Endo-1,3(4)-β-Glucanase-Treatment of Oat β-Glucan Enhances Fermentability by Infant Fecal Microbiota, Stimulates Dectin-1 Activation and Attenuates Inflammatory Responses in Immature Dendritic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Oat β-Glucans and Enzymatic Modification
2.2. Fermentation of Native and Enzymatic-Treated Oat β-Glucans by Infant Fecal Inoculum
2.2.1. Culture Medium
2.2.2. Infant Fecal Inoculum
2.2.3. In Vitro Fermentation
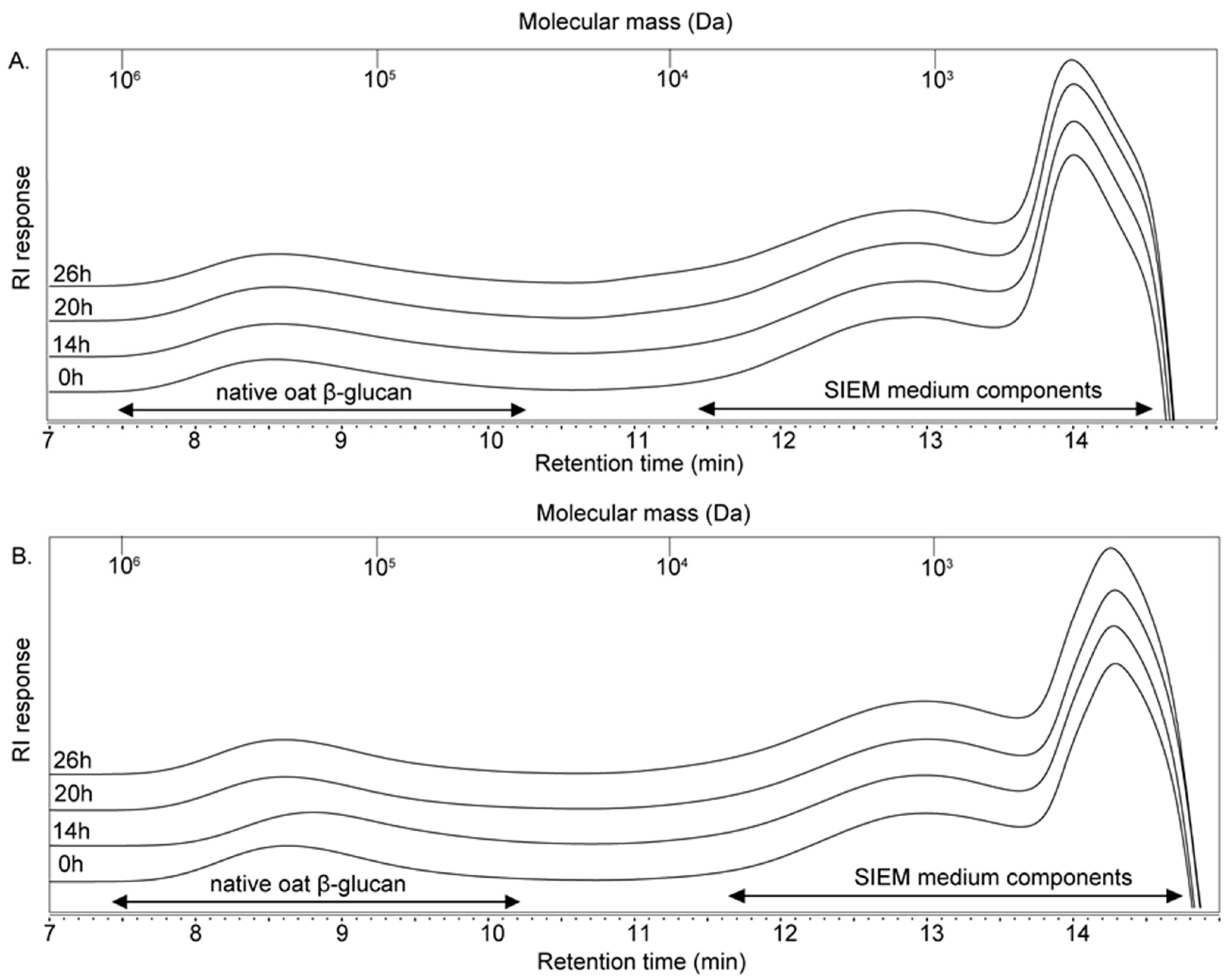
2.3. Fate of OBG and eOBG Upon Fermentation
2.3.1. High Performance Size Exclusion Chromatography (HPSEC)
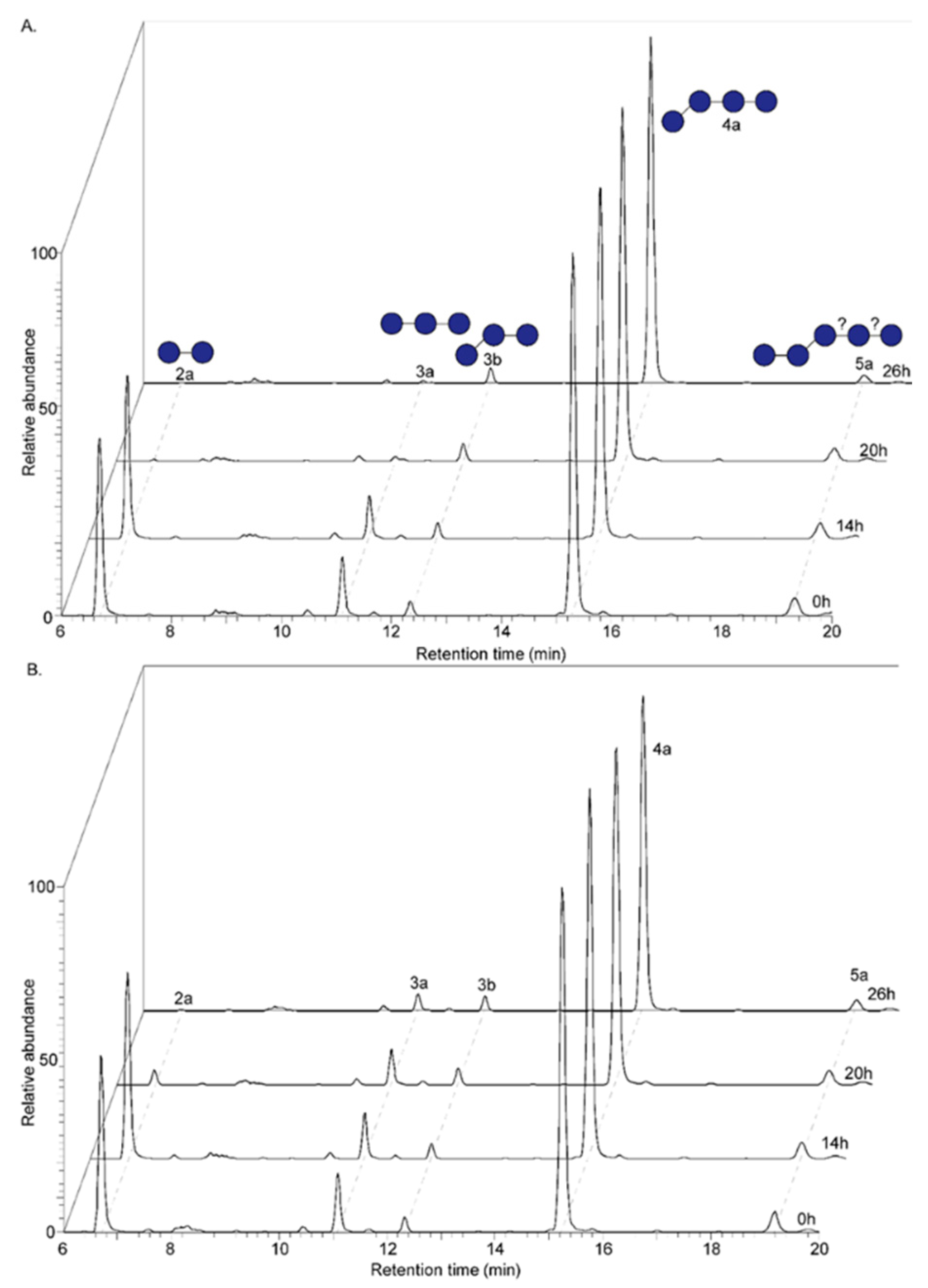
2.3.2. UHPLC-PGC-MS
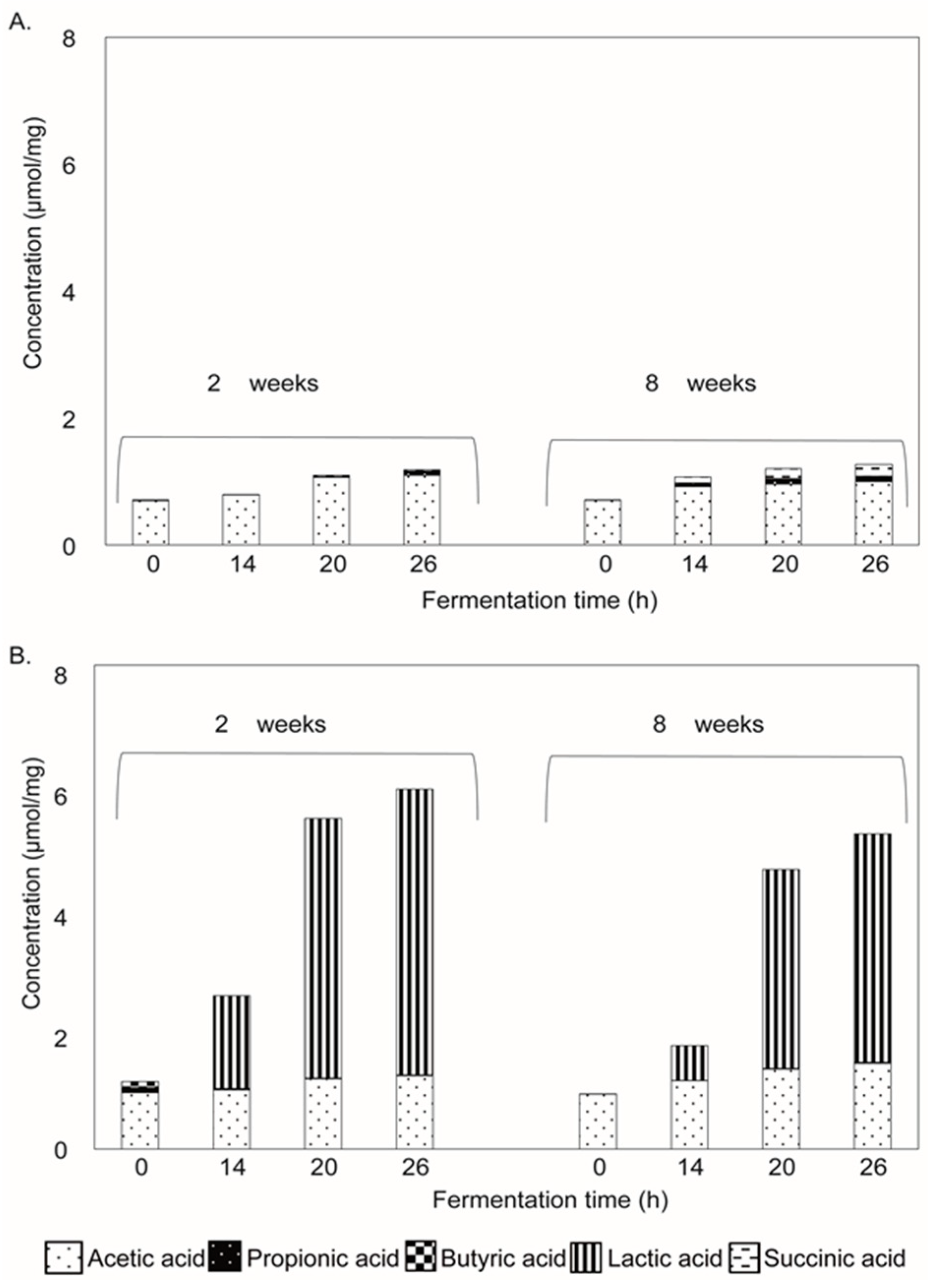
2.4. Production of SCFAs and Other Organic Acids Upon Fermentation
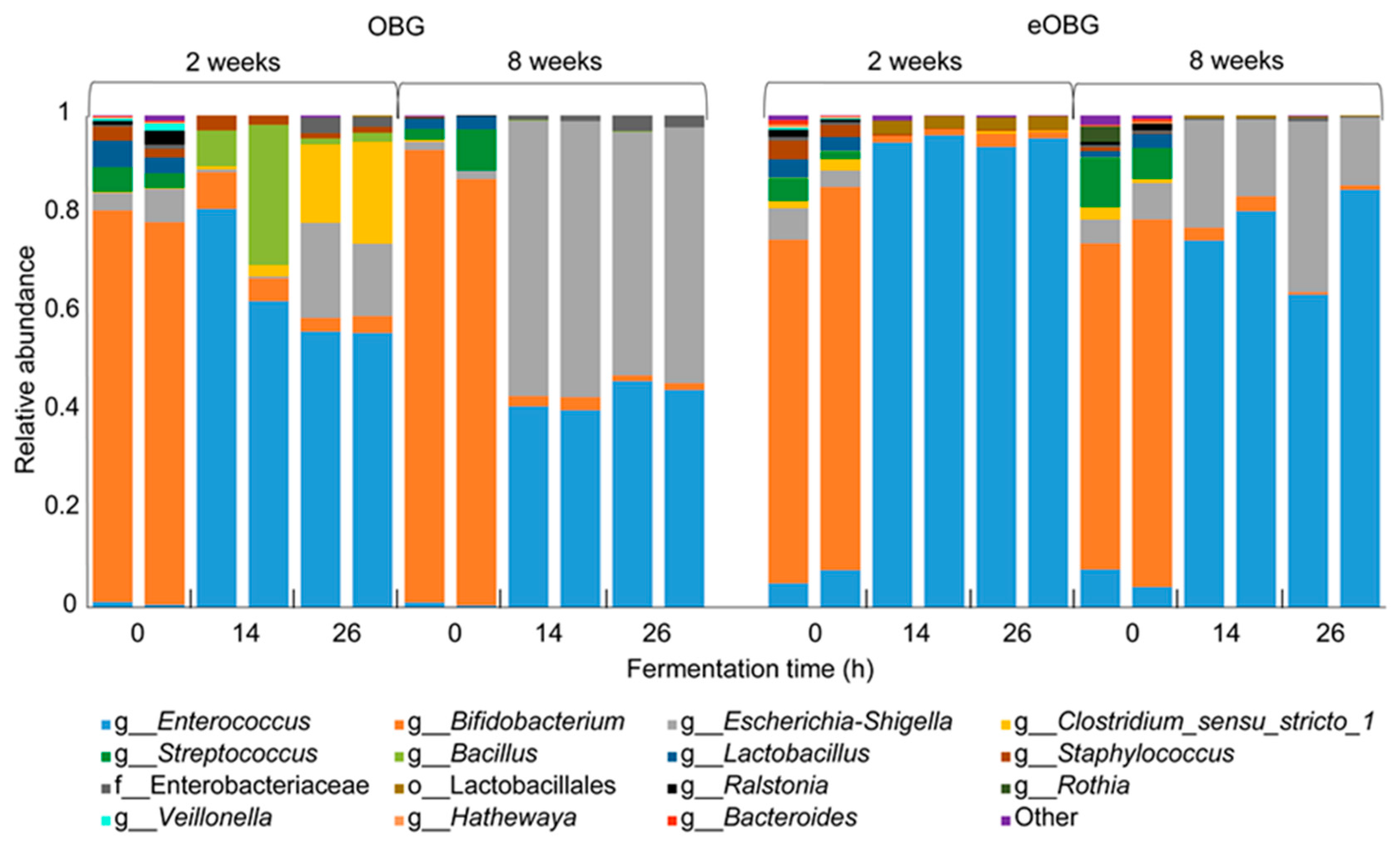
2.5. Microbial Composition Analysis
2.6. Stimulation of DCs with Oat β-Glucan Fermentation Samples
2.6.1. Cell Culture and Stimulation
2.6.2. Assessment of Cytokine Expression
2.7. HEK-Dectin1 Reporter Cell Assays
2.8. Statistical Analysis
3. Results
3.1. Formation of Oligomers Upon Endo-1,3(4)-β-Glucanase Treatment of Native Oat β-Glucan
3.2. Size- and Linkage-Specific Fermentation of Oat β-Glucan (Oligomers) by Infant Fecal Microbiota
3.3. Increase in Enterococcus during Fermentation of Media Supplemented with Native and Enzyme-Treated Oat β-Glucan
3.4. Increase of Specific Enterococcus ASVs during Fermentation of Media Supplemented with Native and Enzyme-Treated Oat β-Glucan is Dependent on Infant Age
3.5. Lactate, a Key Metabolite Formed during Enzyme-Treated Oat β-Glucan Fermentation
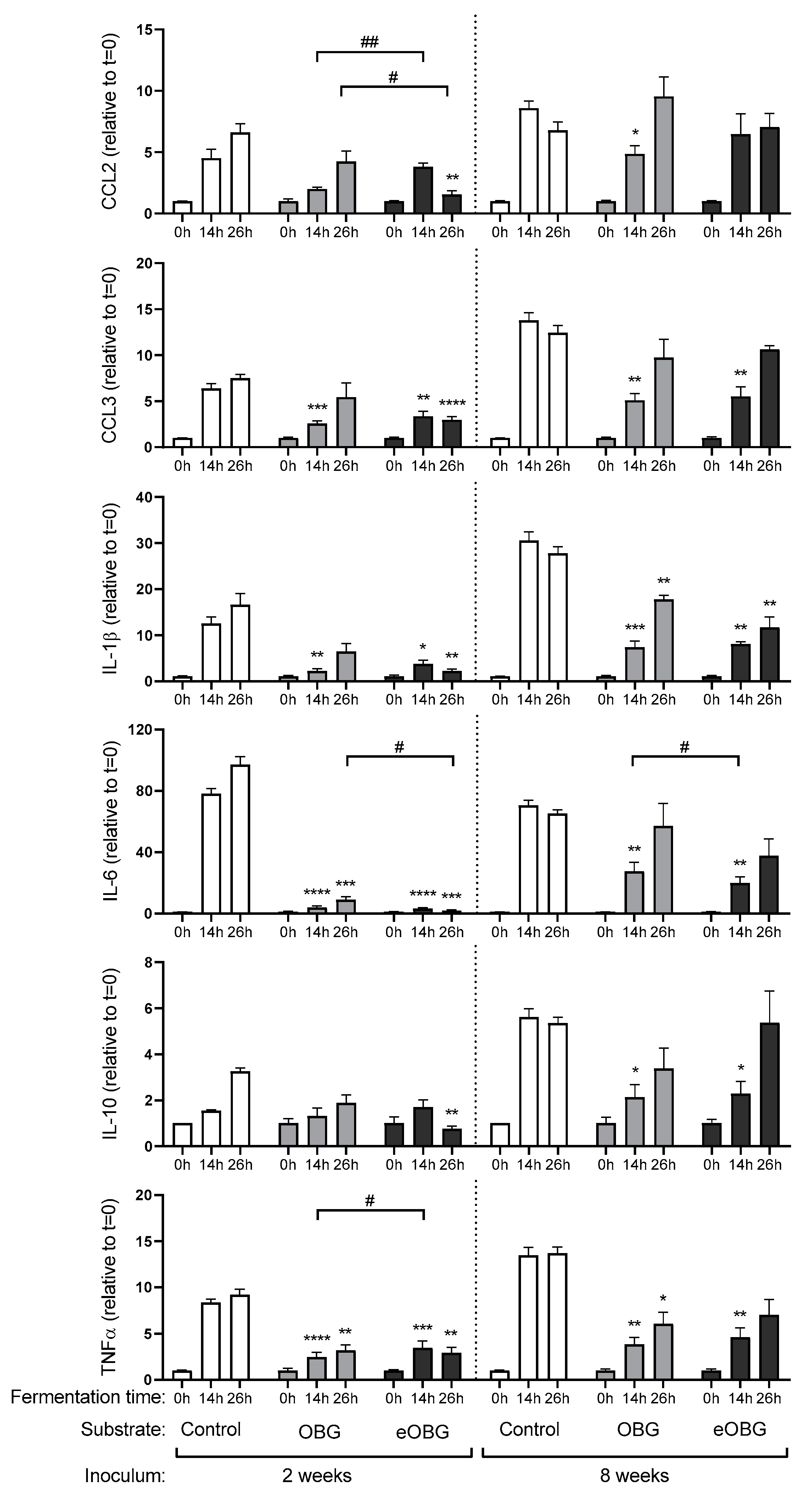
3.6. Cytokine Production by Dendritic Cells (DC) Differs between Fermentation Digesta of Media Supplemented with Native Oat β-Glucan and Enzyme Treated Oat β-Glucan
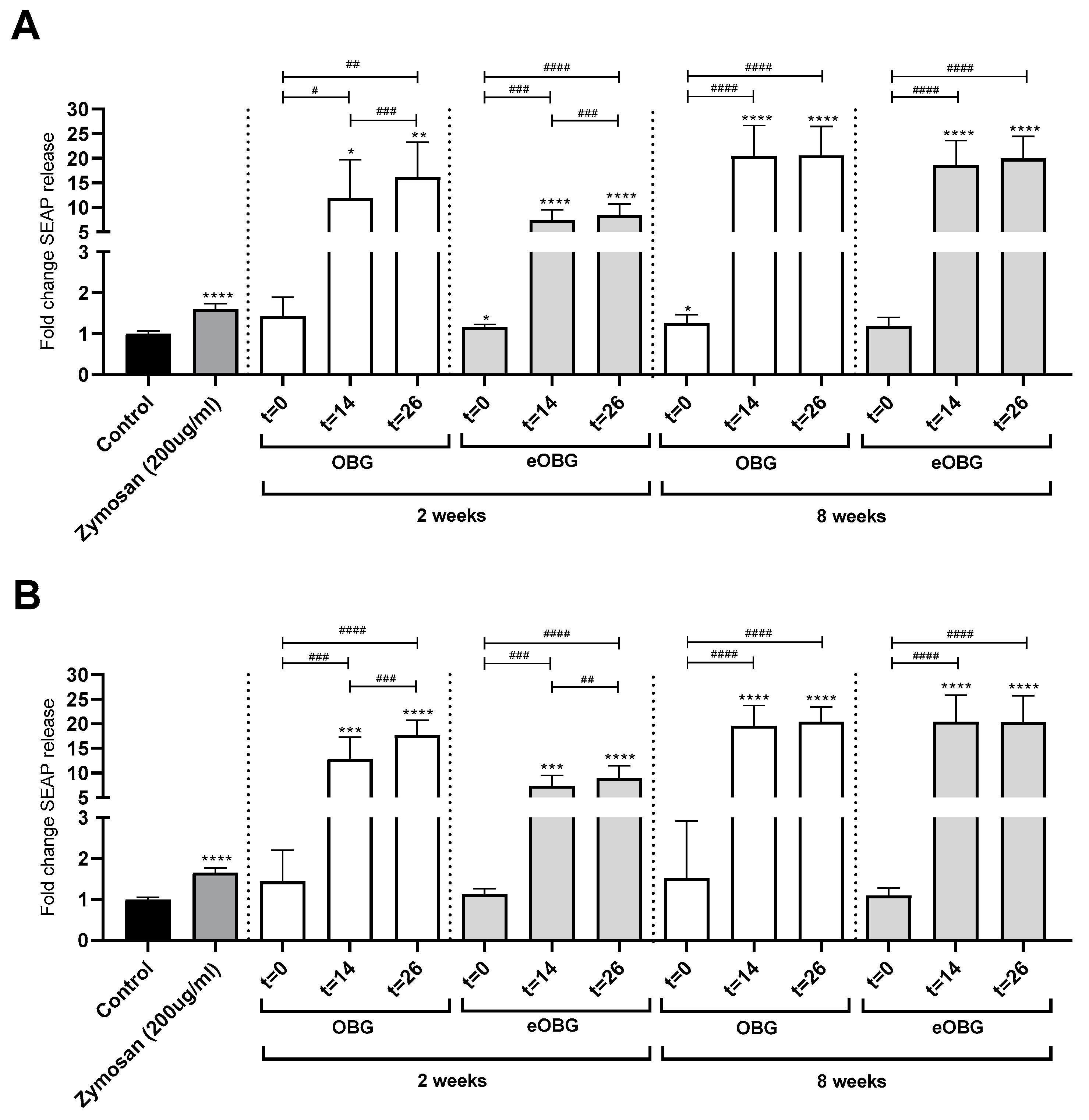
3.7. Fermentation of Media Supplemented with Native Oat β-Glucan and Enzyme-Treated Oat β-Glucan by Infant Fecal Inoculum Enhances Dectin-1 Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rautava, S.; Luoto, R.; Salminen, S.; Isolauri, E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L. Postnatal development of intestinal microflora as influenced by infant nutrition. J. Nutr. 2008, 138, 1791s–1795s. [Google Scholar] [CrossRef]
- WHO. Global Strategy for Infant and Young Child. Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Ann. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Theurich, M.A.; Davanzo, R.; Busck-Rasmussen, M.; Diaz-Gomez, N.M.; Brennan, C.; Kylberg, E.; Baerug, A.; McHugh, L.; Weikert, C.; Abraham, K.; et al. Breastfeeding rates and programs in Europe: A survey of 11 national breastfeeding committees and representatives. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 400–407. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Jeurink, P.V.; van Esch, B.C.; Rijnierse, A.; Garssen, J.; Knippels, L.M. Mechanisms underlying immune effects of dietary oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 572S. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Sahasrabudhe, N.M.; Rosch, C.; Schols, H.A.; Faas, M.M.; de Vos, P. The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.; Yun, C.H.; Van Kessel, A.; Li, B.; Hauta, S.; Laarveld, B. Immunomodulatory activities of oat beta-glucan in vitro and in vivo. Microbiol. Immunol. 1997, 41, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Dokter-Fokkens, J.; de Vos, P. Particulate β-glucans synergistically activate TLR4 and Dectin-1 in human dendritic cells. Mol. Nutr. Food Sci. 2016, 60, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Kanjan, P.; Sahasrabudhe, N.M.; de Haan, B.J.; de Vos, P. Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods 2017, 37, 433–440. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Tian, L.; van den Berg, M.; Bruggeman, G.; Bruininx, E.; Schols, H.A.; Faas, M.M.; de Vos, P. Endo-glucanase digestion of oat β-Glucan enhances Dectin-1 activation in human dendritic cells. J. Funct. Foods 2016, 21, 104–112. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived β-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef]
- Lam, K.L.; Ko, K.C.; Li, X.; Ke, X.; Cheng, W.Y.; Chen, T.; You, L.; Kwan, H.S.; Cheung, P.C. In vitro infant faecal fermentation of low viscosity barley beta-glucan and its acid hydrolyzed derivatives: Evaluation of their potential as novel prebiotics. Molecules 2019, 24, 828. [Google Scholar] [CrossRef]
- Adlerberth, I.; Wold, A.E. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009, 98, 229–238. [Google Scholar] [CrossRef]
- Gu, F.; Borewicz, K.; Richter, B.; van der Zaal, P.H.; Smidt, H.; Buwalda, P.L.; Schols, H.A. In vitro fermentation behavior of isomalto/malto-polysaccharides using human fecal inoculum indicates prebiotic potential. Mol. Nutr. Food Sci. 2018, 62, e1800232. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.M.; Aguirre, M.; Venema, K.; Bosch, G.; Gruppen, H.; Schols, H.A. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agric. Food Chem. 2014, 62, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, M.J.; Vink, J.C.M.; Serierse, R.M.; An, R.; Hermes, G.D.A.; Smidt, H.; Schols, H.A. Pooled faecal inoculum can predict infant prebiotic fermentability despite high inter-individual variability of microbiota composition. 2020. Submitted to Bioactive carbohydrates and dietary fiber. [Google Scholar]
- Borewicz, K.; Gu, F.; Saccenti, E.; Arts, I.C.W.; Penders, J.; Thijs, C.; van Leeuwen, S.S.; Lindner, C.; Nauta, A.; van Leusen, E.; et al. Correlating infant fecal microbiota composition and human milk oligosaccharide consumption by microbiota of 1-month-old breastfed infants. Mol. Nutr. Food Res. 2019, 63, 1801214. [Google Scholar] [CrossRef] [PubMed]
- Ladirat, S.E.; Schuren, F.H.; Schoterman, M.H.; Nauta, A.; Gruppen, H.; Schols, H.A. Impact of galacto-oligosaccharides on the gut microbiota composition and metabolic activity upon antibiotic treatment during in vitro fermentation. FEMS Microbiol. Ecol. 2014, 87, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Nikkilä, J.; Jalanka-Tuovinen, J.; Immonen, O.; Rajilić-Stojanović, M.; Kekkonen, R.A.; Palva, A.; de Vos, W.M. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: Effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J. Microbiol. Methods 2010, 81, 127–134. [Google Scholar] [CrossRef]
- An, R.; Wilms, E.; Smolinska, A.; Hermes, G.D.A.; Masclee, A.A.M.; de Vos, P.; Schols, H.A.; van Schooten, F.J.; Smidt, H.; Jonkers, D.; et al. Sugar beet pectin supplementation did not alter profiles of fecal microbiota and exhaled breath in healthy young adults and healthy elderly. Nutrients 2019, 11, 2193. [Google Scholar] [CrossRef]
- Ramiro-Garcia, J.; Hermes, G.; Giatsis, C.; Sipkema, D.; Zoetendal, E.; Schaap, P.; Smidt, H. NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000Research 2016, 5, 1791. [Google Scholar] [CrossRef]
- Poncheewin, W.; Hermes, G.D.A.; van Dam, J.C.J.; Koehorst, J.J.; Smidt, H.; Schaap, P.J. NG-Tax 2.0: A semantic framework for high-throughput amplicon analysis. Front. Genet. 2020, 10, 1366. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Schols, H.A.; Faas, M.M.; de Vos, P. Arabinoxylan activates Dectin-1 and modulates particulate beta-glucan-induced Dectin-1 activation. Mol. Nutr. Food Sci. 2016, 60, 458–467. [Google Scholar]
- Albrecht, S.; Schols, H.A.; van den Heuvel, E.G.; Voragen, A.G.; Gruppen, H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr. Res. 2011, 346, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; McKain, N.; Wallace, R.J. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Ødum, N.; et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Ghosh, T.S.; Mande, S.S. Global profiling of carbohydrate active enzymes in human gut microbiome. PLoS ONE 2015, 10, e0142038. [Google Scholar] [CrossRef]
- Mittendorf, V.; Thomson, J.A. Cloning of an endo-(1→4)-beta-glucanase gene, celA, from the rumen bacterium Clostridium sp. (‘C. longisporum’) and characterization of its product, CelA, in Escherichia coli. J. Gen. Microbiol. 1993, 139, 3233–3242. [Google Scholar] [CrossRef][Green Version]
- Zappe, H.; Jones, W.A.; Jones, D.T.; Woods, D.R. Structure of an endo-beta-1,4-glucanase gene from Clostridium acetobutylicum P262 showing homology with endoglucanase genes from Bacillus spp. Appl. Environ. Microbiol. 1988, 54, 1289–1292. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Panopoulou, N.; Turunen, K.; Spiliotis, V.; Kyriacou, A. Prebiotic potential of barley derived β-glucan at low intake levels: A randomised, double-blinded, placebo-controlled clinical study. Food Res. Int. 2010, 43, 1086–1092. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Cheng, J.; Ringel, Y.; Salojärvi, J.; Carroll, I.; Palva, A.; de Vos, W.M.; Satokari, R. Intestinal microbiota in healthy U.S. young children and adults—A high throughput microarray analysis. PLoS ONE 2013, 8, e64315. [Google Scholar] [CrossRef]
- Fanaro, S.; Chierici, R.; Guerrini, P.; Vigi, V. Intestinal microflora in early infancy: Composition and development. Acta Paediatr. 2003, 91, 48–55. [Google Scholar] [CrossRef]
- Kirtzalidou, E.I.; Mitsou, E.K.; Pramateftaki, P.; Kyriacou, A. Screening fecal Enterococci from Greek healthy infants for susceptibility to antimicrobial agents. Microb. Drug Resist. 2012, 18, 578–585. [Google Scholar] [CrossRef]
- Obermajer, T.; Grabnar, I.; Benedik, E.; Tušar, T.; Robič Pikel, T.; Fidler Mis, N.; Bogovič Matijašić, B.; Rogelj, I. Microbes in infant gut development: Placing abundance within environmental, clinical and growth parameters. Sci. Rep. 2017, 7, 11230. [Google Scholar] [CrossRef]
- Yiu, J.H.C.; Dorweiler, B.; Woo, C.W. Interaction between gut microbiota and toll-like receptor: From immunity to metabolism. J. Mol. Med. 2016, 95, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.; Hartke, A.; Huycke, M. The physiology and metabolism of Enterococci. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Ratter, J.M.; Rooijackers, H.M.M.; Hooiveld, G.J.; Hijmans, A.G.M.; de Galan, B.E.; Tack, C.J.; Stienstra, R. In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front. Immunol. 2018, 9, 2564. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.C.; Suen, J.-L.; Huang, S.-K.; Huang, S.-C.; Huang, H.-C.; Kuo, H.-C.; Wei, C.-C.; Wang, F.-S.; Yu, H.-R.; Yang, K.D. Dectin-1/Syk signaling is involved in Lactobacillus casei cell wall extract-induced mouse model of Kawasaki disease. Immunobiology 2013, 218, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Bjorksten, B. Evidence of probiotics in prevention of allergy and asthma. Curr. Drug Targets Inflamm. Allergy 2005, 4, 599–604. [Google Scholar] [CrossRef]
- Franz, C.M.; Huch, M.; Abriouel, H.; Holzapfel, W.; Galvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- Are, A.; Aronsson, L.; Wang, S.; Greicius, G.; Lee, Y.K.; Gustafsson, J.-Å.; Pettersson, S.; Arulampalam, V. Enterococcus faecalis from newborn babies regulate endogenous PPARγ activity and IL-10 levels in colonic epithelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1943–1948. [Google Scholar] [CrossRef]
- Wang, S.; Ng, L.H.M.; Chow, W.L.; Lee, Y.K. Infant intestinal Enterococcus faecalis down-regulates inflammatory responses in human intestinal cell lines. World J. Gastroenterol. 2008, 14, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hibberd, M.L.; Pettersson, S.; Lee, Y.K. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS ONE 2014, 9, e97523. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-Glucan receptor, Dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-Glucan size controls Dectin-1-mediated immune responses in human dendritic cells by regulating IL-1β production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- Dzharullaeva, A.S.; Tukhvatulin, A.I.; Erokhova, A.S.; Bandelyuk, A.S.; Polyakov, N.B.; Solovyev, A.I.; Nikitenko, N.A.; Shcheblyakov, D.V.; Naroditsky, B.S.; Logunov, D.Y.; et al. Stimulation of Dectin-1 and Dectin-2 during parenteral immunization, but not mincle, induces secretory IgA in intestinal mucosa. J. Immunol. Res. 2018, 2018, 3835720. [Google Scholar] [CrossRef]
- Mae, M.; Iyori, M.; Yasuda, M.; Shamsul, H.M.; Kataoka, H.; Kiura, K.; Hasebe, A.; Totsuka, Y.; Shibata, K. The diacylated lipopeptide FSL-1 enhances phagocytosis of bacteria by macrophages through a Toll-like receptor 2-mediated signalling pathway. FEMS Immunol. Med. Microbiol. 2007, 49, 398–409. [Google Scholar] [CrossRef]
- Rothfuchs, A.G.; Bafica, A.; Feng, C.G.; Egen, J.G.; Williams, D.L.; Brown, G.D.; Sher, A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J. Immunol. 2007, 179, 3463–3471. [Google Scholar] [CrossRef]
- Hendrickx, A.P.; Schaik, W.v.; Willems, R.J. The cell wall architecture of Enterococcus faecium: From resistance to pathogenesis. Future Microbiol. 2013, 8, 993–1010. [Google Scholar] [CrossRef]
- Elcombe, S.E.; Naqvi, S.; Van Den Bosch, M.W.M.; MacKenzie, K.F.; Cianfanelli, F.; Brown, G.D.; Arthur, J.S.C. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS ONE 2013, 8, e60086. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkerman, R.; Logtenberg, M.J.; An, R.; Van Den Berg, M.A.; de Haan, B.J.; Faas, M.M.; Zoetendal, E.; de Vos, P.; Schols, H.A. Endo-1,3(4)-β-Glucanase-Treatment of Oat β-Glucan Enhances Fermentability by Infant Fecal Microbiota, Stimulates Dectin-1 Activation and Attenuates Inflammatory Responses in Immature Dendritic Cells. Nutrients 2020, 12, 1660. https://doi.org/10.3390/nu12061660
Akkerman R, Logtenberg MJ, An R, Van Den Berg MA, de Haan BJ, Faas MM, Zoetendal E, de Vos P, Schols HA. Endo-1,3(4)-β-Glucanase-Treatment of Oat β-Glucan Enhances Fermentability by Infant Fecal Microbiota, Stimulates Dectin-1 Activation and Attenuates Inflammatory Responses in Immature Dendritic Cells. Nutrients. 2020; 12(6):1660. https://doi.org/10.3390/nu12061660
Chicago/Turabian StyleAkkerman, Renate, Madelon J. Logtenberg, Ran An, Marco Alexander Van Den Berg, Bart J. de Haan, Marijke M. Faas, Erwin Zoetendal, Paul de Vos, and Henk A. Schols. 2020. "Endo-1,3(4)-β-Glucanase-Treatment of Oat β-Glucan Enhances Fermentability by Infant Fecal Microbiota, Stimulates Dectin-1 Activation and Attenuates Inflammatory Responses in Immature Dendritic Cells" Nutrients 12, no. 6: 1660. https://doi.org/10.3390/nu12061660
APA StyleAkkerman, R., Logtenberg, M. J., An, R., Van Den Berg, M. A., de Haan, B. J., Faas, M. M., Zoetendal, E., de Vos, P., & Schols, H. A. (2020). Endo-1,3(4)-β-Glucanase-Treatment of Oat β-Glucan Enhances Fermentability by Infant Fecal Microbiota, Stimulates Dectin-1 Activation and Attenuates Inflammatory Responses in Immature Dendritic Cells. Nutrients, 12(6), 1660. https://doi.org/10.3390/nu12061660







