Dietary Phytase and Lactic Acid-Treated Cereal Grains Differently Affected Calcium and Phosphorus Homeostasis from Intestinal Uptake to Systemic Metabolism in a Pig Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design
2.3. Sample Collection
2.4. Analytical Methods
2.4.1. Proximate Analysis
2.4.2. Content of Phosphorus in the Stomach and Balance Study
2.4.3. Intestinal Electrophysiology
2.4.4. Intestinal Short-Chain Fatty Acids and pH in the Digesta
2.4.5. Serum Parameters
2.4.6. RNA Isolation and Quantitative Real-Time PCR
2.4.7. Metacarpal Bone Measurements
2.4.8. Statistical Analyses
3. Results
3.1. Diets and Animal Performance
3.2. P Content in the Stomach and Mineral Balances
3.3. Intestinal Electrophysiology in the Jejunum, Short-Chain Fatty Acid Concentration, and pH along the Intestinal Tract
3.4. Serum Parameters
3.5. Gene Expression in the Intestinal Tract
3.6. Relative Gene Expression in the Kidney and Metacarpal Bones
3.7. Metric Parameters in Metacarpal Bones
3.8. Pearson’s Correlations between Serum Parameters and Metric Metacarpal Bone Parameters and Genes Expression in Metacarpal Bone and Kidney
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berndt, T.; Kumar, R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology 2009, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Penido, M.G.M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, J.; Labrador, H. Bone health in women. Prim. Care Clin. Off. Pract. 2018, 45, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Quarles, L.D. Novel bone endocrine networks integrating mineral and energy metabolism. Curr. Osteoporos. Rep. 2013, 11, 391–399. [Google Scholar] [CrossRef]
- Doyle, M.E.; de Jan Beur, S.M. The skeleton: Endocrine regulator of phosphate homeostasis. Curr. Osteoporos. Rep. 2008, 6, 134–141. [Google Scholar] [CrossRef]
- Crenshaw, T.D.; Rortvedt-Amundson, L.A.; Cuarón, J.A.; Bergstrom, J.R.; Litta, G. Triennial growth symposium: Vitamin D—Establishing the basics to dispel the hype. J. Anim. Sci. 2014, 92, 883–886. [Google Scholar] [CrossRef]
- Fernández, J. Calcium and phosphorus metabolism in growing pigs. I. Absorption and balance studies. Livest. Prod. Sci. 1995, 41, 233–241. [Google Scholar] [CrossRef]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. FGF23/Klotho axis: Phosphorus, mineral metabolism and beyond. Cytokine Growth Factor Rev. 2012, 23, 37–46. [Google Scholar] [CrossRef]
- Erben, R.G. Update on FGF23 and Klotho signaling. Mol. Cell. Endocrinol. 2016, 432, 56–65. [Google Scholar] [CrossRef]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quarles, L.D. How Fibroblast Growth Factor 23 Works. J. Am. Soc. Nephrol. 2007, 18, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yoshioka, M.; Itoh, N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 2000, 277, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.M.; Zhao, J.P.; Wang, X.J.; Jiao, H.C.; Wu, J.M.; Lin, H. Fibroblast growth factor 23 mRNA expression profile in chickens and its response to dietary phosphorus. Poult. Sci. 2018, 97, 2258–2266. [Google Scholar] [CrossRef]
- De Diaz Barboza, G.; Guizzardi, S.; De Tolosa Talamoni, N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142–7154. [Google Scholar] [CrossRef]
- Kellett, G.L. Alternative perspective on intestinal calcium absorption: Proposed complementary actions of Cav1.3 and TRPV6. Nutr. Rev. 2011, 69, 347–370. [Google Scholar] [CrossRef]
- Schröder, B.; Breves, G. Mechanisms and regulation of calcium absorption from the gastrointestinal tract in pigs and ruminants: Comparative aspects with special emphasis on hypocalcemia in dairy cows. Anim. Health Res. Rev. 2006, 7, 31–41. [Google Scholar] [CrossRef]
- Trautvetter, U.; Ditscheid, B.; Jahreis, G.; Glei, M. Calcium and phosphate metabolism, blood lipids and intestinal sterols in human intervention studies using different sources of phosphate as supplements—Pooled results and literature search. Nutrients 2018, 10, 936. [Google Scholar] [CrossRef]
- Kim, O.H.; Booth, C.J.; Choi, H.S.; Lee, J.; Kang, J.; Hur, J.; Jung, W.J.; Jung, Y.S.; Choi, H.J.; Kim, H.; et al. High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. Elife 2020, 9, e52709. [Google Scholar] [CrossRef]
- Moe, S.M.; Radcliffe, J.S.; White, K.E.; Gattone, V.H.; Seifert, M.F.; Chen, X.; Aldridge, B.; Chen, N.X. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J. Bone Miner. Res. 2011, 26, 2672–2681. [Google Scholar] [CrossRef]
- Dhayat, N.A.; Ackermann, D.; Pruijm, M.; Ponte, B.; Ehret, G.; Guessous, I.; Leichtle, A.B.; Paccaud, F.; Mohaupt, M.; Fiedler, G.M.; et al. Fibroblast growth factor 23 and markers of mineral metabolism in individuals with preserved renal function. Kidney Int. 2016, 90, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Eeckhout, W.; De Paepe, M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 1994, 47, 19–29. [Google Scholar] [CrossRef]
- Herrmann, K.R.; Ruff, A.J.; Infanzón, B.; Schwaneberg, U. Engineered phytases for emerging biotechnological applications beyond animal feeding. Appl. Microbiol. Biotechnol. 2019, 103, 6435–6448. [Google Scholar] [CrossRef] [PubMed]
- Carcea, M. Nutritional Value of Grain-Based Foods. Foods 2020, 9, 504–508. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Dietary change scenarios and implications for environmental, nutrition, human health and economic dimensions of food sustainability. Nutrients 2019, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.D.; Blaabjerg, K.; Feuerstein, D. Comparison of different levels and sources of microbial phytases. Livest. Sci. 2007, 109, 255–257. [Google Scholar] [CrossRef]
- Wise, A.; Gilburt, D.J. Phytate hydrolysis by germfree and conventional rats. Appl. Environ. Microbiol. 1982, 43, 753–756. [Google Scholar] [CrossRef]
- Jones, C.K.; Tokach, M.D.; Dritz, S.S.; Ratliff, B.W.; Horn, N.L.; Goodband, R.D.; DeRouchey, J.M.; Sulabo, R.C.; Nelssen, J.L. Efficacy of different commercial phytase enzymes and development of an available phosphorus release curve for Escherichia coli-derived phytases in nursery pigs. J. Anim. Sci. 2010, 88, 3631–3644. [Google Scholar] [CrossRef]
- Klinsoda, J.; Vötterl, J.; Zebeli, Q.; Metzler-zebeli, B.U. Alterations of the viable ileal microbiota of gut-mucosa-lymph node axis in pigs fed phytase and lactic acid-treated cereals. Appl. Environ. Microbiol. 2020, 86, e02128. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Mann, E.; Schmitz-Esser, S.; Wagner, M.; Ritzmann, M.; Zebeli, Q. Changing dietary calcium-phosphorus level and cereal source selectively alters abundance of bacteria and metabolites in the upper gastrointestinal tracts of weaned pigs. Appl. Environ. Microbiol. 2013, 79, 7264–7272. [Google Scholar] [CrossRef]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.S.; Andlid, T. Phytogenic and microbial phytases in human nutrition. Int. J. Food Sci. Technol. 2002, 37, 823–833. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; El-Salam, S.M.A.; Omran, A.A. Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties. PLoS ONE 2011, 6, e25512. [Google Scholar] [CrossRef] [PubMed]
- Rimsten, L.; Haraldsson, A.K.; Andersson, R.; Alminger, M.; Sandberg, A.S.; Man, P. Effects of malting on β-glucanase and phytase activity in barley grain. J. Sci. Food Agric. 2002, 82, 904–912. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Deckardt, K.; Schollenberger, M.; Rodehutscord, M.; Zebeli, Q. Lactic acid and thermal treatments trigger the hydrolysis of myo-inositol hexakisphosphate and modify the abundance of lower myo-inositol phosphates in barley (Hordeum vulgare L.). PLoS ONE 2014, 9, e101166. [Google Scholar] [CrossRef]
- Vötterl, J.C.; Zebeli, Q.; Hennig-Pauka, I.; Metzler-Zebeli, B.U. Soaking in lactic acid lowers the phytate-phosphorus content and increases the resistant starch in wheat and corn grains. Anim. Feed Sci. Technol. 2019, 252, 115–125. [Google Scholar] [CrossRef]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef]
- Selle, P.H.; Ravindran, V. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 2008, 113, 99–122. [Google Scholar] [CrossRef]
- Patterson, J.K.; Lei, X.G.; Miller, D.D. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp. Biol. Med. 2008, 233, 651–664. [Google Scholar] [CrossRef]
- Roura, E.; Koopmans, S.J.; Lallès, J.P.; Le Huerou-Luron, I.; De Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef] [PubMed]
- Vlot, M.C.; den Heijer, M.; de Jongh, R.T.; Vervloet, M.G.; Lems, W.F.; de Jonge, R.; Obermayer-Pietsch, B.; Heijboer, A.C. Clinical utility of bone markers in various diseases. Bone 2018, 114, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Gallant, K.M.H.; Weaver, C.M.; Towler, D.A.; Thuppal, S.V.; Bailey, R.L. Nutrition in Cardioskeletal Health. Adv. Nutr. 2016, 7, 544–555. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef]
- EFSA Safety and efficacy of Phyzyme XP 10000 (TPT/L), 6-phytase, as feed additive for chickens for fattening, laying hens, ducks for fattening, turkeys for fattening, piglets (weaned), pigs for fattening and sows—Scientific Opinion of the Panel on Additives. EFSA J. 2008, 6, 915.
- National Research Council (NRC). Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998; ISBN 0309059933. [Google Scholar]
- Flachowsky, G.; Pallauf, J.; Pfeffer, E.; Rodehutscord, M.; Schenkel, H.; Staudacher, W.; Susenbeth, A. Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen; DLG-Verlag: Frankfurt am Main, Germany, 2006. [Google Scholar]
- Metzler-Zebeli, B.U.; Hollmann, M.; Aschenbach, J.R.; Zebeli, Q. Comparison of electrogenic glucose transport processes and permeability between proximal and distal jejunum of laying hens. Br. Poult. Sci. 2017, 58, 278–282. [Google Scholar] [CrossRef]
- Newman, M.A.; Zebeli, Q.; Eberspächer, E.; Grüll, D.; Molnar, T.; Metzler-Zebeli, B.U. Transglycosylated starch improves insulin response and alters lipid and amino acid metabolome in a growing pig model. Nutrients 2017, 9, 291. [Google Scholar] [CrossRef]
- Naumann, C.; Bassler, R. Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Newman, M.A.; Grüll, D.; Zebeli, Q. Consumption of transglycosylated starch down-regulates expression of mucosal innate immune response genes in the large intestine using a pig model. Br. J. Nutr. 2018, 119, 1366–1377. [Google Scholar] [CrossRef]
- Quinn, S.J.; Thomsen, A.R.B.; Pang, J.L.; Kantham, L.; Brauner-Osborne, H.; Pollak, M.; Goltzman, D.; Brown, E.M. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E310–E320. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.U.; Kruger, M.C.; Hansen-Møller, J.; Poulsen, H.D. Bone biochemical markers for assessment of bone responses to differentiated phosphorus supply in growing-finishing pigs. J. Anim. Sci. 2018, 96, 4693–4703. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Canibe, N.; Montagne, L.; Freire, J.; Bosi, P.; Prates, J.A.M.; Tanghe, S.; Trevisi, P. Resistant starch reduces large intestinal pH and promotes fecal lactobacilli and bifidobacteria in pigs. Animal 2019, 13, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Miesorski, M.; Gerlinger, C.; Borgelt, L.; Lieboldt, M.; Oster, M.; Wimmers, K.; Wolf, P. Bone mineralization as diagnostic parameter for the assessment of dietary phosphorous supply in pigs—Are there differences between bones? In Proceedings of the 22nd European Society of Veterinary & Comparative Nutrition, Munich, Germany, 6–8 September 2018; p. 183. [Google Scholar]
- Rieger, H. Effects of a Different Phosphorus Supply on the Development and Mineralisation of Several Bones in Growing Pigs. Ph.D. Thesis, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany, 2017. [Google Scholar]
- Skiba, G.; Sobol, M.; Raj, S. Bone mineralization, geometry and strength in pigs growing from 56 to 115 day of life as affected by body fatness. J. Anim. Feed Sci. 2016, 25, 302–308. [Google Scholar] [CrossRef]
- Keçi, M.; Lucke, A.; Paulsen, P.; Zebeli, Q.; Böhm, J.; Metzler-Zebeli, B.U. Deoxynivalenol in the diet impairs bone mineralization in broiler chickens. Toxins 2019, 11, 352. [Google Scholar] [CrossRef]
- Newman, M.A.; Zebeli, Q.; Velde, K.; Grüll, D.; Molnar, T.; Kandler, W.; Metzler-Zebeli, B.U. Enzymatically modified starch favorably modulated intestinal transit time and hindgut fermentation in growing pigs. PLoS ONE 2016, 11, e0167784. [Google Scholar] [CrossRef]
- Kononoff, P.J.; Hanford, K.J. Technical note: Estimating statistical power of mixed models used in dairy nutrition experiments. J. Dairy Sci. 2006, 89, 3968–3971. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Ertl, R.; Klein, D.; Zebeli, Q. Explorative study of metabolic adaptations to various dietary calcium intakes and cereal sources on serum metabolome and hepatic gene expression in juvenile pigs. Metabolomics 2015, 11, 545–558. [Google Scholar] [CrossRef]
- Schneider, S.; Brunlehner, E.-M.; Schäffler, M.; Propstmeier, G.; Preißinger, W.; Harms, K.; Weiß, J.; Jais, C. Futterberechnung für Schweine; 22. Auflage; Bayerische Landesanstalt für Landwirtschaft (LfL): Freising, Germany, 2019. [Google Scholar]
- Kamphues, J.; Wolf, P.; Coenen, M.; Eder, K.; Iben, C.; Kienzle, E.; Liesegang, A.; Männer, K.; Zebeli, Q.; Zentek, J. Supplemente zur Tierernährung, 12th ed.; M. & H. Scharper GmbH: Hannover, Germany, 2014; ISBN 978-3-7944-0241-3. [Google Scholar]
- González-Vega, J.C.; Liu, Y.; McCann, J.C.; Walk, C.L.; Loor, J.J.; Stein, H.H. Requirement for digestible calcium by eleven- to twenty-five–kilogram pigs as determined by growth performance, bone ash concentration, calcium and phosphorus balances, and expression of genes involved in transport of calcium in intestinal and kidney cell. J. Anim. Sci. 2016, 94, 3321–3334. [Google Scholar] [CrossRef]
- Huttunen, M.M.; Tillman, I.; Viljakainen, H.T.; Tuukkanen, J.; Peng, Z.Q.; Pekkinen, M.; Lamberg-Allardt, C.J.E. High dietary phosphate intake reduces bone strength in the growing rat skeleton. J. Bone Miner. Res. 2007, 22, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Klinsoda, J.; Vötterl, J.; Zebeli, Q.; Metzler-zebeli, B.U. Lactic acid treatment of cereals and dietary phytase modified fecal microbiome composition without affecting expression of virulence factor genes in growing pigs. Front. Microbiol. 2019, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.W.; Coudray, C.; Bellanger, J.; Younes, H.; Demigné, C.; Rémésy, C. Intestinal Fermentation Lessens the Inhibitory Effects of Phytic Acid on Mineral Utilization in Rats. J. Nutr. 1998, 128, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Raschka, L.; Daniel, H. Mechanisms underlying the effects of inulin-type fructans on calcium absorption in the large intestine of rats. Bone 2005, 37, 728–735. [Google Scholar] [CrossRef]
- Walk, C.L.; Murphy, M.R.; Stein, H.H. Requirement for digestible calcium by 25 to 50 kg pigs at different dietary concentrations of phosphorus as indicated by growth performance, bone ash concentration, and calcium and phosphorus balances. J. Anim. Sci. 2016, 94, 5272–5285. [Google Scholar]
- Selle, P.H.; Cowieson, A.J.; Ravindran, V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 2009, 124, 126–141. [Google Scholar] [CrossRef]
- DLG Erfolgreiche Mastschweinefütterung; DLG-Verlags-GmbH: Frankfurt am Main, Germany, 2010.
- González-Vega, J.C.; Stein, H.H. Calcium digestibility and metabolism in pigs. Asian-Australasian J. Anim. Sci. 2014, 27, 1–9. [Google Scholar] [CrossRef]
- Kies, A.K.; Gerrits, W.J.J.; Schrama, J.W.; Heetkamp, M.J.W.; van der Linden, K.L.; Zandstra, T.; Verstegen, M.W.A. Mineral absorption and excretion as affected by microbial phytase, and their effect on energy metabolism in young piglets. J. Nutr. 2005, 135, 1131–1138. [Google Scholar] [CrossRef]
- Böswald, L.F.; Dobenecker, B.; Clauss, M.; Kienzle, E. A comparative meta-analysis on the relationship of faecal calcium and phosphorus excretion in mammals. J. Anim. Physiol. Anim. Nutr. 2018, 102, 370–379. [Google Scholar] [CrossRef]
- Mack, J.K.; Alexander, L.G.; Morris, P.J.; Dobenecker, B.; Kienzle, E. Demonstration of uniformity of calcium absorption in adult dogs and cats. J. Anim. Physiol. Anim. Nutr. 2015, 99, 801–809. [Google Scholar] [CrossRef]
- Varley, P.F.; Callan, J.J.; O’Doherty, J.V. Effect of dietary phosphorus and calcium level and phytase addition on performance, bone parameters, apparent nutrient digestibility, mineral and nitrogen utilization of weaner pigs and the subsequent effect on finisher pig bone parameters. Anim. Feed Sci. Technol. 2011, 165, 201–209. [Google Scholar] [CrossRef]
- Breves, G.; Kock, J.; Schröder, B. Transport of nutrients and electrolytes across the intestinal wall in pigs. Livest. Sci. 2007, 109, 4–13. [Google Scholar] [CrossRef]
- Saddoris, K.L.; Fleet, J.C.; Radcliffe, J.S. Sodium-dependent phosphate uptake in the jejunum is post-transcriptionally regulated in pigs fed a low-phosphorus diet and is independent of dietary calcium concentration. J. Nutr. 2010, 140, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Hishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.V.; Picotto, G.; Carpentieri, A.R.; Rivoira, M.A.; Peralta López, M.E.; Tolosa De Talamoni, N.G. Minireview on regulation of intestinal calcium absorption: Emphasis on molecular mechanisms of transcellular pathway. Digestion 2008, 77, 22–34. [Google Scholar] [CrossRef]
- Yang, L.Y.; Wang, S.X.; Min, G.; Shu, X.G.; Liu, Z.Q.; Li, T.J.; Yin, Y.L. Intestinal and renal Type II NaPi co-transporter gene expression patterns in growing pigs fed with different levels of dietary calcium. J. Food Agric. Environ. 2010, 8, 882–886. [Google Scholar]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; Van de Wiele, T.; Schüller, S.; Juge, N.; et al. Experimental models to study intestinal microbes—mucus interactions in health and disease. FEMS Microbiol. Rev. 2019, 43, 457–489. [Google Scholar] [CrossRef]
- Meldrum, O.W.; Yakubov, G.E.; Bonilla, M.R.; Deshmukh, O.; McGuckin, M.A.; Gidley, M.J. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca2+ -mediated links, and hydrogen bonding. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- DeLuca, H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008, 66, S73–S87. [Google Scholar] [CrossRef]
- Erben, R.G.; Andrukhova, O. FGF23 regulation of renal tubular solute transport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, I.; Saini, R.K.; Griffin, K.P.; Whitfield, G.K.; Haussler, M.R.; Jurutka, P.W. FGF23 gene regulation by 1,25-dihyoxyvitamin D: Opposing effects in adipocytes and osteocytes. J. Endocrinol. 2015, 226, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Blau, J.E.; Collins, M.T. The PTH-Vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.W. Kidney and Phosphate Metabolism; Electrolyte Blood Press: Seoul, Korea, 2008; Volume 6, pp. 77–85. [Google Scholar]
- Mahdavi, S.; Bellasi, A.; Nagra, K.; Johnston, L.; Tam, P.; Di Iorio, B.; Sikaneta, T. Associations of calcium from food sources versus phosphate binders with serum calcium and FGF23 in hemodialysis patients. J. Clin. Med. 2019, 8, 1680. [Google Scholar] [CrossRef] [PubMed]
- Burnett, S.A.M.; Gunawardene, S.C.; Bringhurst, F.R.; Jüppner, H.; Lee, H.; Finkelstein, J.S. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 2006, 21, 1187–1196. [Google Scholar] [CrossRef]
- Eklou-Kalonji, E.; Zerath, E.; Colin, C.; Lacroix, C.; Holy, X.; Denis, I.; Pointillart, A. Calcium-regulating hormones, bone mineral content, breaking load and trabecular remodeling are altered in growing pigs fed calcium-deficient diets. J. Nutr. 1999, 129, 188–193. [Google Scholar] [CrossRef]
- Michigami, T.; Ozono, K. Roles of phosphate in skeleton. Front. Endocrinol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Murali, S.K.; Andrukhova, O.; Clinkenbeard, E.L.; White, K.E.; Erben, R.G. Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in hyp mice. PLoS Biol. 2016, 14, 1–24. [Google Scholar] [CrossRef]
- Adeola, O.; Azain, M.J.; Carter, S.D.; Crenshaw, T.D.; Estienne, M.J.; Kerr, B.J.; Lindemann, M.D.; Maxwell, C.V.; Miller, P.S.; Shannon, M.C.; et al. A cooperative study on the standardized total-tract digestible phosphorus requirement of twenty-kilogram pigs. J. Anim. Sci. 2015, 93, 5743–5753. [Google Scholar] [CrossRef]
 ), LA (
), LA ( ), Con-Phy (
), Con-Phy ( ), and LA-Phy (
), and LA-Phy ( ) for 18 days. Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a, b, c).
) for 18 days. Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a, b, c).
 ), LA (
), LA ( ), Con-Phy (
), Con-Phy ( ), and LA-Phy (
), and LA-Phy ( ) for 18 days. Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a, b, c).
) for 18 days. Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a, b, c).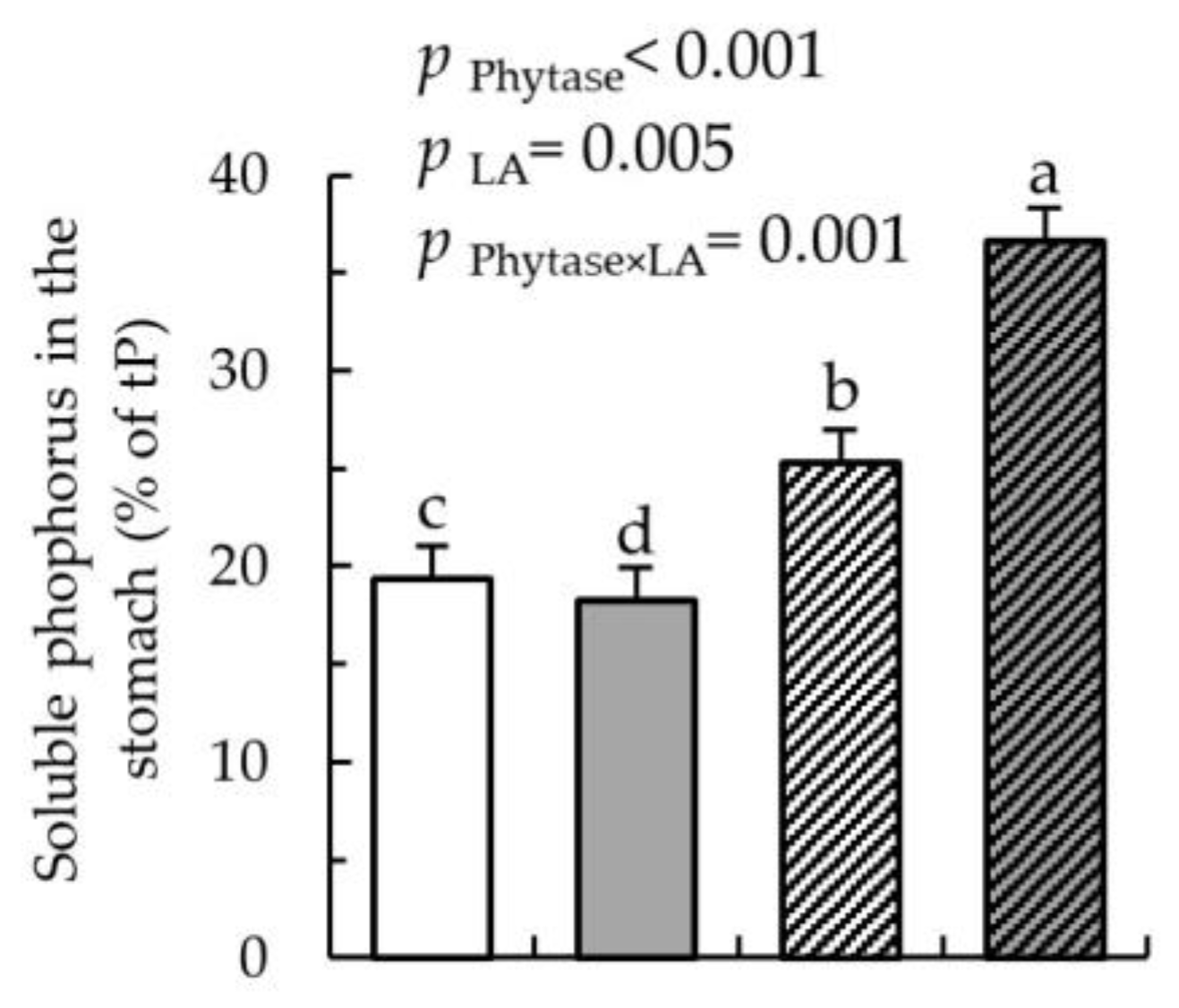
 ), LA (
), LA ( ), Con-Phy (
), Con-Phy ( ), and LA-Phy (
), and LA-Phy ( ). (a) Urinary phosphorus (P) excretion (% of dietary intake); (b) urinary calcium (Ca) excretion; (% of dietary intake); (c) ratio of excreted Ca:P in urine; (d) P absorption (% of dietary intake); (e) Ca absorption (% of dietary intake); (f) ratio of absorbed Ca:P; (g) P retention (% of dietary intake); (h) Ca retention (% of dietary intake); and (i) ratio of retained Ca:P; Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p > 0.05) effects of treatment are indicated by different letters (a,b,c). The nutrient intake, nutrient excretion in feces and urine, as well as absorption and retention were calculated as the mean of the three days of sampling (experimental day 15 to day 17).
). (a) Urinary phosphorus (P) excretion (% of dietary intake); (b) urinary calcium (Ca) excretion; (% of dietary intake); (c) ratio of excreted Ca:P in urine; (d) P absorption (% of dietary intake); (e) Ca absorption (% of dietary intake); (f) ratio of absorbed Ca:P; (g) P retention (% of dietary intake); (h) Ca retention (% of dietary intake); and (i) ratio of retained Ca:P; Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p > 0.05) effects of treatment are indicated by different letters (a,b,c). The nutrient intake, nutrient excretion in feces and urine, as well as absorption and retention were calculated as the mean of the three days of sampling (experimental day 15 to day 17).
 ), LA (
), LA ( ), Con-Phy (
), Con-Phy ( ), and LA-Phy (
), and LA-Phy ( ). (a) Urinary phosphorus (P) excretion (% of dietary intake); (b) urinary calcium (Ca) excretion; (% of dietary intake); (c) ratio of excreted Ca:P in urine; (d) P absorption (% of dietary intake); (e) Ca absorption (% of dietary intake); (f) ratio of absorbed Ca:P; (g) P retention (% of dietary intake); (h) Ca retention (% of dietary intake); and (i) ratio of retained Ca:P; Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p > 0.05) effects of treatment are indicated by different letters (a,b,c). The nutrient intake, nutrient excretion in feces and urine, as well as absorption and retention were calculated as the mean of the three days of sampling (experimental day 15 to day 17).
). (a) Urinary phosphorus (P) excretion (% of dietary intake); (b) urinary calcium (Ca) excretion; (% of dietary intake); (c) ratio of excreted Ca:P in urine; (d) P absorption (% of dietary intake); (e) Ca absorption (% of dietary intake); (f) ratio of absorbed Ca:P; (g) P retention (% of dietary intake); (h) Ca retention (% of dietary intake); and (i) ratio of retained Ca:P; Values are least square means (n = 8/diet), with their SEM represented by vertical bars. Statistically significant (p > 0.05) effects of treatment are indicated by different letters (a,b,c). The nutrient intake, nutrient excretion in feces and urine, as well as absorption and retention were calculated as the mean of the three days of sampling (experimental day 15 to day 17).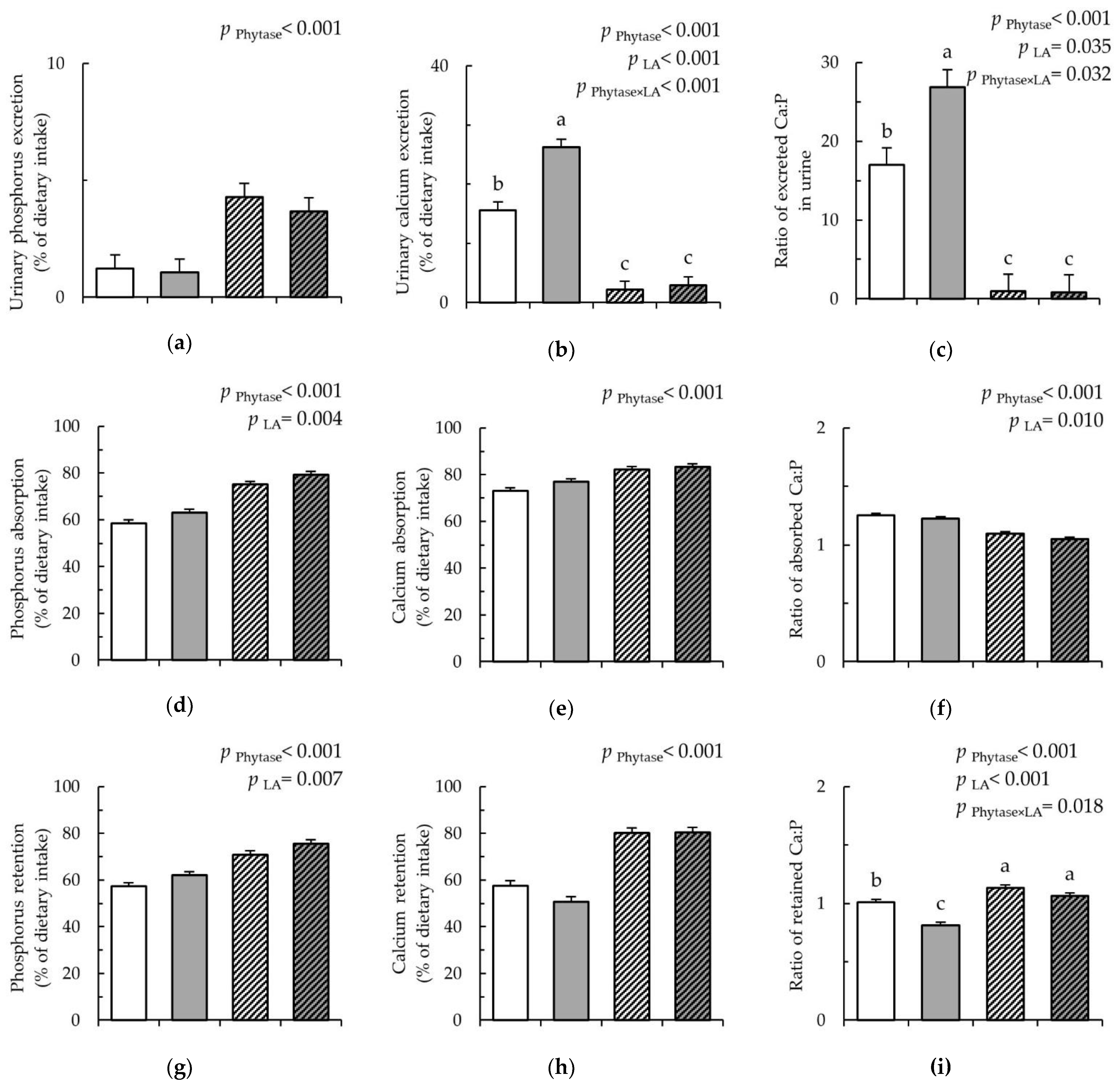
 ), LA (
), LA ( ), Con-Phy (
), Con-Phy ( ), and LA-Phy (
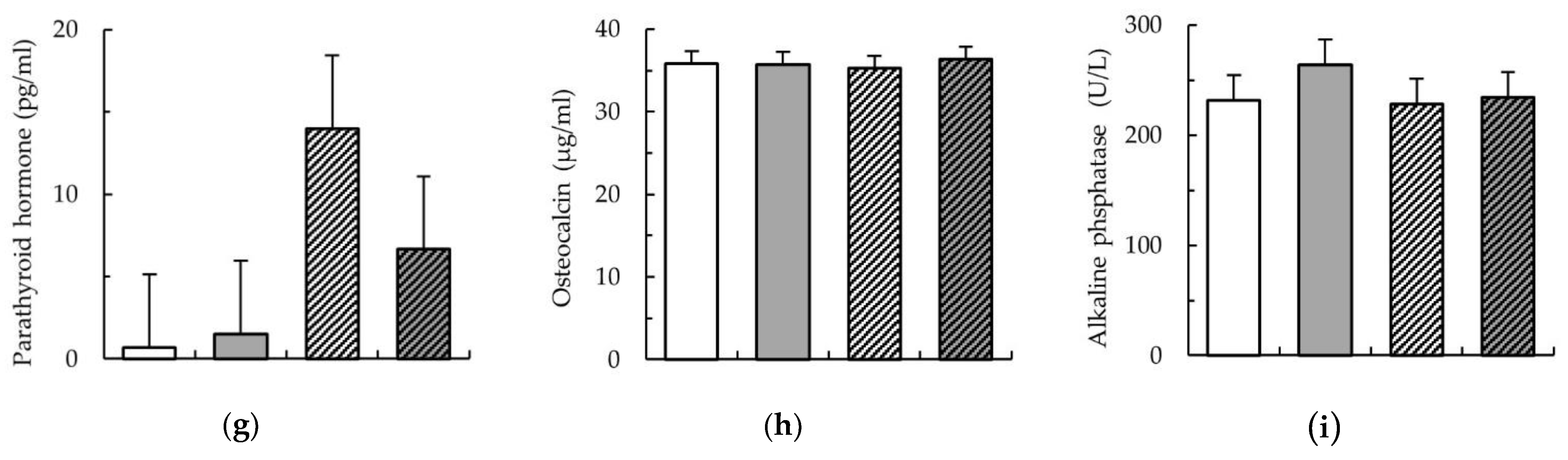
), and LA-Phy ( ) for 18 days. (a) phosphorus (mmol/L); (b) calcium (mmol/L); (c) ratio of Ca:P in serum; (d) product of Ca × P in serum; (e) vitamin D (ng/mL); (f) fibroblast growth factor (pg/mL); (g) parathyroid hormone parathormon (pg/mL); (h) osteocalcin (ng/mL); (i) alkaline phosphatase (U/L). Values are means (n 8/diet), with their standard errors represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a,b,c).
) for 18 days. (a) phosphorus (mmol/L); (b) calcium (mmol/L); (c) ratio of Ca:P in serum; (d) product of Ca × P in serum; (e) vitamin D (ng/mL); (f) fibroblast growth factor (pg/mL); (g) parathyroid hormone parathormon (pg/mL); (h) osteocalcin (ng/mL); (i) alkaline phosphatase (U/L). Values are means (n 8/diet), with their standard errors represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a,b,c).
 ), LA (
), LA ( ), Con-Phy (
), Con-Phy ( ), and LA-Phy (
), and LA-Phy ( ) for 18 days. (a) phosphorus (mmol/L); (b) calcium (mmol/L); (c) ratio of Ca:P in serum; (d) product of Ca × P in serum; (e) vitamin D (ng/mL); (f) fibroblast growth factor (pg/mL); (g) parathyroid hormone parathormon (pg/mL); (h) osteocalcin (ng/mL); (i) alkaline phosphatase (U/L). Values are means (n 8/diet), with their standard errors represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a,b,c).
) for 18 days. (a) phosphorus (mmol/L); (b) calcium (mmol/L); (c) ratio of Ca:P in serum; (d) product of Ca × P in serum; (e) vitamin D (ng/mL); (f) fibroblast growth factor (pg/mL); (g) parathyroid hormone parathormon (pg/mL); (h) osteocalcin (ng/mL); (i) alkaline phosphatase (U/L). Values are means (n 8/diet), with their standard errors represented by vertical bars. Statistically significant (p < 0.05) effects of treatment are indicated by different letters (a,b,c).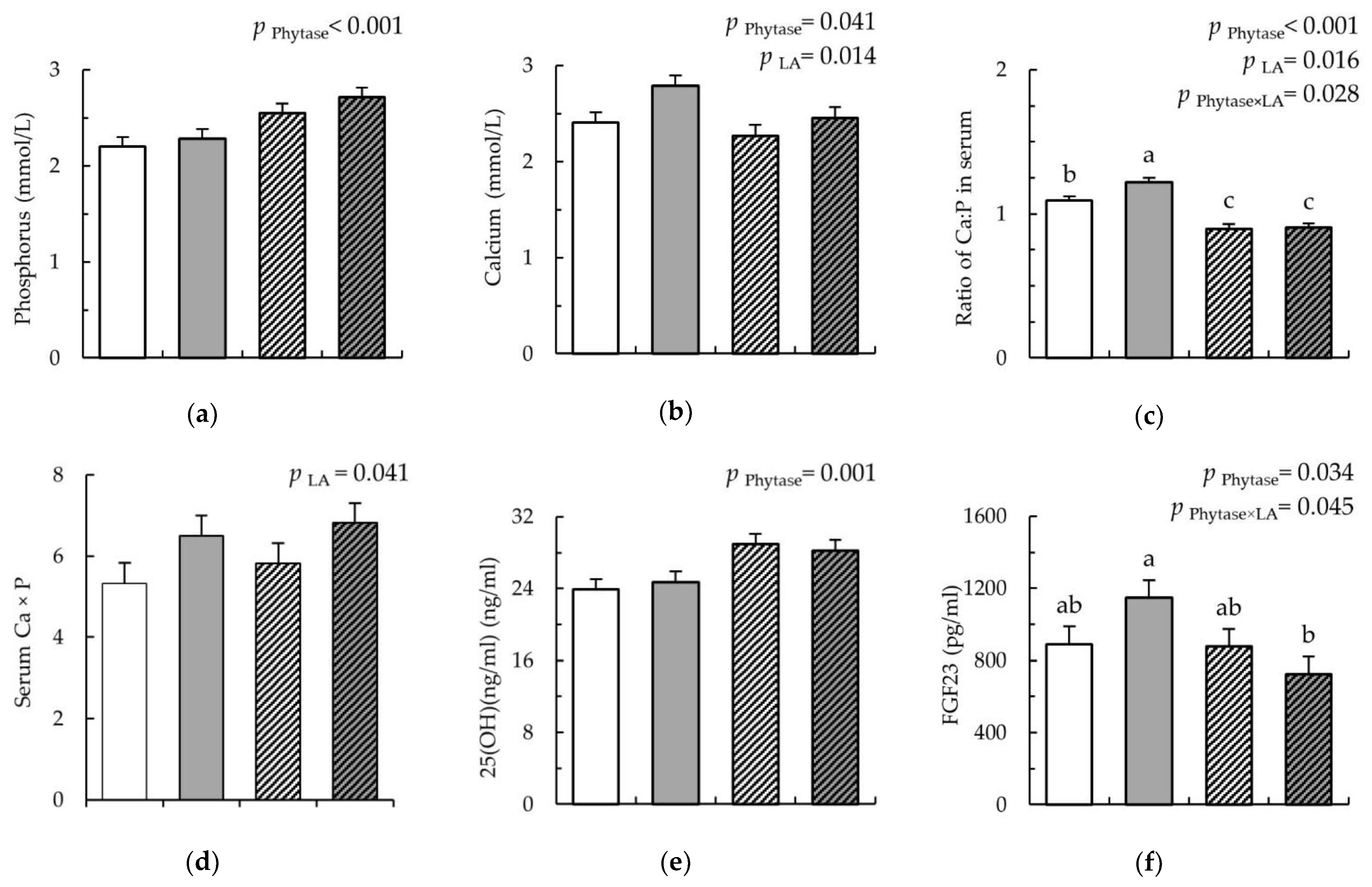
| Dietary Treatment 1 | Con | LA | Con-Phytase | LA-Phytase |
|---|---|---|---|---|
| Ingredient (g/kg) | ||||
| Wheat | 362 | - | 362 | - |
| Maize | 360 | - | 360 | - |
| LA-treated wheat 2 | - | 362 | - | 362 |
| LA-treated maize 2 | - | 360 | - | 360 |
| Soybean meal | 220 | 220 | 220 | 220 |
| Oil | 20 | 20 | 20 | 20 |
| Premix 3 with phytase 4 | - | - | 23 | 23 |
| Premix 3 without phytase | 23 | 23 | - | - |
| Monocalcium phosphate (Ca(H2PO4)2) | 4 | 4 | 4 | 4 |
| Limestone (CaCO3) | 11 | 11 | 11 | 11 |
| Analyzed chemical composition (DM basis, g/kg) | ||||
| Dry matter | 901 | 942 | 900 | 941 |
| Protein | 213 | 202 | 216 | 206 |
| Neutral-detergent fiber | 131 | 122 | 129 | 119 |
| Acid-detergent fiber | 50 | 51 | 52 | 51 |
| Total starch | 519 | 500 | 509 | 493 |
| Non-resistant starch | 510 | 492 | 500 | 485 |
| Resistant starch | 8.2 | 7.7 | 9.2 | 8.3 |
| Ash | 55 | 50 | 54 | 49 |
| Calcium | 6.6 | 6.2 | 6.6 | 6.2 |
| Total phosphorus | 5.5 | 4.9 | 5.5 | 5.0 |
| Available phosphorus 5 | 1.2 | 1.6 | 1.2 | 1.6 |
| Available phosphorus (%)5 | 21 | 32 | 21 | 32 |
| Phytate-phosphorus 5 | 4.3 | 3.3 | 4.3 | 3.3 |
| Phytate (%) 5 | 79 | 68 | 79 | 68 |
| Metabolizable energy (kJ, kg) | 13.7 | 13.7 | 13.7 | 13.7 |
| DCAD 6 | 36 | 37 | 36 | 37 |
| Dietary Treatment 1 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter 2 | Con | LA | Con-Phytase | LA-Phytase | SEM | Phytase | LA | Phytase × LA |
| Na2HPO4 response | ||||||||
| ISC (µA/cm2) | −35.9 | −46.3 | −31.5 | −58.2 | 9.62 | 0.705 | 0.066 | 0.412 |
| ∆ ISC | −0.1 | 0.2 | −0.1 | 0.1 | 0.13 | 0.676 | 0.059 | 0.729 |
| GT (mS/cm2) | 8.3 | 10.8 | 9.2 | 9.0 | 1.06 | 0.685 | 0.295 | 0.204 |
| ∆ GT | −0.4 | −0.6 | −0.5 | −0.4 | 0.10 | 0.718 | 0.778 | 0.208 |
| Mucosal to serosal FITC-dextran flux rates | ||||||||
| JMS (mmol/cm2 × h) | 0.07 | 0.14 | 0.07 | 0.06 | 0.018 | 0.069 | 0.146 | 0.030 |
| Dietary Treatment 1 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Paramter 2 | Con | LA | Con-Phytase | LA-Phytase | SEM | Phytase | LA | Phytase × LA |
| SCFA (µmol/g) | ||||||||
| Stomach | 11.0 | 11.3 | 12.8 | 6.9 | 2.53 | 0.608 | 0.285 | 0.235 |
| Jejunum | 104.6 | 86.7 | 87.9 | 95.4 | 19.35 | 0.837 | 0.789 | 0.535 |
| Ileum | 106.9 | 51.9 | 69.4 | 40.9 | 26.00 | 0.379 | 0.127 | 0.635 |
| Caecum | 169.1 | 198.6 | 164.1 | 184.7 | 6.88 | 0.182 | 0.001 | 0.529 |
| Colon | 172.4 | 191.1 | 192.6 | 187.9 | 9.36 | 0.371 | 0.460 | 0.223 |
| Digesta pH | ||||||||
| Stomach | 4.3 | 3.9 | 4.2 | 3.4 | 0.20 | 0.208 | 0.007 | 0.258 |
| Jejunum | 7.8 | 7.2 | 7.4 | 7.6 | 0.21 | 0.898 | 0.390 | 0.144 |
| Ileum | 8.0 | 7.9 | 7.9 | 7.5 | 0.30 | 0.401 | 0.462 | 0.676 |
| Caecum | 6.3 | 5.9 | 6.0 | 6.1 | 0.15 | 0.653 | 0.428 | 0.219 |
| Colon | 6.6 | 6.6 | 6.6 | 6.6 | 0.06 | 0.750 | 0.392 | 0.854 |
| Dietary Treatment 1 | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter 2 | Gut Site | Con | LA | Con-Phytase | LA-Phytase | SEM | Phytase | LA | Phytase × LA |
| SLC34A1 | Duodenum | 0.04 | 0.03 | 0.11 | 0.06 | 0.037 | 0.233 | 0.377 | 0.627 |
| Jejunum | 0.34 | 0.41 | 0.24 | 0.47 | 0.077 | 0.765 | 0.063 | 0.330 | |
| Ileum | n.d. | n.d. | n.d. | n.d. | . | . | . | . | |
| Caecum | n.d. | n.d. | n.d. | n.d. | . | . | . | . | |
| Colon | n.d. | n.d. | n.d. | n.d. | . | . | . | . | |
| SLC34A2 | Duodenum | 0.73 | 0.81 | 0.77 | 0.59 | 0.098 | 0.370 | 0.628 | 0.212 |
| Jejunum | n.d. | n.d. | n.d. | n.d. | . | . | . | . | |
| Ileum | 0.23 | 0.52 | 0.14 | 0.35 | 0.134 | 0.363 | 0.075 | 0.791 | |
| Caecum | 0.06 | 0.09 | 0.06 | 0.07 | 0.023 | 0.769 | 0.455 | 0.721 | |
| Colon | 0.05 | 0.05 | 0.03 | 0.003 | 0.0018 | 0.372 | 0.936 | 0.936 | |
| TRPV5 | Duodenum | 0.18 | 0.13 | 0.12 | 0.12 | 0.055 | 0.544 | 0.669 | 0.655 |
| Jejunum | 0.29 | 0.46 | 0.43 | 0.39 | 0.063 | 0.600 | 0.305 | 0.137 | |
| Ileum | 0.19 | 0.23 | 0.20 | 0.15 | 0.045 | 0.414 | 0.841 | 0.305 | |
| Caecum | 0.15 | 0.16 | 0.06 | 0.09 | 0.036 | 0.037 | 0.623 | 0.848 | |
| Colon | 0.08 | 0.06 | 0.03 | 0.03 | 0.022 | 0.099 | 0.597 | 0.779 | |
| TRPV6 | Duodenum | 0.23 | 0.24 | 0.22 | 0.25 | 0.09 | 0.908 | 0.512 | 0.773 |
| Jejunum | 0.60 | 0.44 | 0.26 | 0.22 | 0.048 | <0.001 | 0.049 | 0.286 | |
| Ileum | 0.025 | 0.017 | 0.005 | 0.004 | 0.0030 | <0.001 | 0.199 | 0.224 | |
| Caecum | 0.07 | 0.07 | 0.04 | 0.06 | 0.010 | 0.227 | 0.400 | 0.267 | |
| Colon | 0.08 | 0.08 | 0.03 | 0.04 | 0.009 | <0.001 | 0.690 | 0.682 | |
| CALB1 | Duodenum | 0.43 | 0.54 | 0.41 | 0.39 | 0.050 | 0.125 | 0.385 | 0.219 |
| Jejunum | 0.23 | 0.19 | 0.20 | 0.13 | 0.061 | 0.416 | 0.374 | 0.840 | |
| Ileum | 0.003 | 0.005 | 0.004 | 0.003 | 0.0014 | 0.997 | 0.703 | 0.306 | |
| Caecum | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.00002 | 0.075 | 0.840 | 0.484 | |
| Colon | 0.0006 | 0.0004 | 0.0004 | 0.0005 | 0.00015 | 0.801 | 0.737 | 0.317 | |
| PMCA1b | Duodenum | 0.04 | 0.03 | 0.03 | 0.03 | 0.005 | 0.861 | 0.332 | 0.186 |
| Jejunum | 0.22 | 0.27 | 0.19 | 0.32 | 0.063 | 0.912 | 0.165 | 0.496 | |
| Ileum | 0.005 | 0.006 | 0.005 | 0.004 | 0.0018 | 0.550 | 0.989 | 0.785 | |
| Caecum | 0.03 | 0.04 | 0.03 | 0.04 | 0.002 | 0.934 | 0.002 | 0.119 | |
| Colon | 0.04 | 0.04 | 0.04 | 0.03 | 0.004 | 0.060 | 0.260 | 0.073 | |
| VDR | Duodenum | 0.10 | 0.08 | 0.09 | 0.07 | 0.010 | 0.555 | 0.115 | 0.739 |
| Jejunum | 0.22 | 0.26 | 0.24 | 0.41 | 0.054 | 0.121 | 0.059 | 0.217 | |
| Ileum | 0.003 | 0.003 | 0.002 | 0.002 | 0.0005 | 0.006 | 0.904 | 0.347 | |
| Caecum | 0.02 | 0.02 | 0.02 | 0.02 | 0.002 | 0.146 | 0.569 | 0.983 | |
| Colon | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | 0.917 | 0.604 | 0.588 | |
| CYP24A1 | Duodenum | 0.05 | 0.03 | 0.01 | 0.01 | 0.009 | 0.018 | 0.282 | 0.297 |
| Jejunum | 0.35 | 0.29 | 0.06 | 0.01 | 0.065 | <0.001 | 0.405 | 0.989 | |
| Ileum | 0.0267 | 0.0391 | 0.0004 | 0.0006 | 0.01837 | 0.089 | 0.734 | 0.743 | |
| Caecum | 0.0004 | 0.0007 | 0.0005 | 0.0009 | 0.00022 | 0.614 | 0.186 | 0.955 | |
| Colon | 0.0011 | 0.0017 | 0.0015 | 0.0016 | 0.00040 | 0.711 | 0.390 | 0.579 | |
| FGF23 | Duodenum | 0.014 | 0.071 | 0.001 | 0.018 | 0.0335 | 0.332 | 0.284 | 0.550 |
| Jejunum | 0.05 | 0.10 | 0.04 | 0.03 | 0.034 | 0.242 | 0.702 | 0.386 | |
| Ileum | 0.016 | 0.122 | 0.002 | 0.227 | 0.0691 | 0.515 | 0.024 | 0.398 | |
| Caecum | 0.003 | 0.006 | 0.002 | 0.003 | 0.0028 | 0.496 | 0.496 | 0.708 | |
| Colon | 0.008 | 0.018 | 0.004 | 0.013 | 0.0043 | 0.301 | 0.031 | 0.907 | |
| Dietary Treatment 1 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Genes of Interest 2 | Con | LA | Con-Phytase | LA-Phytase | SEM | Phytase | LA | Phytase × LA |
| Relative gene expression in the kidney | ||||||||
| SLC34A1 | 0.58 | 0.65 | 0.63 | 0.61 | 0.065 | 0.998 | 0.704 | 0.521 |
| TRPV5 | 0.20 | 0.15 | 0.52 | 0.54 | 0.043 | <0.001 | 0.636 | 0.408 |
| TRPV6 | 0.43 | 0.35 | 0.30 | 0.31 | 0.061 | 0.184 | 0.583 | 0.466 |
| CALB1 | 0.15 | 0.11 | 0.31 | 0.38 | 0.031 | <0.001 | 0.644 | 0.085 |
| PMCA1b | 0.41 | 0.50 | 0.47 | 0.42 | 0.115 | 0.912 | 0.874 | 0.534 |
| VDR | 0.39 | 0.43 | 0.72 | 0.70 | 0.059 | <0.001 | 0.941 | 0.587 |
| CYP24A1 | 0.38 | 0.58 | 0.27 | 0.65 | 0.074 | 0.785 | <0.001 | 0.222 |
| Relative gene expression in the metacarpal bones | ||||||||
| VDR | 0.23 | 0.14 | 0.29 | 0.09 | 0.072 | 0.918 | 0.058 | 0.429 |
| CYP24A1 | 0.32 | 0.21 | 0.11 | 0.08 | 0.064 | 0.015 | 0.292 | 0.547 |
| CYP27B1 | 0.37 | 0.23 | 0.29 | 0.21 | 0.052 | 0.361 | 0.040 | 0.572 |
| FGF23 | 0.36 | 0.24 | 0.52 | 0.20 | 0.078 | 0.436 | 0.009 | 0.215 |
| OCN | 0.43 | 0.18 | 0.19 | 0.06 | 0.073 | 0.022 | 0.016 | 0.424 |
| OPG | 0.30 | 0.09 | 0.16 | 0.05 | 0.063 | 0.173 | 0.017 | 0.440 |
| RANKL | 0.28 | 0.15 | 0.24 | 0.12 | 0.054 | 0.542 | 0.026 | 0.899 |
| Dietary Treatment 1 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Paramter 2 | Con | LA | Con-Phytase | LA-Phytase | SEM | Phytase | LA | Phytase × LA |
| Length (mm) | 5.54 | 5.54 | 5.60 | 5.50 | 0.166 | 0.944 | 0.769 | 0.769 |
| Weight (g) | 27.25 | 21.38 | 22.36 | 22.89 | 2.777 | 0.549 | 0.338 | 0.260 |
| b (mm) | 7.90 | 8.37 | 8.35 | 8.44 | 0.266 | 0.341 | 0.307 | 0.483 |
| B (mm) | 10.84 | 11.08 | 10.66 | 11.32 | 0.470 | 0.943 | 0.346 | 0.656 |
| h (mm) | 8.48 | 8.70 | 9.05 | 8.54 | 0.324 | 0.534 | 0.654 | 0.266 |
| H (mm) | 11.63 | 11.30 | 11.58 | 11.85 | 0.406 | 0.539 | 0.947 | 0.469 |
| Thickness (mm) | 1.52 | 1.33 | 1.21 | 1.55 | 0.103 | 0.674 | 0.482 | 0.016 |
| Area (mm2) | 46.22 | 41.25 | 37.77 | 48.90 | 3.960 | 0.921 | 0.443 | 0.053 |
| Index | 0.54 | 0.47 | 0.43 | 0.53 | 0.028 | 0.331 | 0.656 | 0.007 |
| Density (g/cm3) | 1.16 | 1.14 | 1.16 | 1.16 | 0.019 | 0.499 | 0.584 | 0.629 |
| DM (%) | 55.44 | 53.76 | 56.66 | 54.56 | 0.920 | 0.283 | 0.051 | 0.822 |
| Ash (%) | 16.06 | 15.28 | 18.15 | 17.29 | 0.420 | <0.001 | 0.063 | 0.913 |
| Calcium oxide (% of ash) | 50.49 | 48.92 | 49.85 | 50.18 | 0.713 | 0.671 | 0.394 | 0.198 |
| Phosphorus pentoxide (% of ash) | 38.55 | 38.50 | 38.74 | 39.26 | 0.492 | 0.344 | 0.634 | 0.564 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vötterl, J.C.; Klinsoda, J.; Zebeli, Q.; Hennig-Pauka, I.; Kandler, W.; Metzler-Zebeli, B.U. Dietary Phytase and Lactic Acid-Treated Cereal Grains Differently Affected Calcium and Phosphorus Homeostasis from Intestinal Uptake to Systemic Metabolism in a Pig Model. Nutrients 2020, 12, 1542. https://doi.org/10.3390/nu12051542
Vötterl JC, Klinsoda J, Zebeli Q, Hennig-Pauka I, Kandler W, Metzler-Zebeli BU. Dietary Phytase and Lactic Acid-Treated Cereal Grains Differently Affected Calcium and Phosphorus Homeostasis from Intestinal Uptake to Systemic Metabolism in a Pig Model. Nutrients. 2020; 12(5):1542. https://doi.org/10.3390/nu12051542
Chicago/Turabian StyleVötterl, Julia C., Jutamat Klinsoda, Qendrim Zebeli, Isabel Hennig-Pauka, Wolfgang Kandler, and Barbara U. Metzler-Zebeli. 2020. "Dietary Phytase and Lactic Acid-Treated Cereal Grains Differently Affected Calcium and Phosphorus Homeostasis from Intestinal Uptake to Systemic Metabolism in a Pig Model" Nutrients 12, no. 5: 1542. https://doi.org/10.3390/nu12051542
APA StyleVötterl, J. C., Klinsoda, J., Zebeli, Q., Hennig-Pauka, I., Kandler, W., & Metzler-Zebeli, B. U. (2020). Dietary Phytase and Lactic Acid-Treated Cereal Grains Differently Affected Calcium and Phosphorus Homeostasis from Intestinal Uptake to Systemic Metabolism in a Pig Model. Nutrients, 12(5), 1542. https://doi.org/10.3390/nu12051542







