Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Intervention Types
2.4. Primary Outcome Measures
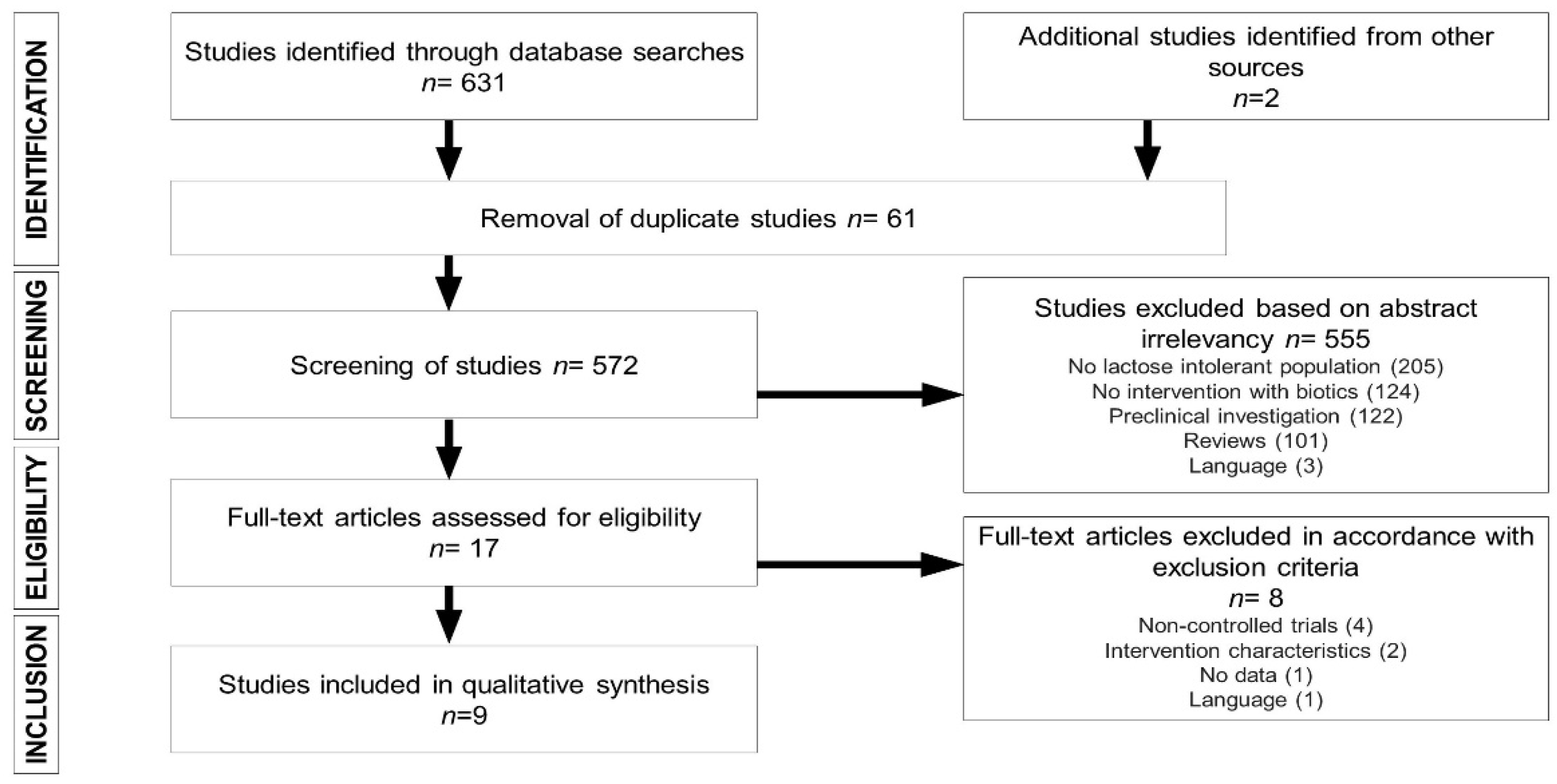
2.5. Study Selection
2.6. Data Extraction
2.7. Assessment of Risk of Bias
3. Results
3.1. Study Characteristics
3.2. Prebiotics, Probiotics, and LI Symptoms
3.3. Prebiotics, Probiotics, and Lactose Digestion
3.4. Risk-of-Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Fassio, F.; Facioni, M.S.; Guagnini, F. Lactose Maldigestion, Malabsorption, and Intolerance: A Comprehensive Review with a Focus on Current Management and Future Perspectives. Nutrients 2018, 10, 1599. [Google Scholar] [CrossRef]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, M.; Berni Canani, R. Lactose Intolerance: Common Misunderstandings. Ann. Nutr. Metab. 2018, 73, 30–37. [Google Scholar] [CrossRef]
- Silberman, E.S.; Jin, J. Lactose Intolerance. JAMA 2019, 322, 1620. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; López, S.; Jones, B.L.; Montalva, N.; Gerbault, P.; Lau, W. World-wide distributions of lactase persistence alleles and the complex effects of recombination and selection. Hum. Genet. 2017, 136, 1445–1453. [Google Scholar] [CrossRef]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef]
- Leis, R.; Tojo, R.; Pavón, P.; Douwes, A. Prevalence of lactose malabsorption in Galicia. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 296–300. [Google Scholar] [CrossRef]
- Ingram, C.J.; Mulcare, C.A.; Itan, Y.; Thomas, M.G.; Swallow, D.M. Lactose digestion and the evolutionary genetics of lactase persistence. Hum. Genet. 2009, 124, 579–591. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, L.; Chang, J.; Liu, J.; Xu, S.; Deng, C.; Yu, F.; Ma, Z.; Wang, G.; Zhang, C. The incidence of infants with rotavirus enteritis combined with lactose intolerance. Pak. J. Pharm. Sci. 2016, 29, 321–323. [Google Scholar]
- Szilagyi, A.; Galiatsatos, P.; Xue, X.A. Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr. J. 2016, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Gabrielli, M.; Migneco, A.; Lauritano, C.; Zocco, M.A.; Scarpellini, E.; Nista, E.C.; Gasbarrini, G.; Gasbarrini, A. Regression of lactose malabsorption in coeliac patients after receiving a gluten-free diet. Scand. J. Gastroenterol. 2008, 43, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.B. Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006, 118, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Rojo, C.; Jaime, F.; Azócar, L.; Hernández, C.; Villagrán, A.; Miquel, J.F.; Arancibia, G. Concordance between Lactose Quick Test, hydrogen-methane breath test and genotyping for the diagnosis of lactose malabsorption in children. Neurogastroenterol. Motil. 2018, 30, e13271. [Google Scholar] [CrossRef]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: Effective management strategies for lactose intolerance. Ann. Intern. Med. 2010, 152, 797–803. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef]
- Hodges, J.K.; Cao, S.; Cladis, D.P.; Weaver, C.M. Lactose Intolerance and Bone Health: The Challenge of Ensuring Adequate Calcium Intake. Nutrients 2019, 11, 718. [Google Scholar] [CrossRef]
- Savaiano, D. Lactose intolerance: An unnecessary risk for low bone density. Nestle Nutr. Workshop Ser. Pediatr. Program 2011, 67, 161–171. [Google Scholar] [CrossRef]
- Grenov, B.; Briend, A.; Sangild, P.T.; Thymann, T.; Rytter, M.H.; Hother, A.L.; Mølgaard, C.; Michaelsen, K.F. Undernourished Children and Milk Lactose. Food Nutr. Bull. 2016, 37, 85–99. [Google Scholar] [CrossRef]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ibba, I.; Gilli, A.; Boi, M.F.; Usai, P. Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting lactose malabsorption and intolerance. BioMed Res. Int. 2014, 2014, 680196. [Google Scholar] [CrossRef] [PubMed]
- de Vrese, M.; Stegelmann, A.; Richter, B.; Fenselau, S.; Laue, C.; Schrezenmeir, J. Probiotics—Compensation for lactase insufficiency. Am. J. Clin. Nutr. 2001, 73, 421S–429S. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L. Yogurt, living cultures, and gut health. Am J Clin Nutr 2014, 99, 1248S–1250S. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Saborido, R.; Leis, R. Yogurt and dietary recommendations for lactose intolerance. Nutr. Hosp. 2018, 35, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A. Lactose—A potential prebiotic. Aliment. Pharmacol. Ther. 2002, 16, 1591–1602. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Sackett, D.L.; Strauss, S.E.; Richardson, W.S. Evidence-Based Medicine: How to Practice and Teach EBM.; Churchill-Livingstone: London, UK, 2000. [Google Scholar]
- Montes, R.G.; Bayless, T.M.; Saavedra, J.M.; Perman, J.A. Effect of milks inoculated with Lactobacillus acidophilus or a yogurt starter culture in lactose-maldigesting children. J. Dairy Sci. 1995, 78, 1657–1664. [Google Scholar] [CrossRef]
- Pakdaman, M.N.; Udani, J.K.; Molina, J.P.; Shahani, M. The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance—A randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 2016, 15, 56–67. [Google Scholar] [CrossRef]
- Roškar, I.; Švigelj, K.; Štempelj, M.; Volfand, J.; Štabuc, B.; Malovrh, Š.; Rogelj, I. Effects of a probiotic product containing Bifidobacterium animalis subsp. animalis IM386 and Lactobacillus plantarum MP2026 in lactose intolerant individuals: Randomized, placebo-controlled clinical trial. J. Funct. Foods 2017, 35, 1–8. [Google Scholar] [CrossRef]
- Ojetti, V.; Gigante, G.; Gabrielli, M.; Ainora, M.E.; Mannocci, A.; Lauritano, E.C.; Gasbarrini, G.; Gasbarrini, A. The effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose intolerant patients: Randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 163–170. [Google Scholar] [PubMed]
- Savaiano, D.A.; Ritter, A.J.; Klaenhammer, T.R.; James, G.M.; Longcore, A.T.; Chandler, J.R.; Walker, W.A.; Foyt, H.L. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): A randomized, double-blind clinical trial. Nutr. J. 2013, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of bifidobacterium longum and lactobacillus rhamnosuson gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms: A randomised, double-blind, Crossover study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Yen, C.L.; Chen, S.H. Management of lactose maldigestion by consuming milk containing lactobacilli. Dig. Dis. Sci. 1998, 43, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Gilliland, S.E. Lactobacillus acidophilus as a dietary adjunct for milk to aid lactose digestion in humans. J. Dairy Sci. 1983, 66, 959–966. [Google Scholar] [CrossRef]
- Lin, M.Y.; Savaiano, D.; Harlander, S. Influence of nonfermented dairy products containing bacterial starter cultures on lactose maldigestion in humans. J. Dairy Sci. 1991, 74, 87–95. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Sterne, J.A.C. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0.; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2006; Available online: www.cochrane-handbook.org (accessed on 9 March 2020).
- He, T.; Priebe, M.G.; Zhong, Y.; Huang, C.; Harmsen, H.J.; Raangs, G.C.; Antoine, J.M.; Welling, G.W.; Vonk, R.J. Effects of yogurt and bifidobacteria supplementation on the colonic microbiota in lactose-intolerant subjects. J. Appl. Microbiol. 2008, 104, 595–604. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Munjal, A.K.; Khandia, R.; Samad, H.A.; Iqbal, H.M.N.; Joshi, S.K. Probiotics in Curing Allergic and Inflammatory Conditions—Research Progress and Futuristic Vision. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 10, 105–118. [Google Scholar] [CrossRef]
- Solomons, N.W. Fermentation, fermented foods and lactose intolerance. Eur. J. Clin. Nutr. 2002, 56, S50–S55. [Google Scholar] [CrossRef]
- Corr, S.; Hill, C.; Gahan, C.G. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 2009, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Savaiano, D.A. Lactose digestion from yogurt: Mechanism and relevance. Am. J. Clin. Nutr. 2014, 99, 1251S–1255S. [Google Scholar] [CrossRef] [PubMed]
- Born, P.; Sekatcheva, M.; Rösch, T.; Classen, M. Carbohydrate malabsorption in clinical routine: A prospective observational study. Hepatogastroenterology 2006, 53, 673–677. [Google Scholar] [PubMed]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W. Probiotics: From Isolation to Application. J. Am. Coll. Nutr. 2017, 36, 666–676. [Google Scholar] [CrossRef]
- Zhu, D.; Sun, Y.; Huo, G.C.; Yang, L.; Liu, F.; Li, A.; Meng, X.C. Complete genome sequence of Bifidobacterium animalis subsp. lactis KLDS 2.0603, a probiotic strain with digestive tract resistance and adhesion to the intestinal epithelial cells. J. Biotechnol. 2016, 220, 49–50. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, C.Y.; He, T.; Harmsen, H.M. Effect of probiotics and yogurt on colonic microflora in subjects with lactose intolerance. Wei Sheng Yan Jiu 2006, 35, 587–591. [Google Scholar]
- Davis, L.M.; Martínez, I.; Walter, J.; Goin, C.; Hutkins, R.W. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE 2011, 6, e25200. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Ritter, A.J.; Savaiano, D.; Monteagudo-Mera, A.; Anderson, C.; Magness, S.T.; Klaenhammer, T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA 2017, 114, E367–E375. [Google Scholar] [CrossRef]
- Arnold, J.W.; Simpson, J.B.; Roach, J.; Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Prebiotics for Lactose Intolerance: Variability in Galacto-Oligosaccharide Utilization by Intestinal Lactobacillus rhamnosus. Nutrients 2018, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.D.; Di Palma, J.A. Carbohydrate challenge tests: Do you need to measure methane? South. Med. J. 2012, 105, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Harvie, R.M.; Tuck, C.J.; Schultz, M. Evaluation of lactulose, lactose, and fructose breath testing in clinical practice: A focus on methane. JGH Open 2019, 4, 198–205. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.P.; Ledochowski, M.; Ratcliffe, N.M. The importance of methane breath testing: A review. J. Breath Res. 2013, 7, 024001. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.L.; Savaiano, D.A.; Levitt, M.D. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N. Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology 2017, 152, 515–532. [Google Scholar] [CrossRef]
- Dainese, R.; Casellas, F.; Mariné-Barjoan, E.; Vivinus-Nébot, M.; Schneider, S.M.; Hébuterne, X.; Piche, T. Perception of lactose intolerance in irritable bowel syndrome patients. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1167–1175. [Google Scholar] [CrossRef]
- Larsen, C.N.; Nielsen, S.; Kaestel, P.; Brockmann, E.; Bennedsen, M.; Christensen, H.R.; Eskesen, D.C.; Jacobsen, B.L.; Michaelsen, K.F. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur. J. Clin. Nutr. 2006, 60, 1284–1293. [Google Scholar]
- Almeida, C.C.; Lorena, S.L.; Pavan, C.R.; Akasaka, H.M.; Mesquita, M.A. Beneficial effects of long-term consumption of a probiotic combination of lactobacillus casei shirota and bifidobacterium breve yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012, 27, 247–251. [Google Scholar] [CrossRef]
- Begtrup, L.M.; de Muckadell, O.B.; Kjeldsen, J.; Christensen, R.D.; Jarbøl, D.E. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome—A randomised, double-blind, placebo controlled trial. Scand. J. Gastroenterol. 2013, 48, 1127–1135. [Google Scholar] [CrossRef]
- Maynard, C.; Weinkove, D. The gut microbiota and ageing. Subcell. Biochem. 2018, 90, 351–371. [Google Scholar] [CrossRef]
| Parameter 1 | Inclusion Criteria |
|---|---|
| Population | Lactose-intolerant subjects |
| Intervention | Controlled intake of biotics |
| Comparison | Non-exposed control group |
| Outcomes | Symptoms of lactose intolerance and signs of lactose malabsorption |
| Settings | Controlled trials |
| Reference | n | Age, y 1 | Intervention | Trial Type (Intervention Duration) | Outcome Measure | Results 2 | Conclusions |
|---|---|---|---|---|---|---|---|
| Montes et al. (1995) [30] | 20 (11F) | 5–16 | IG1: 1010 CFU L. acidophilus IG2: 108 CFU L. acidophilus + 1010 CFU S. thermophilus (250 mL milk) | Crossover RCT (-) | Mean 8-h symptom score for abdominal pain, bloating, borborygmi and flatus (0 = absent, 4 = severe symptoms) after ingestion of 2 g/kg of lactose | Symptom score: IG1 0.9 ± 0.43; IG2 1.62 ± 0.71; CG 4.6 ± 0.73 | Significantly lower symptom score |
| Pakdaman et al. (2016) [31] | 38 | 18–75 | 109 CFU DDS-1 strain of L. acidophilus/day (capsules) | Crossover RCT (4 weeks) | Mean 6-h symptom scores (0 = no symptoms, 10 = most severe symptoms) after ingestion of 25 g of lactose | Abdominal cramping: IG 1.94 ± 2.341; CG 2.39 ± 2.188 Bowel sounds: IG 2.76 ± 2.536; CG 2.86 ± 2.497 Diarrhea: IG 1.34 ± 2.462; CG 1.69 ± 2.558 Flatulence: IG 3.16 ± 2.873; CG 3.21 ± 2.699 Vomiting: IG 0.08 ± 0.379; CG 0.36 ± 0.936 Overall symptoms: IG 9.28 ± 9.202; CG 10.51 ± 9.327 | Significantly less abdominal cramping, diarrhea, vomiting and lower overall symptom score |
| Roškar et al. (2017) [32] | 44 (36F) IG 22 | IG 28 (19–54) CG 31 (18–55) | 1010 CFU L. plantarum + 1010 CFU B. animalis/day (capsules) | RCT (6 weeks) | Mean LI symptom assessment score (0 = absent, 10 = worst) | Abdominal pain: IG 2.4 (1.3–3.4); CG 2.3 (0.9–3.7) Diarrhea: IG 0.3 (−0.1;0.8); CG 0.6 (−0.3;1.5) Flatulence: IG 4.2 (2.9–5.5); CG 4.2 (2.8–5.5) Rumble: IG 3.9 (2.8–5.1); CG 3.6 (2.1–5.1) Vomiting: IG 0.2 (−0.2;0.7); CG 0.2 (−0.1;0.4) Total (Ʃ): IG 11.1 (7.9–14.3); CG: 10.8 (6.4–5.3) | No significant differences |
| Ojetti et al. (2010) [33] | 40 (33F) IG 20 | IG 33 ± 11 CG 32 ± 12 | 8 × 108 CFU L. reuteri/day (capsules) | RCT (10 days) | Mean 8-h symptom scores values (0 = absent, 10 = severe symptoms) after ingestion of 25 g of lactose | Abdominal pain: IG 6.9 ± 1.07; CG 7.1 ± 0.72 Bloating: IG 9.95±0.88; CG 7.1 ± 0.72 Diarrhea: IG 2.95±2.07; CG 5.9 ± 0.85 Flatulence: IG 3.95 ± 1.35; CG: 5.15 ± 0.93 | Significant improvement in abdominal pain, bloating, diarrhea, and flatulence |
| Savaiano et al. (2013) [34] | 85 (49F) IG 57 | 41 | 15 g RP-G28 (95% GOS)/day (capsules) | RCT (35 days) | Rate of disappearance of abdominal pain (%) | Abdominal pain: IG 72%; CG 28% | Significantly higher rate of disappearance of abdominal pain |
| Vitellio et al. (2019) [35] | 23 (19F) | 48 ± 3.1 | 4 × 109 CFU B. longum BB536 + 109 CFU L. rhamnosus/day (packets) | Crossover RCT (4 weeks) | Mean VAS perceived symptom score (0 = absent, 100 = worst) [abdominal pain and bloating] and mean BSFS (1 = constipation, 7 = diarrhea) | Abdominal pain: IG 39 ± 6; CG 53 ± 7 Bloating: IG 60 ± 5; CG 77 ± 4 Bowel movements: IG 3 ± 0; CG 3 ± 0 | Significantly less bloating |
| Lin et al. (1998) [36] | 20 | - | IG1: 4 × 108 CFU L. acidophilus/day IG2: 4 × 109 CFU L. acidophilus/day IG3: 4 × 108 CFU L. bulgaricus/day IG4: 4 × 109 CFU L. bulgaricus/day (400 mL milk) | Crossover RCT (-) | Mean 8-h symptom score for stomach pain, gas, and diarrhea (0 = absent, 5 = severe) after ingestion of 25 g of lactose | Symptom score: IG1 9.8; IG2 6.5; IG3 3.9; IG4 2.8; CG 12.5 | Significantly lower symptom score in IG2, IG3, and IG4 |
| Reference | n | Age, y 1 | Intervention | Trial Type (Intervention Duration) | Outcome Measure | Results 2 | Conclusions |
|---|---|---|---|---|---|---|---|
| Kim et al. (1983) [37] | 24 IG 6 × 3 | 20–31 | IG1: 1.25 × 107 CFU L. acidophilus/kg/day IG2: 1.25 × 108 CFU L. acidophilus/kg/day IG3: 1.25 × 109 CFU L. acidophilus/kg/day (milk 10 mL/kg/day) | RCT (6 days) | Change in mean breath H concentration (ppm) 3 h after ingestion of 5 mL/kg milk | Change in mean breath H concentration: IG1-15.2; IG2-1.1; IG3-19.2; CG-0.3 | Significant change in mean breath H concentration in IG1 and IG3. |
| Lin et al. (1991) [38] | 10 (4F) | 24–40 | IG1: 107 CFU L. acidophilus NCFM/day IG2: 108 CFU L. acidophilus NCFM/day IG3: 107 CFU L. acidophilus LA1/day IG4: 108 CFU L. acidophilus LA1/day IG5: 107 CFU L. acidophilus LA2/day IG6: 108 CFU L. acidophilus LA2/day IG7: 107 CFU S. thermophilus/L. bulgaricus/day IG8: 108 CFU S. thermophilus/L. bulgaricus/day (400 mL milk) | Crossover RCT (-) | Mean individual breath H concentration 8 h after ingestion of 25 g lactose | Breath H concentration: IG1 36.33; IG2 35.08; IG3 27.64; IG4 22.43; IG5 31.03; IG6 25.32; IG7 24.1; IG8 9.81; CG 30.78 | Significantly lower breath H concentration in IG4 and IG8 |
| Ojetti et al. (2010) [33] | 40 (33F) IG 20 | IG 33 ± 11 CG 32 ± 12 | 8 × 108 CFU L. reuteri/day (capsules) | RCT (10 days) | HBT normalization rate (%) Mean peak H2 excretion (ppm) | HBT normalization rate: IG 35%; CG 0% Peak H2: IG 23.1 ± 7.85; CG 31.7 ± 8.3 | Significantly higher HBT normalization rate and reduced mean peak H2 excretion |
| Savaiano et al. (2013) [34] | 85 (49F) IG 57 | 41 | 15 g RP-G28 (95% GOS)/day (capsules) | RCT (35 days) | Mean change in HBT values 2 h after ingestion of 25 g lactose | HBT change: IG-10.12; CG 13.95 | No significant differences |
| Lin et al. (1998) [36] | 20 | - | IG1: 4 × 108 CFU L. acidophilus/day IG2: 4 × 109 CFU L. acidophilus/day IG3: 4 × 108 CFU L. bulgaricus/day IG4: 4 × 109 CFU L. bulgaricus/day (400 mL milk) | Crossover RCT (-) | Mean hourly breath H concentration 8 h after ingestion of 25 g lactose | Breath H: IG1 262; IG2 231; IG3 188; IG4 135; CG 280 | Significantly lower breath H concentration in IG3 and IG4 (L. bulgaricus) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leis, R.; de Castro, M.-J.; de Lamas, C.; Picáns, R.; Couce, M.L. Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients 2020, 12, 1487. https://doi.org/10.3390/nu12051487
Leis R, de Castro M-J, de Lamas C, Picáns R, Couce ML. Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients. 2020; 12(5):1487. https://doi.org/10.3390/nu12051487
Chicago/Turabian StyleLeis, Rosaura, María-José de Castro, Carmela de Lamas, Rosaura Picáns, and María L. Couce. 2020. "Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials" Nutrients 12, no. 5: 1487. https://doi.org/10.3390/nu12051487
APA StyleLeis, R., de Castro, M.-J., de Lamas, C., Picáns, R., & Couce, M. L. (2020). Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients, 12(5), 1487. https://doi.org/10.3390/nu12051487







