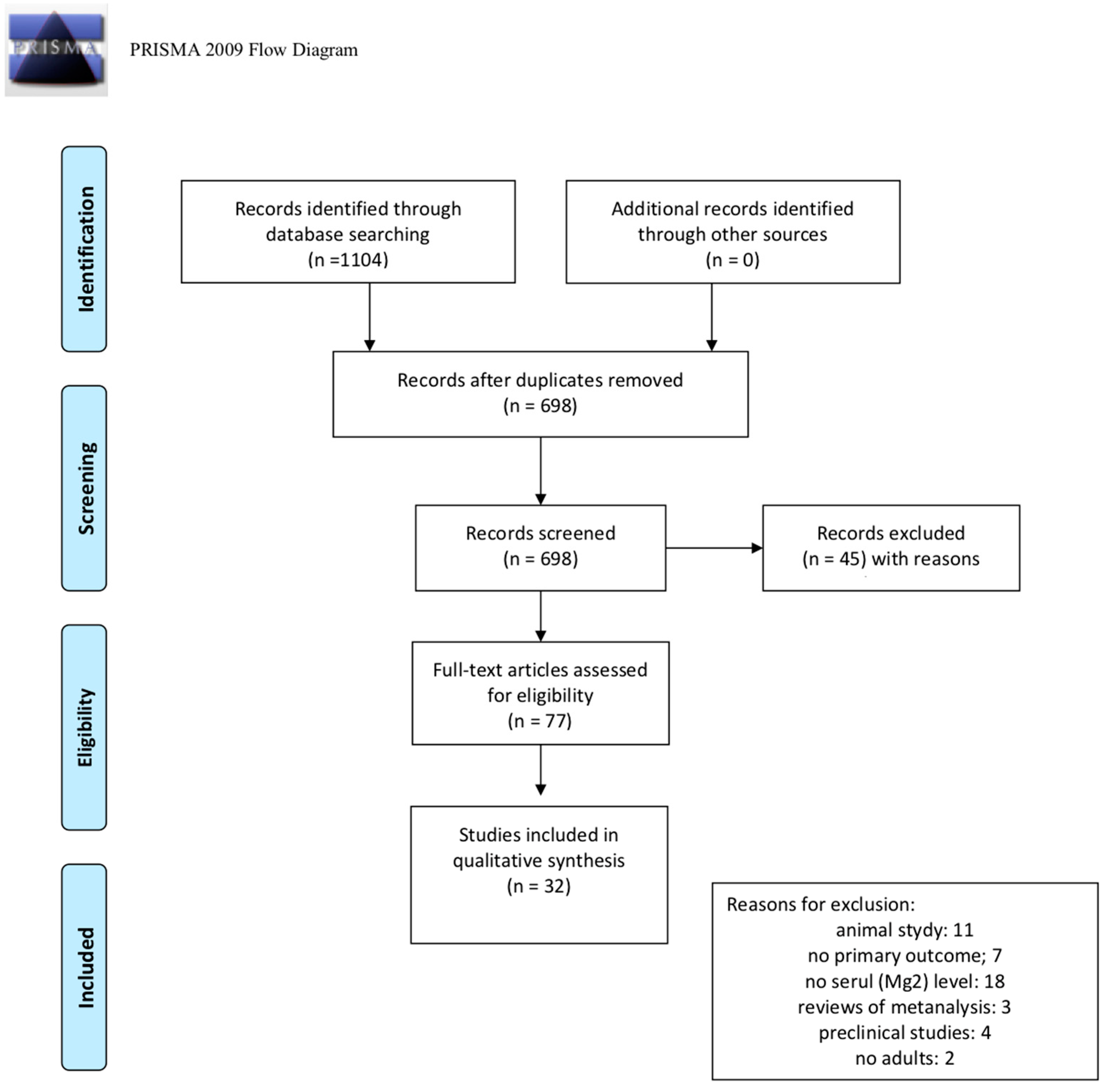
2. Materials and Methods
3. Results
3.1. Depression
3.2. Other Psychiatric Disorders
3.2.1. Anxiety Disorders
3.2.2. Obsessive–Compulsive Disorder (OCD)
3.2.3. Schizophrenia
3.2.4. Eating Disorders
3.2.5. Attention Deficit Hyperactivity Disorder (ADHD)
3.2.6. Autism Spectrum Disorder (ASD)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016, 3, 171–178. [Google Scholar] [CrossRef]
- Doran, C.M.; Kinchin, I. A review of the economic impact of mental illness. Aust. Health Rev. 2019, 43, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Leucht, S.; Leucht, C.; Huhn, M.; Chaimani, A.; Mavridis, D.; Helfer, B.; Samara, M.; Rabaioli, M.; Bächer, S.; Cipriani, A.; et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: Systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 2017, 174, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Murru, A.; Pacchiarotti, I.; Undurraga, J.; Veronese, N.; Fornaro, M.; Stubbs, B.; Monaco, F.; Vieta, E.; Vseeman, M.; et al. Safety, tolerability, and risks associated with first-and second-generation antipsychotics: A state- of-the-art clinical review. Ther. Clin. Risk Manag. 2017, 13, 757–777. [Google Scholar] [CrossRef]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101. [Google Scholar] [CrossRef]
- Balon, R. The inexplicable rise of medication prices. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2018, 30, 165–166. [Google Scholar]
- Philipson, T.J.; Snider, J.T.; Lakdawalla, D.N.; Stryckman, B.; Goldman, D.P. Impact of oral nutritional supplementation on hospital outcomes. Am. J. Manag. Care 2013, 19, 121–128. [Google Scholar] [CrossRef]
- Elia, M.; Normand, C.; Norman, K.; Laviano, A. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin. Nutr. 2016, 35, 370–380. [Google Scholar] [CrossRef]
- Sarris, J. Nutritional Psychiatry: From Concept to the Clinic. Drugs 2019, 79, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Teasdale, S.B.; Allott, K.; Siskind, D.; Marx, W.; Cotter, J.; Veronese, N.; Schuch, F.; Smith, L.; Solmi, M.; et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019, 18, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Ciappolino, V.; Delvecchio, G.; Agostoni, C.; Mazzocchi, A.; Altamura, A.C.; Brambilla, P. The role of n-3 polyunsaturated fatty acids (n-3PUFAs) in affective disorders. J. Affect. Disord. 2017, 224, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9. [Google Scholar] [CrossRef]
- Guu, T.W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R.H.; et al. A multi-national, multi-disciplinary Delphi consensus study on using omega-3 polyunsaturated fatty acids (n-3 PUFAs) for the treatment of major depressive disorder. J. Affect. Disord. 2020, 265, 233–238. [Google Scholar] [CrossRef]
- Ryan, M.F. The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 1991, 28, 19–26. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Srivali, N.; Ungprasert, P.; Varothai, N.; Sanguankeo, A.; Kittanamongkolchai, W.; Erickson, S.B. Hypomagnesaemia linked to depression: A systematic review and meta-analysis. Intern. Med. J. 2015, 45, 436–440. [Google Scholar] [CrossRef]
- Ordak, M.; Matras, J.; Muszynska, E.; Nasierowski, T.; Bujalska-Zadrozny, M. Magnesium in schizophrenia. Pharmacol. Rep. 2017, 69, 929–934. [Google Scholar] [CrossRef]
- Nechifor, M. Magnesium in addiction—A general view. Magnes. Res. 2018, 31, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Młyniec, K.; Gaweł, M.; Doboszewska, U.; Starowicz, G.; Nowak, G. The Role of Elements in Anxiety. Vitam. Horm. 2017, 103, 295–326. [Google Scholar] [CrossRef]
- Effatpanah, M.; Rezaei, M.; Effatpanah, H.; Effatpanah, Z.; Varkaneh, H.K.; Mousavi, S.M.; Fatahi, S.; Rinaldi, G.; Hashemi, R. Magnesium status and attention deficit hyperactivity disorder (ADHD): A meta-analysis. Psychiatry Res. 2019, 274, 228–234. [Google Scholar] [CrossRef]
- Boyle, N.B.; Lawton, C.; Dye, L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress-A Systematic Review. Nutrients 2017, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Molero, P.; Martínez-González, M.A.; Molendijk, M. Magnesium and mood disorders: Systematic review and meta-analysis. BJPsych Open 2018, 4, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Ordak, M.; Muszynska, E.; Nasierowski, T.; Maj-Zurawska, M.; Bujalska-Zadrozny, M. Level of magnesium in psychiatry—What is the cause of ambiguous results? Gen. Hosp. Psychiatry 2018, 51, 136. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. CKJ Clin. Kidney J. 2012, 5 (Suppl. 1). [Google Scholar] [CrossRef]
- Cowan, J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. BioMetals 2002, 15, 225–235. [Google Scholar] [CrossRef]
- Stangherlin, A.; O’Neill, J.S. Signal Transduction: Magnesium Manifests as a Second Messenger. Curr. Biol. 2018, 28, R1403–R1405. [Google Scholar] [CrossRef]
- Seyama, T.; Kamei, Y.; Iriyama, T.; Imada, S.; Ichinose, M.; Toshimitsu, M.; Fujii, T.; Asou, H. Pretreatment with magnesium sulfate attenuates white matter damage by preventing cell death of developing oligodendrocytes. J. Obstet. Gynaecol. Res. 2018, 44, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Weinger, J.G.; Mao, F.; Liu, G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology 2016, 108, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Iezhitsa, I.N.; Kravchenko, M.S.; Kharitonova, M.V. Features of central neurotransmission in animals in conditions of dietary magnesium deficiency and after its correction. Neurosci. Behav. Physiol. 2009, 39, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium is a key player in neuronal maturation and neuropathology. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Türkyilmaz, C.; Türkyilmaz, Z.; Atalay, Y.; Söylemezoglu, F.; Celasun, B. Magnesium pre-treatment reduces neuronal apoptosis in newborn rats in hypoxia-ischemia. Brain Res. 2002, 955, 133–137. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.G.; et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol. Brain 2014, 7, 1–20. [Google Scholar] [CrossRef]
- Xu, Z.P.; Li, L.; Bao, J.; Wang, Z.H.; Zeng, J.; Liu, E.J.; Li, X.G.; Huang, R.X.; Gao, D.; Li, M.Z.; et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef]
- Wu, C.; Xue, L.D.; Su, L.W.; Xie, J.L.; Jiang, H.; Yu, X.J.; Liu, H.M. Magnesium promotes the viability and induces differentiation of neural stem cells both in vitro and in vivo. Neurol. Res. 2019, 41, 208–215. [Google Scholar] [CrossRef]
- Vennemeyer, J.J.; Hopkins, T.; Kuhlmann, J.; Heineman, W.R.; Pixley, S.K. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci. Res. 2014, 84, 72–78. [Google Scholar] [CrossRef]
- Slutsky, I.; Abumaria, N.; Wu, L.J.; Huang, C.; Zhang, L.; Li, B.; Zhao, X.; Govindarajan, A.; Zhao, M.G.; Zhuo, M.; et al. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron 2010, 65, 165–177. [Google Scholar] [CrossRef]
- Pochwat, B.; Szewczyk, B.; Sowa-Kucma, M.; Siwek, A.; Doboszewska, U.; Piekoszewski, W.; Gruca, P.; Papp, M.; Nowak, G. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: Alterations in the NMDA receptor subunits. Int. J. Neuropsychopharmacol. 2014, 17, 393–405. [Google Scholar] [CrossRef]
- Sartori, S.B.; Whittle, N.; Hetzenauer, A.; Singewald, N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology 2012, 62, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Abumaria, N.; Yin, B.; Zhang, L.; Li, X.Y.; Chen, T.; Descalzi, G.; Zhao, L.; Ahn, M.; Luo, L.; Ran, C.; et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J. Neurosci. 2011, 31, 14871–14881. [Google Scholar] [CrossRef] [PubMed]
- Poleszak, E. Modulation of antidepressant-like activity of magnesium by serotonergic system. J. Neural Transm. 2007, 114, 1129–1134. [Google Scholar] [CrossRef]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sorensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kućma, M.; Szewczyk, B.; Sadlik, K.; Piekoszewski, W.; Trela, F.; Opoka, W.; Poleszak, E.; Pilc, A.; Nowak, G. Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. J. Affect. Disord. 2013, 151, 924–931. [Google Scholar] [CrossRef]
- Nechifor, M.; Vaideanu, C.; Palamaru, I.; Borza, C.; Mindreci, I. The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia. J. Am. Coll. Nutr. 2004, 23, 549S–551S. [Google Scholar] [CrossRef]
- Ang, A.W.K.; Ko, S.M.; Tan, C.H. Calcium, magnesium, and psychotic symptoms in a girl with idiopathic hypoparathyroidism. Psychosom. Med. 1995, 57, 299–302. [Google Scholar] [CrossRef]
- Kronbauer, M.; Metz, V.G.; Roversi, K.; Dias, V.T.; de David Antoniazzi, C.T.; da Silva Barcelos, R.C.; Burger, M.E. Influence of magnesium supplementation on movement side effects related to typical antipsychotic treatment in rats. Behav. Brain Res. 2017, 320, 400–411. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta- Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.R.; Melaku, Y.A.; Page, A.; Gill, T.K. Association between dietary patterns and adult depression symptoms based on principal component analysis, reduced-rank regression and partial least-squares. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Cho, S.E.; Kang, S.G.; Cho, S.J.; Na, K.S. Decreased serum magnesium levels in depression: A systematic review and meta-analysis. Nord. J. Psychiatry 2018, 72, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, A.H.; Al-Hakeim, H.K.; Twayej, A.J.; Maes, M. Total and ionized calcium and magnesium are significantly lowered in drug-naïve depressed patients: Effects of antidepressants and associations with immune activation. Metab. Brain Dis. 2019, 34, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Yasmin, F.; Manzoor, N. Biomarkers in Drug Free Subjects with Depression: Correlation with Tryptophan. Psychiatry Investig. 2019, 16, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Ahmed, M.U.; Mitu, S.A.; Islam, M.S.; Rahman, G.K.; Qusar, M.M.; Hasnat, A. Comparative analysis of serum zinc, copper, manganese, iron, calcium, and magnesium level and complexity of interelement relations in generalized anxiety disorder patients. Biol. Trace Elem. Res. 2013, 154, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.M.A.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.K.M.; Islam, M.S.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef]
- Woodward, G.; Wan, J.C.M.; Viswanath, K.; Zaman, R. Serum Vitamin D and Magnesium levels in a psychiatric cohort. Psychiatr. Danub. 2019, 31 (Suppl. 3), 221–226. [Google Scholar]
- Huang, J.H.; Lu, Y.F.; Cheng, F.C.; Lee, J.N.; Tsai, L.C. Correlation of magnesium intake with metabolic parameters, depression and physical activity in elderly type 2 diabetes patients: A cross-sectional study. Nutr. J. 2012, 11, 41. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, S.K. Low dietary calcium is associated with self-rated depression in middle-aged Korean women. Nutr. Res. Pract. 2012, 6, 527–533. [Google Scholar] [CrossRef]
- Mohaddesi, H.; Saei Ghare Naz, M.; Najarzadeh, M.; Yeganehpour, M.; Khalkhali, H. Correlation between Depression with Serum Levels of Vitamin D, Calcium and Magnesium in Women of Reproductive Age. J. Caring Sci. 2019, 8, 117–119. [Google Scholar] [CrossRef]
- Camardese, G.; De Risio, L.; Pizi, G.; Mattioli, B.; Buccelletti, F.; Serrani, R.; Leone, B.; Sgambato, A.; Bria, P.; Janiri, L. Plasma magnesium levels and treatment outcome in depressed patients. Nutr. Neurosci. 2012, 15, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Verma, P.; Gupta, S.; Saxena, J. Variability in serum electrolytes in different grades of depression. Indian J. Physiol. Pharmacol. 2011, 55, 67–71. [Google Scholar] [PubMed]
- Szkup, M.; Jurczak, A.; Brodowska, A.; Brodowsska, A.; Noceń, I.; Chlubek, D.; Laszczyńska, M.; Karakiewicz, B.; Grochans, E. Analysis of Relations Between the Level of Mg, Zn, Ca, Cu, and Fe and Depressiveness in Postmenopausal Women. Biol. Trace Elem. Res. 2017, 176, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Kennedy, A.G.; Rose, G.L.; Crocker, A.; Littenberg, B. The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Pourmehr, H.; Dolatkhah, N.; Gassab-Abdollahi, N.; Farrin, N.; Mojtahedi, M.; Farshbaf-Khalili, A. Screening of depression in overweight and obese pregnant women and its predictors. J. Obstet. Gynaecol. Res. 2019, 45, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Nahar, Z.; Azad, M.A.; Rahman, M.A.; Rahman, M.A.; Bari, W.; Islam, S.N.; Islam, M.S.; Hasnat, A. Comparative analysis of serum manganese, zinc, calcium, copper and magnesium level in panic disorder patients. Biol. Trace Elem. Res. 2010, 133, 284–290. [Google Scholar] [CrossRef]
- Garalejić, E.; Bojović-Jović, D.; Damjanović, A.; Arsić, B.; Pantić, I.; Turjacanin-Pantelić, D.; Perović, M. Hamilton anxiety scale (HAMA) in infertile women with endometriosis and its correlation with magnesium levels in peritoneal fluid. Psychiatr. Danub. 2010, 21, 64–67. [Google Scholar]
- Shohag, H.; Ullah, A.; Qusar, S.; Rahman, M.; Hasnat, A. Alterations of serum zinc, copper, manganese, iron, calcium, and magnesium concentrations and the complexity of interelement relations in patients with obsessive-compulsive disorder. Biol. Trace Elem. Res. 2012, 148, 275–280. [Google Scholar] [CrossRef]
- Ruljancic, N.; Mihanovic, M.; Cepelak, I.; Bakliza, A. Platelet and serum calcium and magnesium concentration in suicidal and non-suicidal schizophrenic patients. Psychiatry Clin. Neurosci. 2013, 67, 154–159. [Google Scholar] [CrossRef]
- Raj, K.S.; Keane-Miller, C.; Golden, N.H. Hypomagnesemia in adolescents with eating disorders hospitalized for medical instability. Nutr. Clin. Pract. 2012, 27, 689–694. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Gao, W.; Lin, N.; Li, R.; Zhao, Z. Blood Levels of Trace Elements in Children with Attention-Deficit Hyperactivity Disorder: Results from a CaseControl Study. Biol. Trace Elem. Res. 2019, 187, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.M.; El-Mazary, A.A.; Maher, R.M.; Saber, M.M. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011, 37, 60. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chao, J.C.; Chernova, L.N.; Shakieva, R.A.; Kopylov, P.Y.; Skalny, A.A. Serum zinc, copper, zinc-to-copper ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD). J. Trace Elem. Med. Biol. 2020, 58, 126445. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Mao, S.S.; Lin, X.; Yang, R.W.; Zhu, Z.W. Evaluation of Whole Blood Trace Element Levels in Chinese Children with Autism Spectrum Disorder. Biol. Trace Elem. Res. 2019, 191, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Saldanha Tschinkel, P.F.; Bjørklund, G.; Conón, L.Z.Z.; Chirumbolo, S.; Nascimento, V.A. Plasma concentrations of the trace elements copper, zinc and selenium in Brazilian children with autism spectrum disorder. Biomed. Pharmacother. 2018, 106, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, L.; Zhang, Q.; Chen, L.; Dai, Y.; Liu, L.; Feng, J.; Cai, X.; Cheng, Q.; Chen, J.; et al. Vitamin and mineral status of children with autism spectrum disorder in Hainan Province of China: Associations with symptoms. Nutr. Neurosci. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Littenberg, B.; MacLean CDKennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef]
- Ryszewska-Pokraśniewicz, B.; Mach, A.; Skalski, M.; Januszko, P.; Wawrzyniak, Z.M.; Poleszak, E.; Nowak, G.; Pilc, A.; Radziwoń-Zaleska, M. Effects of Magnesium Supplementation on Unipolar Depression: A Placebo-Controlled Study and Review of the Importance of Dosing and Magnesium Status in the Therapeutic Response. Nutrients 2018, 10, 1014. [Google Scholar] [CrossRef]
- Fard, F.E.; Mirghafourvand, M.; Mohammad-Alizadeh Charandabi, S.; Farshbaf-Khalili, A.; Javadzadeh, Y.; Asgharian, H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health 2017, 57, 1115–1128. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Egglestonb, M.J.F.; Darlinga, K.A.; Stevensc, A.J.; Kennedyc, M.A.; Frampton, C.M. Can we predict treatment response in children with ADHD to a vitamin-mineral supplement? An investigation ino pre-treatment nutrient serum levels, MTHFR status, clinical correlates and demographic variables. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.M.; Atlas, S.E.; Qadir, S.; Musselman, D.; Goldberg, S.; Woolger, J.M.; Corredor, R.; Abbas, M.H.; Arosemena, L.; Caccamo, S.; et al. Double-blind, andomized crossover study of intravenous infusion of magnesium sulfate versus 5% dextrose on depressive symptoms in adults with treatment-resistant depression. Psychiatry Clin. Neurosci. 2017, 71, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Jabotinsky-Rubin, K.; Durst, R.; Levitin, L.A.; Moscovich, D.G.; Silver, H.; Lerner, J.; Van Praag, H.; Gardner, E.L. Effects of haloperidol on human plasma magnesium. J. Psychiatr. Res. 1993, 27, 155–159. [Google Scholar] [CrossRef]
- Athanassenas, G.; Papadopoulos, E.; Kourkoubas, A.; Tsitourides, S.; Gabriel, J.; Hoïdas, S.; Frangos, E. Serum calcium and magnesium levels in chronic schizophrenics. J. Clin. Psychopharmacol. 1983, 3, 212–216. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Verlaet, A.A.; Noriega, D.B.; Hermans, N.; Savelkoul, H.F. Nutrition, immunological mechanisms and dietary immunomodulation in ADHD. Eur. Child Adolesc. Psychiatry 2014, 23, 519–529. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; pp. 5–25. ISBN 978-0-89042-555-8. [Google Scholar]
- Mlyniec, K. Zinc in the glutamatergic theory of depression. Curr. Neuropharmacol. 2015, 13, 505–513. [Google Scholar] [CrossRef]
- Ehlert, U.; Gaab, J.; Heinrichs, M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus-pituitary-adrenal axis. Biol. Psychol. 2001, 57, 141–152. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Coan, E.; Collingridge, G. Magnesium ions block an N-methyl-D-aspartate receptor- mediated component of synaptic transmission in rat hippocampus. Neurosci. Lett. 1985, 53, 21–26. [Google Scholar] [CrossRef]
- Billyard, A.J.; Eggett, D.L.; Franz, K.B. Dietary magnesium deficiency decreases plasma melatonin in rats. Magnes. Res. 2006, 19, 157–161. [Google Scholar]
- Serefko, A.; Szopa, A.; Poleszak, E. Magnesium and depression. Magnes. Res. 2016, 29, 112–119. [Google Scholar] [CrossRef]
- Poleszak, E.; Wlaź, P.; Kedzierska, E.; Radziwon-Zaleska, M.; Pilc, A.; Fidecka, S.; Nowak, G. Effects of acute and chronic treatment with magnesium in the forced swim test in rats. Pharmacol. Rep. 2005, 57, 654–658. [Google Scholar] [PubMed]
- Poleszak, E.; Wlaź, P.; Kedzierska, E.; Nieoczym, D.; Wyska, E.; Szymura-Oleksiak, J.; Fidecka, S.; Radziwoń-Zaleska, M.; Nowak, G. Immobility stress induces depression-like behavior in the forced swim test in mice: Effect of magnesium and imipramine. Pharmacol. Rep. 2006, 58, 746–752. [Google Scholar]
- Singewald, N.; Sinner, C.; Hetzenauer, A.; Sartori, S.B.; Murck, H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice-influence of desipramine and Hypericum perforatum extract. Neuropharmacology 2004, 47, 1189–1197. [Google Scholar] [CrossRef]
- Poleszak, E.; Wlaź, P.; Kedzierska, E.; Nieoczym, D.; Wróbel, A.; Fidecka, S.; Pilc, A.; Nowak, G. NMDA/glutamate mechanism of antidepressant-like action of magnesium in forced swim test in mice. Pharmacol. Biochem. Behav. 2007, 88, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Ketamine, magnesium and major depression--from pharmacology to pathophysiology and back. J. Psychiatr. Res. 2013, 47, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: The Hordaland Health Study. Aust. N. Z. J. Psychiatry 2009, 431, 45–52. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Title | Sample | Study Type | Psychiatric Disorder | Psychopathological Scale | Results | Direction of Evidence |
|---|---|---|---|---|---|---|---|
| [53] | Total and ionized calcium and magnesium are significantly lowered in drug-naïve depressed patients: effects of antidepressants and associations with immune activation | (a) 140 MDD + 40 hc; treatment in 48 MDD; (b) 44 patients, 2 months with blood samplings (baseline and during treatment) | (a) case-control study; (b) prospective study | Depression | BDI-II | In MDD patients Serum Ca and Mg (total and ionized) were significantly lower compared with controls. Antidepressants increased Ca and lowered Mg levels. Significant and inverse correlations between the BDI-II scores from baseline to endpoint and Ca (both total and ionized), but not Mg levels. Antidepressants probably reduced Mg levels as a side effect | (+) |
| [54] | Biomarkers in Drug Free Subjects with Depression: Correlation with Tryptophan | 96 (48 depression, 48 controls) | cross-sectional study | Depression | HAMD | Depression is associated with deficiency of TRP, Se, Vit D, Mg. (The association among TRP and other biomarkers is non-significant) | (+) |
| [55] | Comparative analysis of serum zinc, copper, manganese, iron, calcium, and magnesium level and complexity of interelement relations in generalized anxiety disorder patients | 50 GAD, 51 hc | comparative study | GAD | DSM-IV criteria | Ca and Mg concentration between patient and control groups were not significant (p > 0.05) | (−) |
| [56] | Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: a case-control study | 247 patients and 248 | prospective case-control study | Depression | DSM-5 criteria | Decreased concentrations of Ca and Mg, Fe, manganese, selenium, and zinc in MDD patients compared with control subjects. Data obtained from different inter-element relations in MDD patients and control subjects strongly suggest that there is a disturbance in the element homeostasis | (+) |
| [57] | Serum Vitamin D and Magnesium levels in a psychiatric cohort | 73 psychiatric day treatment unit cohort | cross-sectional analysis | Miscellaneous | ICD-10 criteria | The percentage of patients who were magnesium deficient was 78.6% (n = 22/28) | (+) |
| [58] | Depression in elderly type 2 diabetes | 210 type 2 diabetes patients aged 65 years and above | cross-sectional study | Depression in elderly type 2 diabetes | DSM-IV criteria | Among all patients, 88.6% had magnesium intake which was less than the dietary reference intake, and 37.1% had hypomagnesaemia. The odds of depression, central obesity, high body fat percentage, and high body mass index were significantly lower with increasing quartile of magnesium intake (p for trend < 0.05). The majority of elderly type 2 diabetes who have low magnesium intake may compound this deficiency with metabolic abnormalities and depression | (+) |
| [59] | Low dietary calcium is associated with self-rated depression in middle-aged Korean women | 105 women age 41–57; 51 pre-meno-pausal, 54 post-meno-pausal | case-control study | Depression in middle-aged women | SDS | No significant differences in serum levels of Ca and Mg among the three groups with different severity of symptoms. Negative correlations between SDS and Ca intake and animal Ca after adjusting for age, menopause and energy intake | (−) |
| [60] | Correlation between Depression with Serum Levels of Vitamin D, Calcium and Magnesium in Women of Reproductive Age | 100 women 15–44 years old | cross-sectional study | Depression | BDI-II | Women’s depression scores showed a significant inverse correlation with the serum level of vitamin D (r = -0.21, p = 0.03). No significant correlations with serum levels of calcium and magnesium | (−) |
| [61] | Plasma magnesium levels and treatment outcome in depressed patients | 123 outpatients | observational study | Depression | HAMD; HAMA; (DRRS); (SHAPS) | No association between Mg levels and psychopathological severity Patients who responded to antidepressant treatment showed higher Mg levels and higher retardation scores at basal evaluation in comparison with non-responders | (−) |
| [62] | Variability in serum electrolytes in different grades of depression | 100 MDD (age 35–45), 100 hc matched) | cross-sectional study | Depression | DSM-IV, ICD-10 criteria; HAMD | All the depression patients were having higher level of Na, K, and Ca and lower level of Mg. Multiple comparison revealed highly statistically significant difference between the levels of serum Ca and Mg in three levels of severity (mild, moderate and severe depression) | (+) |
| [63] | Analysis of Relations Between the Level of Mg, Zn, Ca, Cu, and Fe and Depressiveness in Postmenopausal Women | 198 healthy post-meno-pausal women (age 56.26 ± 5.55) | cross-sectional study | Depression | Depressive symptoms in postmenopausal women | Women with depressive symptoms had the lowest Mg levels (14.28 ± 2.13 mg/l), the highest in women without depressive symptoms (16.30 ± 3.51 mg/L), (p ≤ 0.05). Authors indicate a higher vulnerability to depression in a group of women with lower levels of Mg and higher levels of Cu | (+) |
| [64] | The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population | 3604 adults | cross-sectional analysis | Depression | PHQ | The relationship between serum magnesium and depression using univariate analyses showed a significant effect when measured by the PHQ-2 (−0.19 points/mg/dL; 95% CI −0.31, −0.07; p = 0.001) and the PHQ-9 (−0.93 points/mg/dL; 95% CI −1.81, −0.06; p = 0.037). This relationship was strengthened after adjusting for covariates | (+) |
| [65] | Screening depression in overweight and obese pregnant women and its predicts | 232 overweight or obese pregnant women | cross-sectional study | Depression in pregnancy | Edinburgh Postnatal Depression Scale | Protein, fat, magnesium had positive significant correlation with depression | (+) |
| [66] | Comparative analysis of serum manganese, zinc, calcium, copper and magnesium level in panic disorder patients | 54 panic disorder. + 52 hc | comparative analysis | Panic disorder | DSM-IV criteria | Serum concentration of Zn decreased significantly (p = 0.001) in patient group. Otherwise concentration of Mn, Ca, Cu, and Mg were not significant. | (−) |
| [67] | Hamilton anxiety scale (HAMA) in infertile women with endometriosis and its correlation with magnesium levels in peritoneal fluid | 40 endo-metriosis, 47 hc undergoing laparo-scopy (other causes of infertility) | prospective study | Anxiety disorders | HAMA | In infertile women without endometriosis there was a correlation between Mg concentration in peritoneal fluid and HAMA score. No such correlation was found in the women with endometriosis | (+) |
| [68] | Alterations of serum zinc, copper, manganese, iron, calcium, and magnesium concentrations and the complexity of interelement relations in patients with obsessive-compulsive disorder | 48 OCD + 48 hc | cross-sectional study | OCD | Yale-Brown Obsessive Compulsive Scale (YBOCS) | In patients’ serum, zinc, iron, and magnesium concentrations decreased significantly (p < 0.05) compared to the controls | (+) |
| [69] | Platelet and serum calcium and magnesium concentration in suicidal and non-suicidal schizophrenic patients | 23 schizophrenics (ICD-10) with attempted suicide + 48 without suicidal behavior + 99 hc | cross sectional study, with 3 groups used for comparison | Suicidal and non-suicidal schizophrenic patients | semi-structured interview (ICD-10 criteria); | A higher Ca/Mg ratio in the platelets of non-suicidal patients confirms indirect higher Ca concentration. Higher Mg concentration in the platelets of suicidal patients, considered a Ca antagonist, may represent a compensatory attempt to restrain Ca activity | (±) |
| [70] | Hypomagnesemia in adolescents with eating disorders hospitalized for medical instability | 541 hospitalized adolescents aged 10–21 years with an eating disorder from 2007 to 2010 | retrospective study | Eating disorders | DSM-IV criteria; clinical characteristics | 15.9% developed hypomagnesemia. Compared with those with normal serum magnesium levels, patients with hypomagnesemia were older (p = 0.0001), ill longer (p = 0.001), more likely to be purging (p = 0.04), and more likely to have an alkaline urine (p = 0.01). They did not differ in eating disorder diagnosis, BMI, or other electrolyte disturbances. Hypomagnesemia is prevalent in adolescents hospitalized for an eating disorder and is associated with purging and alkaline urine | (±) |
| [71] | Blood Levels of Trace Elements in Children with Attention-Deficit Hyperactivity Disorder: Results from a Case-Control Study | 419 ADHD, 395 hc | case-control study | ADHD | Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales, Conners’ Parent and Teacher Rating Scales (Chinese version), SNAP-IV, Raven’s Progressive Matrices. | Lower zinc levels (p < 0.001) and the number out of normal ranges (p = 0.015) were found in children with ADHD when compared with the normal control group. The difference remained when adjusting the factor of BMI z-score. No significant between-group differences were found in levels of other elements | (+) |
| [72] | Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder | 58 ADHD, age 5–15 + 25 hc, matched | case-control study | ADHD | Conner’s Rating Scales, discriminating between children with ADHD and h.c., as well as severity of ADHD. | Zinc, ferritin and Mg levels were significantly lower than controls (p value 0.04, 0.03 and 0.02 respectively). No significant differences between sub-groups of ADHD | (+) |
| [73] | Serum zinc, copper, zinc-to-copper ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD) | 136 (children) (68 ADHD, 68 hc, matched) | cross-sectional study | ADHD | CD-10 criteria (F90.0) | Cr, Mg, and Zn levels in children with ADHD were 21% (p = 0.010), 4% (p = 0.005), and 7% (p = 0. 001) lower as compared to the healthy controls, respectively. However, the patterns of trace element and mineral levels in ADHD were significantly affected by gender and age. Hypothetically, the observed decrease in essential trace elements, namely Mg and Zn, may significantly contribute to the risk of ADHD or its severity and/or comorbidity | (±) |
| [74] | Evaluation of Whole Blood Trace Element Levels in Chinese Children with Autism Spectrum Disorder | 113 ASD children, 141 age- and gender-matched neurotypical children | ASD | No significant differences in the whole blood Cu, Zn/Cu ratio, Fe, or Mg was detected between the ASD group and the control group | (−) | ||
| [75] | Plasma concentrations of the trace elements copper, zinc and selenium in Brazilian children with autism spectrum disorder. | 23 ASD | cross-sectional study | ASD | DSM-5 criteria | The cohort did not show a marked difference in micro-nutrient intake in relation with their resident geographical area and their dietary habit or metabolic state; a slight difference in the levels of magnesium and phosphorus was retrieved due to sex difference | (−) |
| [76] | Vitamin and mineral status of children with autism spectrum disorder in Hainan Province of China: associations with symptoms. | 274 ASD, 97 age-matched hc (typically developed) | Interventional study | ASD | DSM-5 criteria; (ABC), (SRS), (GDS) | The levels of Ca, Mg, Fe, and zinc in children with ASD were significantly lower than those in TD children | (+) |
| Author, Year | Sample | Study Type | Psychiatric Disorder | Psycho-Pathological Scale | Treatment | Treatment Duration | Results | Outcome | Direction of Evidences |
|---|---|---|---|---|---|---|---|---|---|
| [77] | 30 patients (7 M, 19 F; mean age 32.20 ± 9.54) + 30 hc (7 M, 20 F; mean age 32.07 ± 7.69) | Double-blind, placebo-controlled trial | Depression | BDI-II | Mg oxide 250 mg/die | 8 weeks | BDI score significantly declined in patients treated with Mg compared to placebo. | Mg supplementation was effective on depression status in depressed patients with Mg deficiency | (+) |
| [78] | 55 randomized to Immediate treatment (22 M, 33 F; mean age 55.2 ± 12.3) + 57 randomized to Delayed treatment (22 M, 35 F; mean age 50.1 ± 13.0) | Randomized case-control clinical trial | Depression | PHQ-9, GAD-7 | 248 mg of elemental Mg/die | 6 weeks | Clinically significant net improvement in PHQ-9 scores of -6.0 points and (GAD-7 in scores of -4.5 points. Similar effects were observed regardless of age, gender, baseline severity of depression, baseline Mg level, or use of antidepressants. Effects were observed within two weeks. | Mg is effective for mild-to-moderate depression in adults | (+) |
| [79] | 17 patients (6 M, 11 F; mean age 48.1±15.5) + 20 hc (10 M, 10 F; mean age 49.7±12.3) | Placebo-controlled study and review | Depression | HAMD, HAMA, CGI | Fluoxetine (20 to 40 mg/die) treatment was augmented with either placebo or Mg 40 mg ×3/die (equivalent to 3.30 mEq of Mg-aspartate) | 8 weeks | Fluoxetine + Mg group showed improvement in HDRS scores at week 8 than the fluoxetine+placebo group, but the difference was not statistically significant. | No significant superiority of Mg augmentation therapy on depressive and anxiety symptoms | (±) |
| [80] | 99 women (3 groups, mean age 29.4 ± 5.4, 26.4 ± 4.8, and 27.6 ± 5.1 respectively) | Randomized controlled clinical trial | Postpartum Depression and Anxiety | EPDS, SSTAI | 27 mg Zn-sulfate or 320 mg Mg-sulfate/die | 8 weeks | No significant difference in EPDS and SSTAI scores between groups. | Mg and zinc did not reduce postpartum anxiety and depressive symptoms | (−) |
| [81] | 71 medication-free ADHD (55 M, 16 F; mean age 9.7 ± 1.5) | randomized clinical trial | ADHD | ADHD-RS-IV, CGI, CGAS | Broad spectrum micronutrient formula (Daily Essential Nutrients, DEN) up to 12 cp/die, vs placebo (no Mg doses available) | 10 weeks | Most children entered the trial with nutrient levels falling within expected ranges. Regression analyses showed varying predictors across outcomes with no one of the predictors being consistently identified across different variables. | Limited value of using serum nutrient levels to predict treatment response, although Authors cannot rule out that other non-assayed nutrient levels may be more valuable | (±) |
| [82] | 264 (69 M, 195 F; mean age 31.6±8.5) | Randomized, single-blind clinical trial | Depressive, anxiety and stress symptoms | DASS-42 | Mg–vitamin B6 combination, respectively 300 mg/die and 30 mg/die or Mg alone (Magnespasmyl) 300 mg/die | 8 weeks | Both treatment arms reduced DASS-42 stress subscale score from baseline to Week 8. Adults with high stress score had a 24% greater improvement with Mg-vitamin B6 versus Mg at Week 8. | Mg supplementation alleviated stress in healthy adults with hypomagnesemia. Addition of vitamin B6 to Mg was not superior to Mg supplementation alone | (+) |
| [83] | 12 patients (3 M, 9 F, mean age 46.5±9) | Double-blind crossover trial | Treatment-Resistant Depression | PHQ-9, HAMD | 4 g Mg-sulfate/die in 5% dextrose or placeboinfusion of 5% dextrose | 8 days intervention periods with a 5 days washoutin between periods | No changes were recorded on the HAMD or PHQ-9 24 h post- treatment; serum Mg increased from baseline to day 7, PHQ-9 decreased from baseline to day 7. | Intravenous infusion of Mg- sulfate did not reduce depressive symptoms in adults with treatment-resistant depression | (−) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. https://doi.org/10.3390/nu12061661
Botturi A, Ciappolino V, Delvecchio G, Boscutti A, Viscardi B, Brambilla P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients. 2020; 12(6):1661. https://doi.org/10.3390/nu12061661
Chicago/Turabian StyleBotturi, Andrea, Valentina Ciappolino, Giuseppe Delvecchio, Andrea Boscutti, Bianca Viscardi, and Paolo Brambilla. 2020. "The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review" Nutrients 12, no. 6: 1661. https://doi.org/10.3390/nu12061661
APA StyleBotturi, A., Ciappolino, V., Delvecchio, G., Boscutti, A., Viscardi, B., & Brambilla, P. (2020). The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients, 12(6), 1661. https://doi.org/10.3390/nu12061661








