Consummatory, Feeding Microstructural, and Metabolic Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
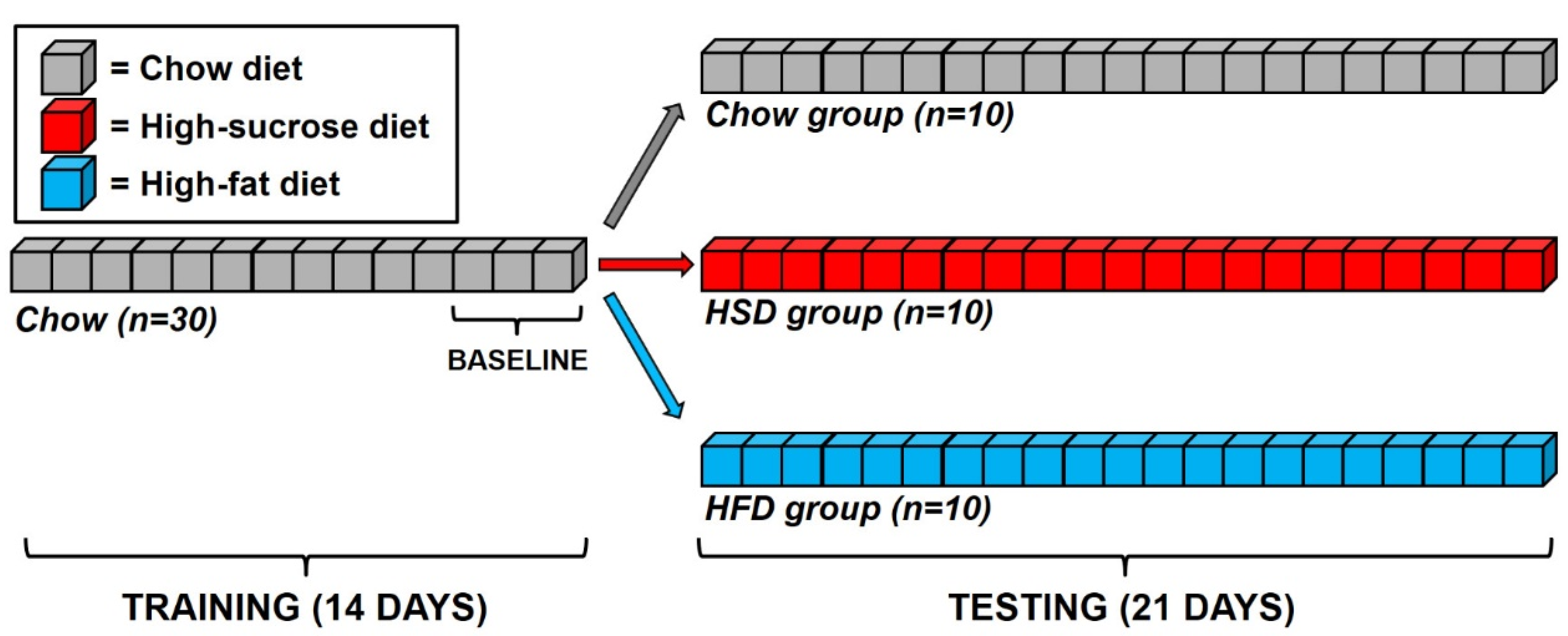
2.1. Subjects
2.2. Apparatus for Self-Administration Procedures
2.3. Operant Binge-Like Eating Procedure in Ad Libitum Fed Rats
2.4. Rate and Regularity of Sustained Eating: Inter Food Interval Analysis
2.5. Home-Cage Food Intake and Body Weights
2.6. Statistical Analysis
3. Results
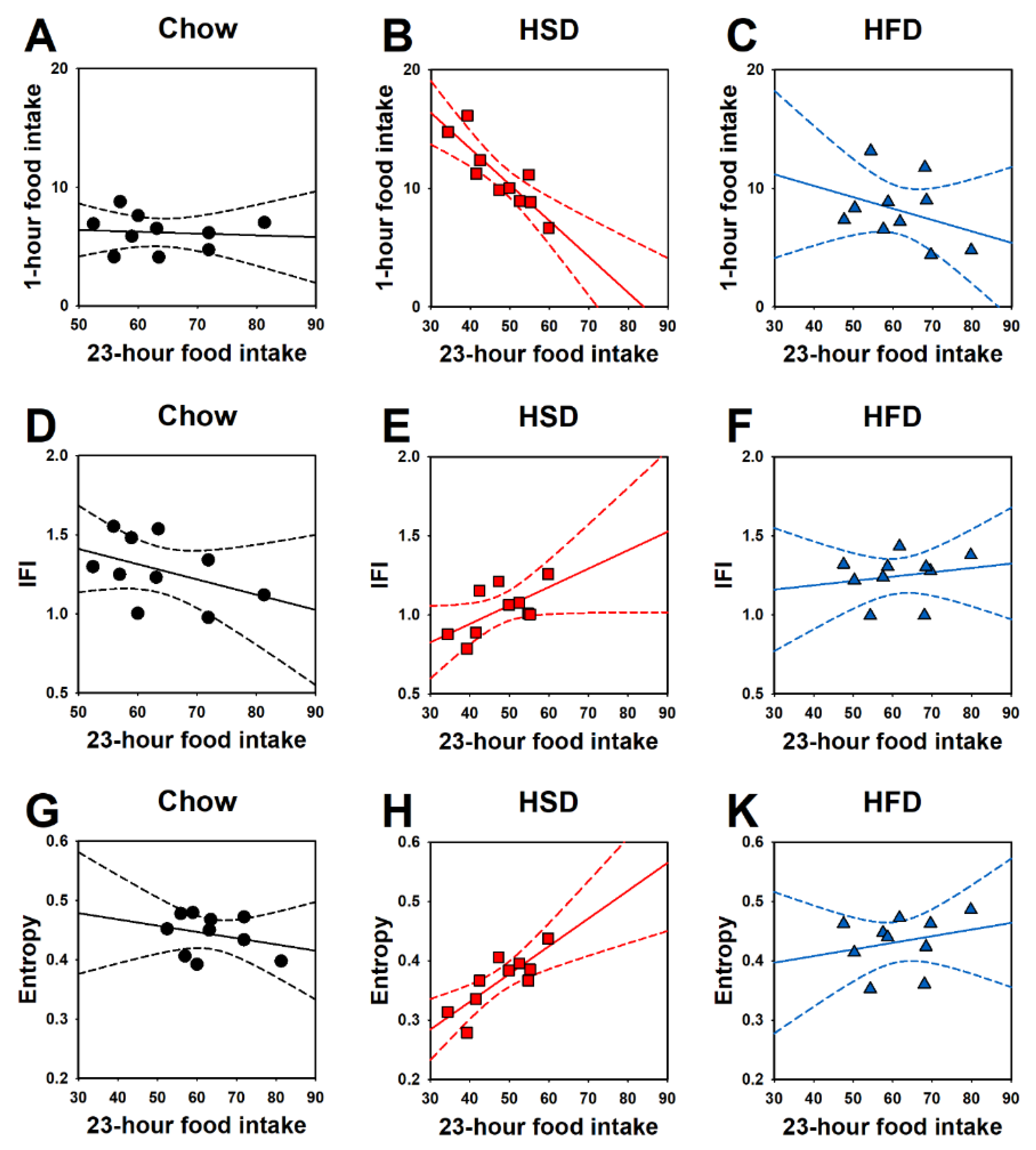
3.1. Consummatory Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet
3.2. Feeding Microstructural Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet
3.3. Metabolic Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet
4. Discussion
4.1. Binge-Like Eating Behavior Development
4.2. Cumulative Intake, Body Weight Gain, and Feed Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Corwin, R.L.; Buda-Levin, A. Behavioral models of binge-type eating. Physiol. Behav. 2004, 82, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yanovski, S.Z. Binge eating disorder and obesity in 2003: Could treating an eating disorder have a positive effect on the obesity epidemic? Int. J. Eat. Disord. 2003, 34 (Suppl. 1), S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Cottone, P.; Sabino, V.; Steardo, L.; Zorrilla, E.P. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.A.; Chiu, W.T.; Deitz, A.C.; Hudson, J.I.; Shahly, V.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry 2013, 73, 904–914. [Google Scholar] [CrossRef]
- Becker, D.F.; Grilo, C.M. Comorbidity of mood and substance use disorders in patients with binge-eating disorder: Associations with personality disorder and eating disorder pathology. J. Psychosom. Res. 2015, 79, 159–164. [Google Scholar] [CrossRef]
- Corwin, R.L.; Wojnicki, F.H. Binge eating in rats with limited access to vegetable shortening. Curr. Protoc. Neurosci. 2006, 36, 9–23. [Google Scholar] [CrossRef]
- Cifani, C.; Polidori, C.; Melotto, S.; Ciccocioppo, R.; Massi, M. A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: Effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology 2009, 204, 113–125. [Google Scholar] [CrossRef]
- Corwin, R.L.; Avena, N.M.; Boggiano, M.M. Feeding and reward: Perspectives from three rat models of binge eating. Physiol. Behav. 2011, 104, 87–97. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Guerdjikova, A.I.; Mori, N.; Casuto, L.S.; McElroy, S.L. Update on Binge Eating Disorder. Med. Clin. N. Am. 2019, 103, 669–680. [Google Scholar] [CrossRef]
- Moore, C.F.; Blasio, A.; Sabino, V.; Cottone, P. Impulsive choice does not predict binge-like eating in rats. Behav. Pharmacol. 2018, 29, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Ferragud, A.; Howell, A.D.; Moore, C.F.; Ta, T.L.; Hoener, M.C.; Sabino, V.; Cottone, P. The Trace Amine-Associated Receptor 1 Agonist RO5256390 Blocks Compulsive, Binge-like Eating in Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Sanchez, C.; Santos, J.W.; Smith, K.L.; Ferragud, A.; Sabino, V.; Cottone, P. Seeking behavior, place conditioning, and resistance to conditioned suppression of feeding in rats intermittently exposed to palatable food. Behav. Neurosci. 2015, 129, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Rao, R.R.; Velazquez-Sanchez, C.; Valenza, M.; Giuliano, C.; Everitt, B.J.; Sabino, V.; Cottone, P. The uncompetitive N-methyl-D-aspartate antagonist memantine reduces binge-like eating, food-seeking behavior, and compulsive eating: Role of the nucleus accumbens shell. Neuropsychopharmacology 2015, 40, 1163–1171. [Google Scholar] [CrossRef]
- Velazquez-Sanchez, C.; Ferragud, A.; Moore, C.F.; Everitt, B.J.; Sabino, V.; Cottone, P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014, 39, 2463–2472. [Google Scholar] [CrossRef]
- Blasio, A.; Steardo, L.; Sabino, V.; Cottone, P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict. Biol. 2014, 19, 652–662. [Google Scholar] [CrossRef]
- Cottone, P.; Wang, X.; Park, J.W.; Valenza, M.; Blasio, A.; Kwak, J.; Iyer, M.R.; Steardo, L.; Rice, K.C.; Hayashi, T.; et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology 2012, 37, 2593–2604. [Google Scholar] [CrossRef]
- Blasio, A.; Rice, K.C.; Sabino, V.; Cottone, P. Characterization of a shortened model of diet alternation in female rats: Effects of the CB1 receptor antagonist rimonabant on food intake and anxiety-like behavior. Behav. Pharm. 2014, 25, 609–617. [Google Scholar] [CrossRef]
- Cottone, P.; Sabino, V.; Steardo, L.; Zorrilla, E.P. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1066–R1076. [Google Scholar] [CrossRef]
- Cottone, P.; Sabino, V.; Steardo, L.; Zorrilla, E.P. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology 2009, 34, 38–49. [Google Scholar] [CrossRef]
- Cottone, P.; Sabino, V.; Steardo, L.; Zorrilla, E.P. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: Evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology 2007, 32, 1069–1081. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cottone, P.; Sabino, V.; Nagy, T.R.; Coscina, D.V.; Zorrilla, E.P. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: Central effects of urocortin 2. J. Physiol. 2007, 583, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Wassum, K.M.; Ostlund, S.B.; Maidment, N.T.; Balleine, B.W. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc. Natl. Acad. Sci. USA 2009, 106, 12512–12517. [Google Scholar] [CrossRef]
- Yoneda, T.; Saitou, K.; Asano, H.; Mizushige, T.; Matsumura, S.; Eguchi, A.; Manabe, Y.; Tsuzuki, S.; Inoue, K.; Fushiki, T. Assessing palatability of long-chain fatty acids from the licking behavior of BALB/c mice. Physiol. Behav. 2009, 96, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Dekhuijzen, A.J.; Bagust, J. Analysis of neural bursting: Nonrhythmic and rhythmic activity in isolated spinal cord. J. Neurosci. Methods 1996, 67, 141–147. [Google Scholar] [CrossRef]
- Iemolo, A.; Ferragud, A.; Cottone, P.; Sabino, V. Pituitary Adenylate Cyclase-Activating Peptide in the Central Amygdala Causes Anorexia and Body Weight Loss via the Melanocortin and the TrkB Systems. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Dore, R.; Valenza, M.; Wang, X.; Rice, K.C.; Sabino, V.; Cottone, P. The inverse agonist of CB1 receptor SR141716 blocks compulsive eating of palatable food. Addict. Biol. 2014, 19, 849–861. [Google Scholar] [CrossRef]
- Anastasio, N.C.; Stutz, S.J.; Price, A.E.; Davis-Reyes, B.D.; Sholler, D.J.; Ferguson, S.M.; Neumaier, J.F.; Moeller, F.G.; Hommel, J.D.; Cunningham, K.A. Convergent neural connectivity in motor impulsivity and high-fat food binge-like eating in male Sprague-Dawley rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1752–1761. [Google Scholar] [CrossRef]
- Davis, J.F.; Melhorn, S.J.; Shurdak, J.D.; Heiman, J.U.; Tschop, M.H.; Clegg, D.J.; Benoit, S.C. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiol. Behav. 2007, 92, 924–930. [Google Scholar] [CrossRef]
- Corwin, R.L. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite 2004, 42, 139–142. [Google Scholar] [CrossRef]
- Archer, Z.A.; Rayner, D.V.; Barrett, P.; Balik, A.; Duncan, J.S.; Moar, K.M.; Mercer, J.G. Hypothalamic energy balance gene responses in the Sprague-Dawley rat to supplementation of high-energy diet with liquid ensure and subsequent transfer to chow. J. Neuroendocr. 2005, 17, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Cottone, P.; Sabino, V.; Roberto, M.; Bajo, M.; Pockros, L.; Frihauf, J.B.; Fekete, E.M.; Steardo, L.; Rice, K.C.; Grigoriadis, D.E.; et al. CRF system recruitment mediates dark side of compulsive eating. Proc. Natl. Acad. Sci. USA 2009, 106, 20016–20020. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, C.F.; Coppotelli, C.; Grigson, P.S.; Mitchell, C.; Flaherty, J.E. Investigation of the devaluation interpretation of anticipatory negative contrast. J. Exp. Psychol Anim Behav Process. 1995, 21, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Grigson, P.S.; Spector, A.C.; Norgren, R. Microstructural analysis of successive negative contrast in free-feeding and deprived rats. Physiol. Behav. 1993, 54, 909–916. [Google Scholar] [CrossRef]
- Blasio, A.; Iemolo, A.; Sabino, V.; Petrosino, S.; Steardo, L.; Rice, K.C.; Orlando, P.; Iannotti, F.A.; Di Marzo, V.; Zorrilla, E.P.; et al. Rimonabant precipitates anxiety in rats withdrawn from palatable food: Role of the central amygdala. Neuropsychopharmacology 2013, 38, 2498–2507. [Google Scholar] [CrossRef]
- Kreisler, A.D.; Mattock, M.; Zorrilla, E.P. The duration of intermittent access to preferred sucrose-rich food affects binge-like intake, fat accumulation, and fasting glucose in male rats. Appetite 2018, 130, 59–69. [Google Scholar] [CrossRef]
- Raymond, N.C.; Neumeyer, B.; Warren, C.S.; Lee, S.S.; Peterson, C.B. Energy intake patterns in obese women with binge eating disorder. Obes. Res. 2003, 11, 869–879. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Leet, M.; Yanovski, J.A.; Flood, M.; Gold, P.W.; Kissileff, H.R.; Walsh, B.T. Food selection and intake of obese women with binge-eating disorder. Am. J. Clin. Nutr. 1992, 56, 975–980. [Google Scholar] [CrossRef]
- Cooke, E.A.; Guss, J.L.; Kissileff, H.R.; Devlin, M.J.; Walsh, B.T. Patterns of food selection during binges in women with binge eating disorder. Int. J. Eat. Disord. 1997, 22, 187–193. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Giunti, E.; Sabino, V.; Cottone, P. Consummatory, Feeding Microstructural, and Metabolic Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet. Nutrients 2020, 12, 1610. https://doi.org/10.3390/nu12061610
Lee HS, Giunti E, Sabino V, Cottone P. Consummatory, Feeding Microstructural, and Metabolic Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet. Nutrients. 2020; 12(6):1610. https://doi.org/10.3390/nu12061610
Chicago/Turabian StyleLee, Harrison Sunjoon, Elisa Giunti, Valentina Sabino, and Pietro Cottone. 2020. "Consummatory, Feeding Microstructural, and Metabolic Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet" Nutrients 12, no. 6: 1610. https://doi.org/10.3390/nu12061610
APA StyleLee, H. S., Giunti, E., Sabino, V., & Cottone, P. (2020). Consummatory, Feeding Microstructural, and Metabolic Effects Induced by Limiting Access to Either a High-Sucrose or a High-Fat Diet. Nutrients, 12(6), 1610. https://doi.org/10.3390/nu12061610



