Abstract
Background: Binge-eating disorder is a pervasive addiction-like disorder that is defined by excessive and uncontrollable consumption of food within brief periods of time. The aim of the current study was to examine the role of the brain noradrenergic system in binge-like eating through the use of the alpha-1 adrenergic receptor antagonist prazosin. Methods: For this purpose, we employed a limited access model whereby male Wistar rats were allowed to nosepoke for either chow (Chow rats) or a sugary, highly palatable food (Palatable rats) for 1 h/day. The effects of prazosin (0, 0.5, 1 and 2 mg/kg, i.p.) were tested in a fixed ratio 1 (FR1) and progressive ratio (PR) schedule of reinforcement. Results: The results show that prazosin preferentially reduced the responses for palatable food in a FR1 reinforcement schedule; when tested in a PR schedule of reinforcement, prazosin increased breakpoint in both Chow and Palatable rats, but more potently and more efficaciously in the latter. Our results suggest that prazosin treatment preferentially increased the motivational properties of the palatable diet. Conclusions: The current findings provide the characterization of the effects of prazosin on binge-like eating and offer support to the existing literature showing the important role of the noradrenergic system in addiction-like behavior.
1. Introduction
Binge-eating disorder (BED) currently affects approximately 8 million people in the United States, and is characterized by excessive and uncontrollable consumption of food within brief periods of time (Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM–5); [1]. Accumulating evidence indicates that BED displays the hallmarks of an addiction-like disorder, as many of the core symptoms of binge eating mirror those seen in drug-addicted individuals [2]. Moreover, evidence suggests that binge eating is associated with neuroadaptations in discrete brain regions that are also present in drug and alcohol addiction [3].
The brain noradrenergic system is well known to play a central role in drug and alcohol abuse and has been implicated in all stages of drug addiction [4]. Interestingly, the noradrenergic system has also been linked to the excessive consumption of highly palatable foods in eating disorders [5]. Indeed, this system stimulates the preferential intake of sugar- and/or fat-rich highly palatable foods, and conversely consumption of palatable foods increases central release of noradrenaline [6].
Prazosin is a centrally acting alpha-1 adrenergic receptor antagonist that has been shown to modulate addiction-like behaviors. Prazosin has been shown to reduce alcohol consumption in both a genetic [7] and environmental [8] model of alcoholism, to inhibit drug-primed reinstatement of cocaine-seeking behavior [9] and to reduce heroin self-administration in rats given extended access to the drug [10]. Importantly, however, to the best of our knowledge, the effects of prazosin specifically on binge eating have not yet been examined.
In the current study, we therefore aimed to determine the effects of systemically administered prazosin on binge-like eating in male rats induced by limiting access to highly palatable food [11,12,13,14,15,16,17]. We used a self-administration model of binge-like eating and employed a fixed ratio 1 (FR1) and progressive ratio (PR) schedule of reinforcement.
2. Materials and Methods
2.1. Animals
Male Wistar rats obtained from Charles River Laboratories (Wilmington, MA) were aged 45 days upon arrival and double- or triple-housed in a humidity- (60 ± 2%) and temperature-controlled (22 ± 1 °C) vivarium maintained on a reverse 12:12-h light/dark cycle (lights off at 11:00-h). Upon arrival, rats were given ad libitum access to water and standard laboratory chow. After a period of acclimation, the standard chow was replaced with an AIN-76A-based diet (5TUM diet formulated as 4–5 g extruded pellets; TestDiet, Richmond, IN), hereafter referred to as ‘Chow A/I’. All procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care, and were approved by the Boston University Medical Campus Institutional Animal Care and Use Committee.
2.2. Drugs
Prazosin hydrochloride was purchased from Alfa Aesar (Ward Hill, MA) and was dissolved in a 10% dimethyl sulfoxide (DMSO) and 90% filtered sterile water vehicle. It was given by intraperitoneal (i.p.) injection at a volume of 1 mL/kg. The doses of prazosin to be administered (0.5, 1 and 2 mg/kg) were based on previous studies [8,10,18].
2.3. Operant Binge-Like Eating Procedure
The rats (n = 39) were allowed to self-administer food and water (100 μL) in daily 1-h sessions in individual operant test chambers previously described in detail by Cottone et al. [19]. All sessions were conducted prior to the onset of the dark cycle. For the FR1 and PR tests, prazosin was administered using a within-subject Latin-square design 30 min prior to the start of the binge sessions. Drug injections were separated by 1–3 washout days after ensuring that responding had returned to baseline.
2.4. Training Phase
The rats were trained on a FR1 reinforcement schedule to nosepoke for 45 mg precision food pellets (5TUM diet; TestDiet, Richmond, IN, USA) that were identical to the home cage ~5 g extruded diet. This was to ensure that the food intake of Chow rats was driven exclusively by homeostatic needs [19,20].
2.5. Fixed Ratio 1 Testing
Once responding in the training phase had stabilized, rats were separated into two groups, ‘Chow’ (n = 19) and ‘Palatable’ (n = 20), matched for body weight, food and water intake in the self-administration sessions. Chow rats served as the control group and received the same 45 mg chow pellets used in the training phase. The Palatable group, on the other hand, were given a chocolate-flavored high sucrose (50% kcal) AIN-76A-based diet (5TUL diet formulated as 45 mg precision food pellets; TestDiet, Richmond, IN), comparable in macronutrient composition and energy density to the chow diet. We have shown that rats strongly prefer this chocolate-flavored diet [19,21]. Drug testing commenced once responding in both groups had stabilized.
2.6. Progressive Ratio Testing
Once testing under FR1 was complete, rats were trained on a PR schedule of reinforcement. The PR sessions employed the same general procedure and length (1 hour) as the FR1 sessions, except that the number of responses required to produce a food pellet increased with successive food reinforcers based on the following shallow exponential progression: response ratio = [4·(e# of reinforcer*0.075) − 3.8], rounded to the nearest integer for more details, see [16,19]. Drug injections commenced after the response rates of Chow (n = 19) and Palatable (n = 20) rats had stabilized.
2.7. Locomotor Activity Test
To determine whether there were any non-specific behavioral disturbances on food responses as a result of prazosin treatment, rats were tested in a 1-h locomotor activity test as previously described [15]. The rats were tested for locomotor activity on two occasions, with vehicle and 2 mg/kg prazosin administered in a within-subject, Latin-square design. The drugs were injected 30 min prior to the start of the test, and the injections were separated by 2 washout days to ensure adequate drug clearance.
2.8. Statistical Analysis
The effects of prazosin on FR1 water responses were analyzed using a two-way mixed design analysis of variance (ANOVA). FR1 food responses, PR breakpoint, and locomotor counts were measured across six 10 min bins and the incremental values were analyzed using a three-way mixed design ANOVA. The cumulative values for each group were analyzed by one-way repeated measures ANOVAs with Bonferroni post-hoc analyses. Homogeneity of variance was assessed and when this requirement was not satisfied, the variables were analyzed as ranked values. All analyses were performed using SPSS v. 19 (SPSS Inc., IBM, Chicago, IL) with significance set at p < 0.05.
3. Results
3.1. Prazosin Effects on FR1 Responding
Although Palatable rats, overall, made a greater number of food responses compared to Chow rats (Figure 1a; diet history: F(1,37) = 11.53, p < 0.01), prazosin reduced responses irrespective of diet history (treatment: F(3,111) = 16.78, p < 0.001) (diet history × treatment: F(3,111) = 0.20, (n.s.). The analysis of the time-course revealed that the effects of prazosin were time-dependent (treatment x time: F(15,555) = 3.64, p < 0.001). Post-hoc tests showed that 2 mg/kg prazosin significantly reduced the food responses of Chow rats in the first 10 min, compared to vehicle. In Palatable rats, prazosin significantly affected the number of food responses at all time points examined; in the first 10 and 20 min, all 3 doses of prazosin reduced responses relative to vehicle treatment. In addition, the 2 mg/kg dose continued to reduce responding for the remainder of the session.
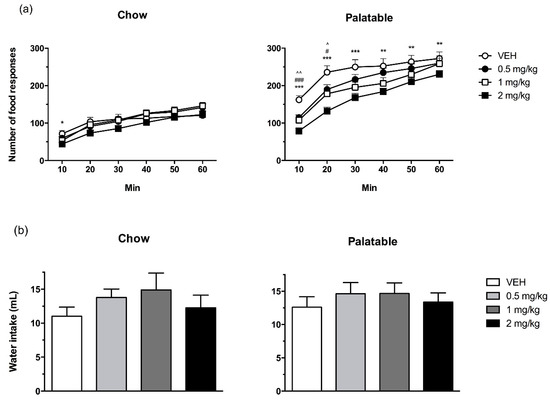
Figure 1.
The effects of prazosin on food responses’ time course (a) and cumulative water intake (b) in Chow and Palatable rats in a fixed ratio 1 reinforcement schedule. Prazosin produced a more pronounced reduction in food responses in Palatable relative to Chow rats, but did not significantly affect water intake in either group. Data are presented as mean + SEM. Symbols indicate a significant difference from vehicle (VEH) within each group; 0.5 mg/kg: ^ p < 0.05, ^^ p < 0.01; 1 mg/kg: # p < 0.05, ### p ≤ 0.001; 2 mg/kg: * p < 0.05, ** p < 0.01, *** p < 0.001.
Chow and Palatable rats did not differ in their responses for water, and prazosin treatment did not reliably affect this measure (Figure 1b; diet history: F(1,37) = 1.01, n.s.; treatment: F(3,111) = 2.64, n.s.; diet history x treatment: F(3,111) = 0.29, (n.s.)).
3.2. Prazosin Effects on PR Responding
Overall, the breakpoint of the Palatable rats was higher than that of Chow rats (Figure 2a; diet history: F(1,37) = 27.94, p < 0.001). Moreover, breakpoint was increased by prazosin preferentially in Palatable rats as compared to Chow rats (treatment: F(3,111) = 18.23, p < 0.001; diet history x treatment: F(3,111) = 3.43, p < 0.02). The analysis of the time-course revealed that the effects of prazosin were time-dependent (treatment × time: F(15,555) = 2.61, p < 0.001). Post-hoc tests revealed that prazosin increased breakpoint in Palatable rats at all doses tested since the first 10 minutes of test and lasted throughout the entire session. On the other hand, prazosin increased the breakpoint in Chow rats only at the highest dose tested (2 mg/kg) starting from the 30-minute bin and until the end of the session.
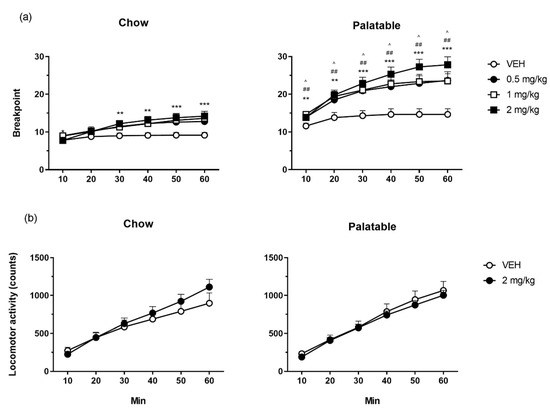
Figure 2.
The effects of prazosin on breakpoint time course in a progressive ratio schedule of reinforcement task (a) and on counts’ time course in a locomotor activity test (b), in Chow and Palatable rats. Prazosin dose-dependently increased PR breakpoint, more potently and more efficaciously in Palatable rats than Chow rats, without significantly altering locomotor activity levels. The data are presented as mean + SEM. Symbols indicate a significant difference from vehicle (VEH) within each group; 0.5 mg/kg: ^ p < 0.05; 1 mg/kg: ## p ≤ 0.01; 2 mg/kg: ** p < 0.01, *** p < 0.001.
3.3. Prazosin Effects on Locomotor Activity
Chow and Palatable rats did not differ significantly in locomotor activity (Figure 2b; diet history: F(1,35) = 0.07, n.s.). There was also no significant effect of prazosin on activity irrespective of diet history and time (treatment: F(1,35) = 0.55, n.s.; diet history x treatment: F(1,35) = 1.91, n.s.; time (diet history x treatment: F(5,175) = 1.39, n.s.).
4. Discussion
We show that systemic administration of the alpha-1 adrenergic receptor antagonist prazosin modulates the motivational component of feeding in an operant model of binge eating. Specifically, prazosin produced a more marked reduction in the number of food responses of Palatable rats as compared to Chow rats under an FR1 reinforcement schedule, while at the same time increased the motivation of Palatable rats to work to obtain food on a PR schedule more potently and more efficaciously than Chow rats. These effects occurred independently of any changes in general behavioral activation as prazosin did not significantly affect water intake, nor did it alter locomotor activity, consistent with what has been shown previously [22].
A time bin analysis of FR1 food responding revealed that prazosin reduced palatable food intake at all doses tested. Drug treatment affected FR1 chow responding as well, but very transiently during the first 10 min of the session and only at the highest dose administered (i.e., 2 mg/kg).
Prazosin increased motivation for food irrespective of the type of diet self-administered; however, drug treatment was more potent and more efficacious in Palatable rats as compared to Chow rats. Indeed, prazosin increased the breakpoint in Palatable rats at all doses tested, since the first 10 min bin, and up to 89.8% of the vehicle condition, while the breakpoint in Chow rats increased only following administration of the highest dose in the second half of the session, and only up to 55.2% of the vehicle condition. Despite the relatively abundant literature on the effects of prazosin on self-administration behavior on an FR1 schedule, the reports on the effects on PR are sparser and to the best of our knowledge, the effects of prazosin in a PR schedule of reinforcement for food have, to our knowledge, not yet been reported.
The opposite effects of prazosin on FR1 and PR responding may, at first, seem conflicting. However, several drug manipulations have been shown to have an effect on PR which is inversely related to their effect on FR1, such that drugs that increase reinforcing efficacy of a substance reduce their self-administration in FR1, and vice versa [23,24,25,26,27,28,29,30,31,32,33,34]. In such cases, while changes in self-administration behavior on an FR1 schedule can be difficult to interpret, alterations in reinforcing efficacy can be better understood using PR schedules [27,28]. In our experiments we show that prazosin increases overall the breakpoint for food, with greater potency and efficacy that for the palatable diet as compared to chow. This effect is by itself a direct evidence for an increase of reinforcing efficacy of food induced by prazosin treatment. Therefore, in absence of any other locomotor, time-related, or rebound effect, the most plausible interpretation of the decrease in high-palatable food self-administration observed in FR1 following prazosin treatment is a compensatory response to an increase in the reinforcing efficacy of food (as measured by breakpoint in PR) [27,28]. A potential argument against this interpretation is that reinforcing efficacy in PR should be affected only by substances which have per se rewarding/reinforcing effects [23,24,25,26], while prazosin does not appear to [35]. The counterargument is that not all drugs that increase a substance’s reinforcing efficacy are rewarding/reinforcing per se [30,36,37,38,39].
The current findings add to an increasing body of literature showing a central role for the brain noradrenergic system in the excessive consumption of highly palatable foods. Prazosin has previously been reported to attenuate cocaine-induced hypophagia [22], reduce self-administration of sucrose solutions [40,41] and inhibit stress-induced reinstatement of food seeking [18]. Moreover, previous studies show that drugs that act as agonists at the alpha-1 adrenergic receptor [42], or block the reuptake of noradrenaline [43,44], reliably induce hypophagia.
A limitation of this study is related to the fact that only male subjects were used. Future studies will be needed to investigate any sex differences in the effects of prazosin through direct comparison between female and male subjects.
In summary, using a limited access model we show that systemic administration of the alpha-1 adrenergic receptor antagonist prazosin dose-dependently decreases palatable food self-administration in FR1 in a binge-like eating model and increased reinforcing efficacy of palatable food, as measured by PR. Prazosin yielded more pronounced effects on the maladaptive feeding behavior of male rats given highly palatable food in comparison to those given regular chow. The present findings provide further evidence of the involvement of the brain noradrenergic system in binge eating and contribute to an expanding body of literature showing a central role for this system in addictive behaviors.
Author Contributions
C.H., V.S., P.C. conceived and designed research. C.H. performed the study. C.H. performed the analyses. C.H., V.S., P.C. prepared and finalized the original manuscript. V.S., P.C. provided funding. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by grants (DA030425, MH091945 and MH093650) from the National Institute on Drug Abuse (NIDA) and the National Institute of Mental Health (NIMH), and by Boston University’s Undergraduate Research Opportunities Program (UROP). PC is currently supported by the Peter Paul Career Development Professorship, and vs. receives funding from the McManus Charitable Trust. The authors declare no conflicts of interest.
Acknowledgments
We are especially grateful to Malk Beydoun and Adam Howell for their technical assistance and help in data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Corwin, R.L.; Grigson, P.S. Symposium overview—food addiction: Fact or fiction? J. Nutr. 2009, 139, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Robbins, T.W. The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biol. Psychiatry 2013, 73, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J. Elevated norepinephrine may be a unifying etiological factor in the abuse of a broad range of substances: Alcohol, nicotine, marijuana, heroin, cocaine, and caffeine. Subst. Abus. Res. Treat. 2013, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Gestel, M.; Kostrzewa, E.; Adan, R.; Janhunen, S. Pharmacological manipulations in animal models of anorexia and binge eating in relation to humans. Br. J. Pharmacol. 2014, 171, 4767–4784. [Google Scholar] [CrossRef] [PubMed]
- Paez, X.; Stanley, B.G.; Leibowitz, S.F. Microdialysis analysis of norepinephrine levels in the paraventricular nucleus in association with food intake at dark onset. Brain Res. 1993, 606, 167–170. [Google Scholar] [CrossRef]
- Rasmussen, D.D.; Alexander, L.L.; Raskind, M.A.; Froehlich, J.C. The α1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in Alcohol-Preferring (P) rats. Alcohol. Clin. Exp. Res. 2009, 33, 264–272. [Google Scholar] [CrossRef]
- Walker, B.M.; Rasmussen, D.D.; Raskind, M.A.; Koob, G.F. α1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol 2008, 42, 91–97. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Kosten, T.A. Prazosin, an α-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol. Psychiatry 2005, 57, 1202–1204. [Google Scholar] [CrossRef]
- Greenwell, T.N.; Walker, B.M.; Cottone, P.; Zorrilla, E.P.; Koob, G.F. The α1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol. Biochem. Behav. 2009, 91, 295–302. [Google Scholar] [CrossRef]
- Moore, C.F.; Blasio, A.; Sabino, V.; Cottone, P. Impulsive choice does not predict binge-like eating in rats. Behav. Pharmacol. 2018, 29, 726–731. [Google Scholar] [CrossRef]
- Ferragud, A.; Howell, A.D.; Moore, C.F.; Ta, T.L.; Hoener, M.C.; Sabino, V.; Cottone, P. The Trace Amine-Associated Receptor 1 Agonist RO5256390 Blocks Compulsive, Binge-like Eating in Rats. Neuropsychopharmacology 2017, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Sanchez, C.; Santos, J.W.; Smith, K.L.; Ferragud, A.; Sabino, V.; Cottone, P. Seeking behavior, place conditioning, and resistance to conditioned suppression of feeding in rats intermittently exposed to palatable food. Behav. Neurosci. 2015, 129, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Rao, R.R.; Velazquez-Sanchez, C.; Valenza, M.; Giuliano, C.; Everitt, B.J.; Sabino, V.; Cottone, P. The uncompetitive N-methyl-D-aspartate antagonist memantine reduces binge-like eating, food-seeking behavior, and compulsive eating: Role of the nucleus accumbens shell. Neuropsychopharmacology 2015, 40, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Sanchez, C.; Ferragud, A.; Moore, C.F.; Everitt, B.J.; Sabino, V.; Cottone, P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology 2014, 39, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Blasio, A.; Steardo, L.; Sabino, V.; Cottone, P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict. Biol. 2014, 19, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Cottone, P.; Wang, X.; Park, J.W.; Valenza, M.; Blasio, A.; Kwak, J.; Iyer, M.R.; Steardo, L.; Rice, K.C.; Hayashi, T.; et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology 2012, 37, 2593–2604. [Google Scholar] [CrossRef]
- Le, A.; Funk, D.; Juzytsch, W.; Coen, K.; Navarre, B.M.; Cifani, C.; Shaham, Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology 2011, 218, 89–99. [Google Scholar] [CrossRef]
- Cottone, P.; Sabino, V.; Steardo, L.; Zorrilla, E.P. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2008, 295, R1066–R1076. [Google Scholar] [CrossRef]
- Cottone, P.; Sabino, V.; Roberto, M.; Bajo, M.; Pockros, L.; Frihauf, J.B.; Fekete, E.M.; Steardo, L.; Rice, K.C.; Grigoriadis, D.E.; et al. CRF system recruitment mediates dark side of compulsive eating. Proc. Natl. Acad. Sci. USA 2009, 106, 20016–20020. [Google Scholar] [CrossRef]
- Cottone, P.; Sabino, V.; Steardo, L.; Zorrilla, E.P. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology 2008, 33, 524–535. [Google Scholar] [CrossRef]
- Wellman, P.; Ho, D.; Cepeda-Benito, A.; Bellinger, L.; Nation, J. Cocaine-induced hypophagia and hyperlocomotion in rats are attenuated by prazosin. Eur. J. Pharmacol. 2002, 455, 117–126. [Google Scholar] [CrossRef]
- Allen, R.M.; Carelli, R.M.; Dykstra, L.A.; Suchey, T.L.; Everett, C.V. Effects of the competitive N-methyl-D-aspartate receptor antagonist, LY235959 [(-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid], on responding for cocaine under both fixed and progressive ratio schedules of reinforcement. J. Pharmacol. Exp. Ther. 2005, 315, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Hyytiä, P.; Bäckström, P.; Liljequist, S. Site-specific NMDA receptor antagonists produce differential effects on cocaine self-administration in rats. Eur. J. Pharmacol. 1999, 378, 9–16. [Google Scholar] [CrossRef]
- Ranaldi, R.; French, E.; Roberts, D. Systemic pretreatment with MK-801 (dizocilpine) increases breaking points for self-administration of cocaine on a progressive-ratio schedule in rats. Psychopharmacology 1996, 128, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.C.; Meil, W.M.; Kalivas, P.W. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacology 1997, 133, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.R.; Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef]
- Arnold, J.M.; Roberts, D.C. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol. Biochem. Behav. 1997, 57, 441–447. [Google Scholar] [CrossRef]
- Yokel, R.A.; Wise, R.A. Increased lever pressing for amphetamine after pimozide in rats: Implications for a dopamine theory of reward. Science 1975, 187, 547–549. [Google Scholar] [CrossRef]
- Loh, E.A.; Fitch, T.; Vickers, G.; Roberts, D.C. Clozapine increases breaking points on a progressive-ratio schedule reinforced by intravenous cocaine. Pharmacol. Biochem. Behav. 1992, 42, 559–562. [Google Scholar] [CrossRef]
- Depoortere, R.Y.; Li, D.H.; Lane, J.D.; Emmett-Oglesby, M.W. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol. Biochem. Behav. 1993, 45, 539–548. [Google Scholar] [CrossRef]
- Hubner, C.B.; Moreton, J.E. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology (Berl) 1991, 105, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.C.; Vickers, G. Atypical neuroleptics increase self-administration of cocaine: An evaluation of a behavioural screen for antipsychotic activity. Psychopharmacology (Berl) 1984, 82, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.R.; Smith, A.M.; Roberts, D.C. A single injection of either flupenthixol decanoate or haloperidol decanoate produces long-term changes in cocaine self-administration in rats. Drug Alcohol Depend. 1994, 36, 23–25. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Bahreini, T.; Adl, M. Effect of imipramine on the expression and acquisition of morphine-induced conditioned place preference in mice. Pharmacol. Biochem. Behav. 2002, 73, 941–949. [Google Scholar] [CrossRef]
- Thrasher, M.J.; Freeman, P.A.; Risinger, F.O. Clozapine’s effects on ethanol’s motivational properties. Alcohol Clin. Exp. Res. 1999, 23, 1377–1385. [Google Scholar] [PubMed]
- Sabino, V.; Cottone, P.; Blasio, A.; Iyer, M.R.; Steardo, L.; Rice, K.C.; Conti, B.; Koob, G.F.; Zorrilla, E.P. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology 2011, 36, 1207–1218. [Google Scholar] [CrossRef]
- Romieu, P.; Phan, V.L.; Martin-Fardon, R.; Maurice, T. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: Possible dependence on dopamine uptake blockade. Neuropsychopharmacology 2002, 26, 444–455. [Google Scholar] [CrossRef]
- Maurice, T.; Casalino, M.; Lacroix, M.; Romieu, P. Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacol. Biochem. Behav. 2003, 74, 869–876. [Google Scholar] [CrossRef]
- Verplaetse, T.L.; Rasmussen, D.D.; Froehlich, J.C.; Czachowski, C.L. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 2012, 36, 881–886. [Google Scholar] [CrossRef]
- Verplaetse, T.L.; Czachowski, C.L. Low-dose prazosin alone and in combination with propranolol or naltrexone: Effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology (Berl) 2015, 232, 2647–2657. [Google Scholar] [CrossRef]
- Wellman, P.J.; Davies, B.T.; Morien, A.; McMahon, L. Modulation of feeding by hypothalamic paraventricular nucleus α1- and α2-adrenergic receptors. Life Sci. 1993, 53, 669–679. [Google Scholar] [CrossRef]
- Bello, N.T.; Yeh, C.-Y.; Verpeut, J.L.; Walters, A.L. Binge-like eating attenuates nisoxetine feeding suppression, stress activation, and brain norepinephrine activity. PLoS ONE 2014, 9, e93610. [Google Scholar] [CrossRef] [PubMed]
- Strack, A.M.; Shu, J.; Camacho, R.; Gorski, J.N.; Murphy, B.; MacIntyre, D.E.; Hickey, G.J. Regulation of body weight and carcass composition by sibutramine in rats. Obes. Res. 2002, 10, 173–181. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).