Tart Cherry Increases Lifespan in Caenorhabditis elegans by Altering Metabolic Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tart Cherry (TC) Extract
2.2. Strains and Growth Conditions
2.3. Lifespan Assay
2.4. Gene Expression Analyses
2.5. Mitochondrial Respiration Analysis
2.6. Statistical Analysis
3. Results
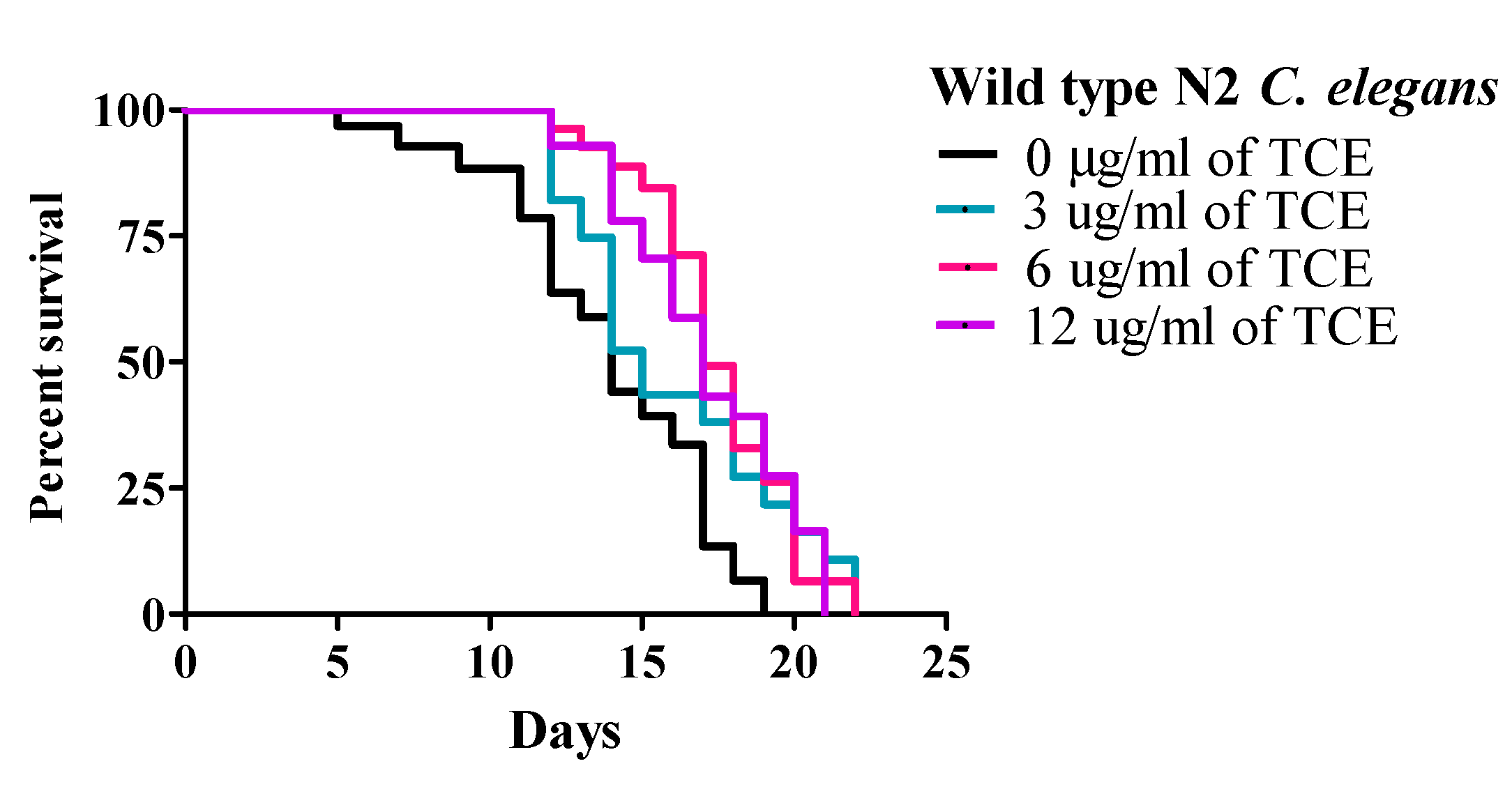
3.1. Tart Cherry Extends Mean Lifespan of Wild Type N2 C. elegans in a Dose-Dependent Manner
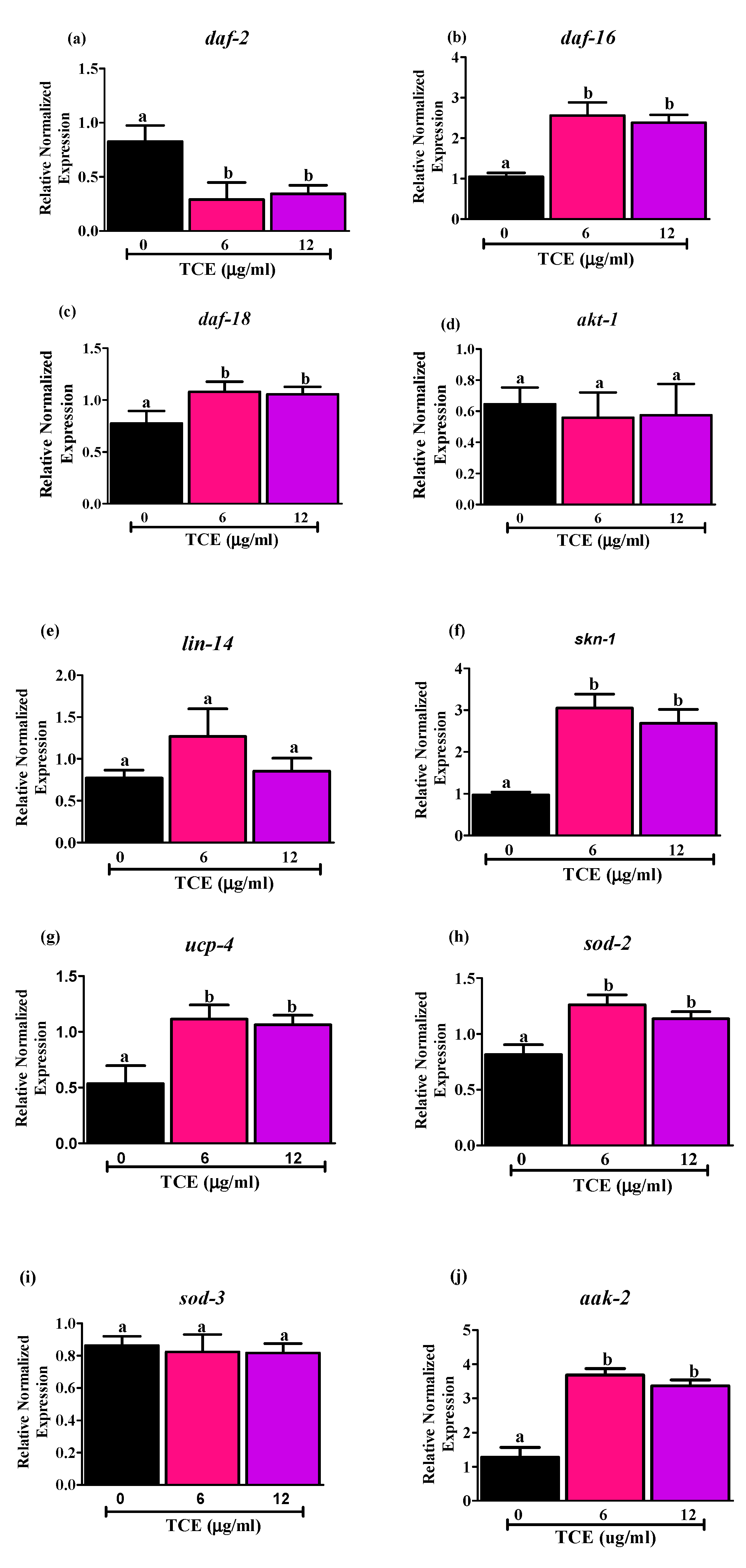
3.2. Tart Cherry Effects on Genes Related to Aging
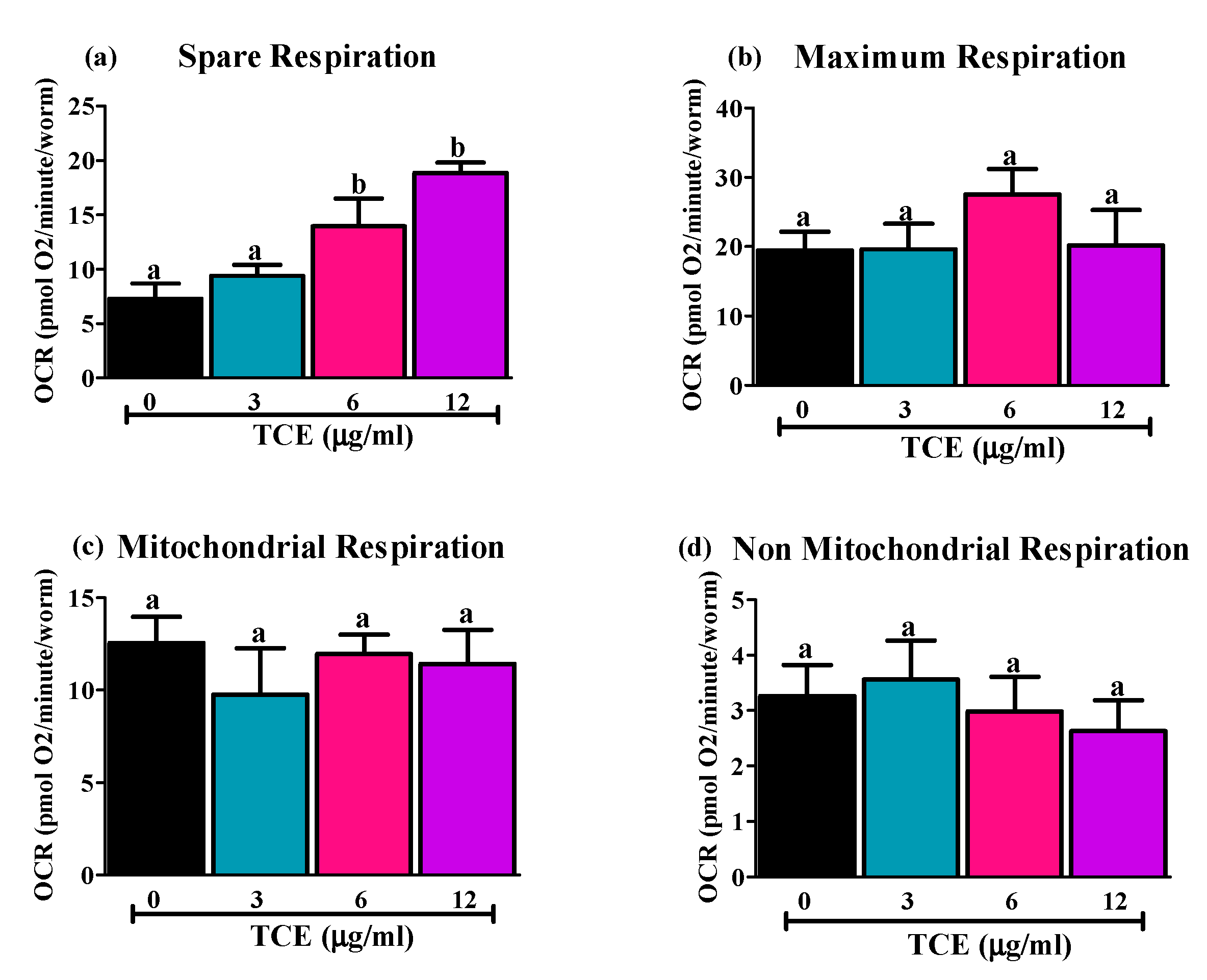
3.3. Effects of TCE on C. elegans Respiration
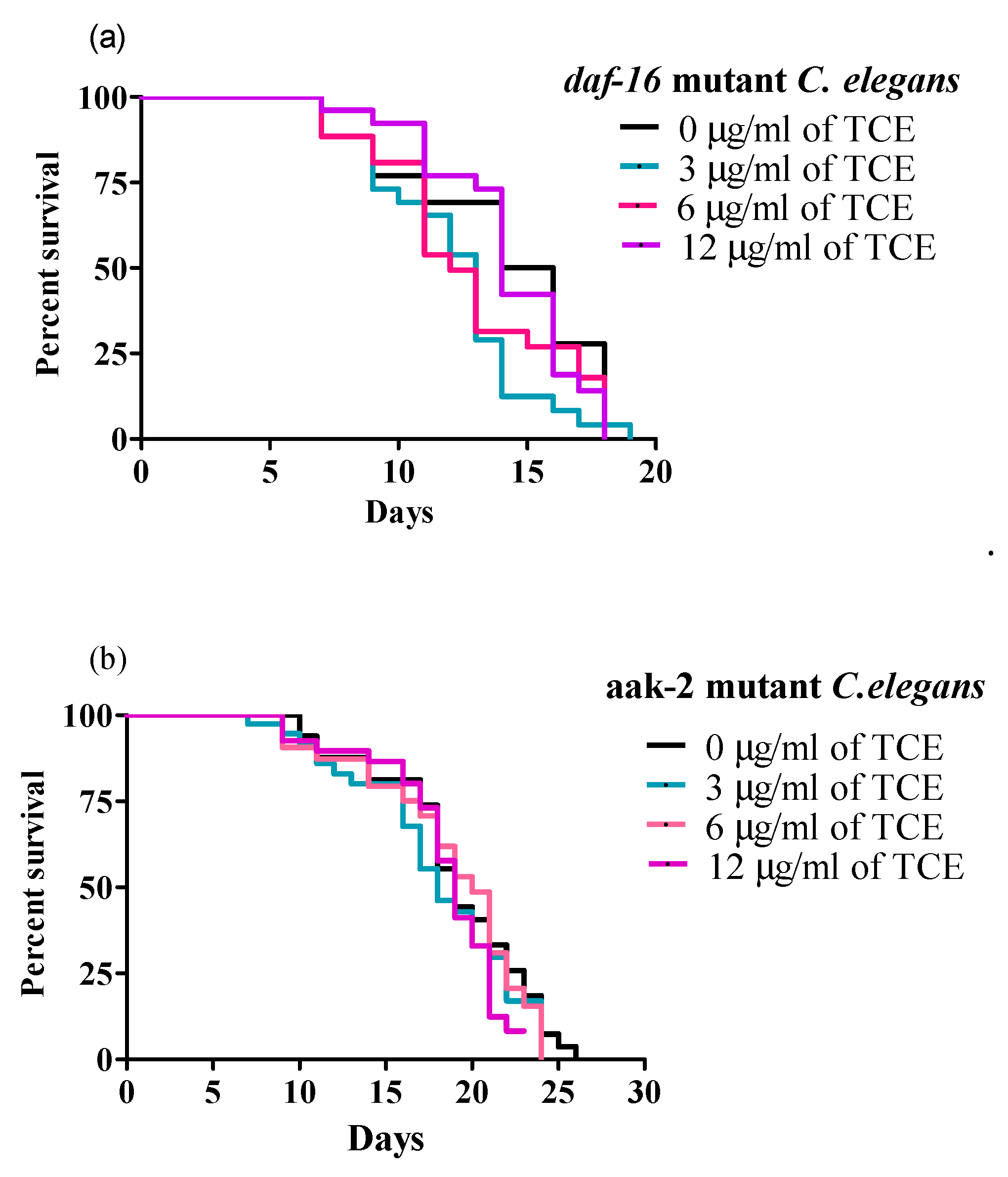
3.4. TCE Did Not Extend Mean Lifespan in Daf-16 and Aak-2 Mutant Worms
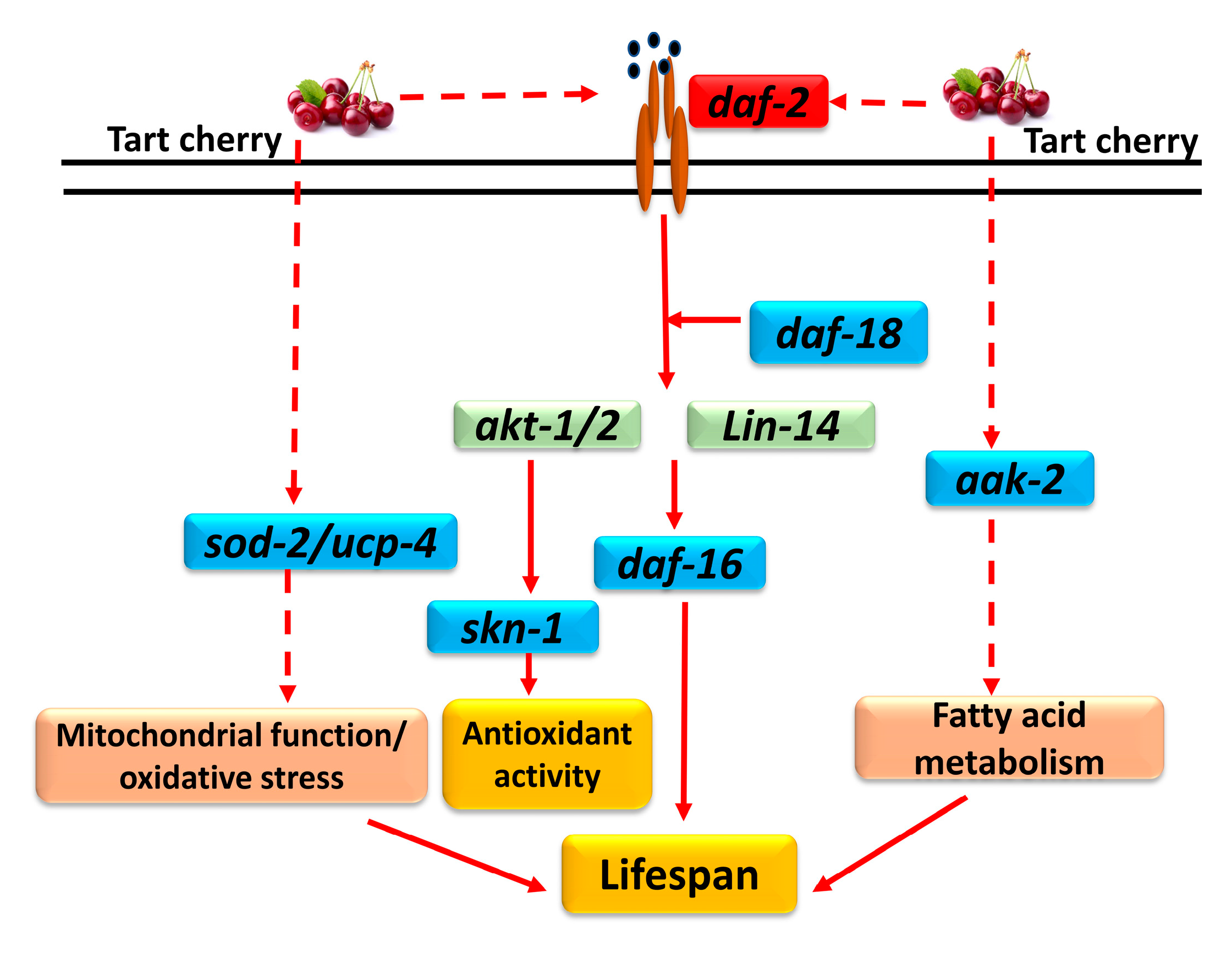
4. Discussion
4.1. TCE Increases Lifespan through IIS and DAF-16 Pathway
4.2. Involvement of AMPK/AAK-2 in Lifespan Determination
4.3. Role of Mitochondria in Lifespan Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mico, V.; Berninches, L.; Tapia, J.; Daimiel, L. NutrimiRAging: Micromanaging nutrient sensing pathways through nutrition to promote healthy aging. Int. J. Mol. Sci. 2017, 18, 915. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet: Aging in the United States. 2019. Available online: https://www.prb.org/aging-unitedstates-fact-sheet (accessed on 13 January 2020).
- United Nations: Global Issues, Ageing. 2019. Available online: https://www.un.org/en/sections/issues-depth/ageing/ (accessed on 12 August 2019).
- Lu, Y.; Krishnan, A.; Brommer, B.; Tian, X.; Meer, M.; Vera, D.; Wang, C.; Zeng, Q.; Yu, D.; Bonkowski, M.; et al. Reversal of ageing- and injury-induced vision loss by Tet-dependent epigenetic reprogramming. BioRxiv 2019. [Google Scholar] [CrossRef]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Wei, C.C.; Liao, V.H. Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated by age-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Radic. Res. 2014, 48, 371–379. [Google Scholar] [CrossRef]
- Larsen, P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 8905–8909. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.M.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Chen, Z.-Y. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Exp. Gerontol. 2015, 69, 189–195. [Google Scholar] [CrossRef]
- Masoro, E.J. Caloric restriction and aging: An update. Exp. Gerontol. 2000, 35, 299–305. [Google Scholar] [CrossRef]
- Keenan, K.P.; Soper, K.A.; Hertzog, P.R.; Gumprecht, L.A.; Smith, P.F.; Mattson, B.A.; Ballam, G.C.; Clark, R.L. Diet, overfeeding, and moderate dietary restriction in control sprague-dawley Rats: II. Effects on age-related proliferative and degenerative lesions. Toxicol. Pathol. 1995, 23, 287–302. [Google Scholar] [CrossRef]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric restriction and aging: Studies in mice and monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Guha, S.; Cao, M.; Kane, R.M.; Savino, A.M.; Zou, S.; Dong, Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age 2013, 35, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 target genes that control C. Elegans life-span and metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Gusarov, I.; Pani, B.; Gautier, L.; Smolentseva, O.; Eremina, S.; Shamovsky, I.; Katkova-Zhukotskaya, O.; Mironov, A.; Nudler, E. Glycogen controls Caenorhabditis elegans lifespan and resistance to oxidative stress. Nat. Commun. 2017, 8, 15868. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M.; Hekimi, S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000361. [Google Scholar] [CrossRef]
- Peng, C.; Zuo, Y.; Kwan, K.M.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.-Y. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp. Gerontol. 2012, 47, 170–178. [Google Scholar] [CrossRef]
- Sun, Y.; Yolitz, J.; Alberico, T.; Sun, X.; Zou, S. Lifespan extension by cranberry supplementation partially requires SOD2 and is life stage independent. Exp. Gerontol. 2014, 50, 57–63. [Google Scholar] [CrossRef][Green Version]
- Tambara, A.L.; de Los Santos Moraes, L.; Dal Forno, A.H.; Boldori, J.R.; Gonçalves Soares, A.T.; de Freitas Rodrigues, C.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C.C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.-G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002, 419, 808–814. [Google Scholar] [CrossRef]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-inflammatory and anti-obesity properties of food bioactive components: Effects on adipose tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Oboh, G.; Ogunsuyi, O.; Abolaji, A.O.; Udofia, A. Curcumin-supplemented diets improve antioxidant enzymes and alter acetylcholinesterase genes expression level in Drosophila melanogaster model. Metab. Brain Dis. 2018, 33, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Natarajan, O.; Murbach, C.G.; Dinh, J.; Wilson, E.C.; Cao, M.; Zou, S.; Dong, Y. Supplement timing of cranberry extract plays a key role in promoting Caenorhabditis elegans healthspan. Nutrients 2014, 6, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, Y.; Azat, R.; Zheng, X. Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 Pathways. Oxid. Med. Cell. Longev. 2017, 2017, 7956158. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Gutierrez, E.; Ramos Solano, B.; Seymour, E.M.; Bolling, S.F. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chem. 2018, 252, 142–146. [Google Scholar] [CrossRef]
- Desai, T.; Bottoms, L.; Roberts, M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur. J. Appl. Physiol. 2018, 118, 2523–2539. [Google Scholar] [CrossRef]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Wooden, A. Tart cherry juice induces differential dose-dependent effects on apoptosis, but not cellular proliferation, in MCF-7 human breast cancer cells. J. Med. Food 2012, 15, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, S.; Stull, A.J.; Miranda, A.; Scoggin, S.; Claycombe-Larson, K.; Kim, J.H.; Moustaid-Moussa, N. Tart cherry reduces inflammation in adipose tissue of zucker fatty rats and cultured 3T3-L1 adipocytes. Nutrients 2018, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Dowlatshahi, D.; Banko, M.R.; Villen, J.; Hoang, K.; Blanchard, D.; Gygi, S.P.; Brunet, A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007, 17, 1646–1656. [Google Scholar] [CrossRef]
- Guarente, L.; Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 2000, 408, 255–262. [Google Scholar] [CrossRef]
- Lai, C.H.; Chou, C.Y.; Ch’ang, L.Y.; Liu, C.S.; Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef]
- Rahman, M.; Hewitt, J.E.; Van-Bussel, F.; Edwards, H.; Blawzdziewicz, J.; Szewczyk, N.J.; Driscoll, M.; Vanapalli, S.A. NemaFlex: A microfluidics-based technology for standardized measurement of muscular strength of C. elegans. Lab Chip 2018, 18, 2187–2201. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Pollard, A.K.; Lesanpezeshki, L.; Deane, C.S.; Gaffney, C.J.; Etheridge, T.; Szewczyk, N.J.; Vanapalli, S.A. Muscle strength deficiency and mitochondrial dysfunction in a muscular dystrophy model of Caenorhabditis elegans and its functional response to drugs. Dis. Models Mech. 2018, 11. [Google Scholar] [CrossRef]
- Rahman, M.; Edwards, H.; Birze, N.; Gabrilska, R.; Rumbaugh, K.P.; Blawzdziewicz, J.; Szewczyk, N.J.; Driscoll, M.; Vanapalli, S.A. NemaLife: A structured microfluidic culture device optimized for aging studies in crawling C. elegans. bioRxiv 2019, 675827. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Iezzoni, A.F.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Quantification and characterization of anthocyanins in balaton tart cherries. J. Agric. Food Chem. 1997, 45, 2556–2560. [Google Scholar] [CrossRef]
- Zhou, Z.; Nair, M.G.; Claycombe, K.J. Synergistic inhibition of interleukin-6 production in adipose stem cells by tart cherry anthocyanins and atorvastatin. Phytomed. Int. J. Phytother. Phytopharm. 2012, 19, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Navas, M.J.; Jiménez-Moreno, A.M.; Asuero, A.M.J.-M.; Agustín, G. Anthocyanin pigments: Importance, sample preparation and extraction. Phenolic Compd. Nat. Sources Importance Appl. 2017. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Koopman, M.; Michels, H.; Dancy, B.M.; Kamble, R.; Mouchiroud, L.; Auwerx, J.; Nollen, E.A.; Houtkooper, R.H. A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat. Protoc. 2016, 11, 1798–1816. [Google Scholar] [CrossRef] [PubMed]
- Masse, I.; Molin, L.; Billaud, M.; Solari, F. Lifespan and dauer regulation by tissue-specific activities of Caenorhabditis elegans DAF-18. Dev. Biol. 2005, 286, 91–101. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Luz, A.L.; Smith, L.L.; Rooney, J.P.; Meyer, J.N. Seahorse Xfe 24 extracellular flux analyzer-based analysis of cellular respiration in caenorhabditis elegans. Curr. Protoc. Toxicol. 2015, 66, 25.7.1–25.7.15. [Google Scholar] [CrossRef]
- Apfeld, J.; O’Connor, G.; McDonagh, T.; DiStefano, P.S.; Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004, 18, 3004–3009. [Google Scholar] [CrossRef]
- Thangthaeng, N.; Poulose, S.M.; Gomes, S.M.; Miller, M.G.; Bielinski, D.F.; Shukitt-Hale, B. Tart cherry supplementation improves working memory, hippocampal inflammation, and autophagy in aged rats. Age 2016, 38, 393–404. [Google Scholar] [CrossRef]
- Thibado, S.P.; Thornthwaite, J.T.; Ballard, T.K.; Goodman, B.T. Anticancer effects of Bilberry anthocyanins compared with NutraNanoSphere encapsulated Bilberry anthocyanins. Mol. Clin. Oncol. 2018, 8, 330–335. [Google Scholar] [CrossRef] [PubMed]
- van de Klashorst, D.; van den Elzen, A.; Weeteling, J.; Roberts, M.; Desai, T.; Bottoms, L.; Hughes, S. Montmorency tart cherry (Prunus cerasus L.) acts as a calorie restriction mimetic that increases intestinal fat and lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103890. [Google Scholar] [CrossRef]
- Smith, B.J.; Crockett, E.K.; Chongwatpol, P.; Graef, J.L.; Clarke, S.L.; Rendina-Ruedy, E.; Lucas, E.A. Montmorency tart cherry protects against age-related bone loss in female C57BL/6 mice and demonstrates some anabolic effects. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xie, Q.; Ding, Y.H.; Li, S.T.; Peng, S.; Zhang, Y.P.; Tan, D.; Yuan, Z.; Dong, M.Q. CAMKII and calcineurin regulate the lifespan of Caenorhabditis elegans through the FOXO transcription factor DAF-16. Elife 2013, 2, e00518. [Google Scholar] [CrossRef]
- Jia, K.; Chen, D.; Riddle, D.L. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 2004, 131, 3897–3906. [Google Scholar] [CrossRef]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, L.; Zhou, L. Oleanolic acid activates daf-16 to increase lifespan in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2015, 468, 843–849. [Google Scholar] [CrossRef]
- Bass, T.M.; Weinkove, D.; Houthoofd, K.; Gems, D.; Partridge, L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007, 128, 546–552. [Google Scholar] [CrossRef]
- Moraes, D.P.; Lozano-Sánchez, J.; Machado, M.L.; Vizzotto, M.; Lazzaretti, M.; Leyva-Jimenez, F.J.J.; da Silveira, T.L.; Ries, E.F.; Barcia, M.T. Characterization of a new blackberry cultivar BRS Xingu: Chemical composition, phenolic compounds, and antioxidant capacity in vitro and in vivo. Food Chem. 2020, 322, 126783. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; An, S.W.A.; Artan, M.; Seo, M.; Hwang, A.B.; Jeong, D.-E.; Son, H.G.; Hwang, W.; Lee, D.; Seo, K.; et al. Genes and pathways that influence longevity in Caenorhabditis elegans. In Aging Mechanisms: Longevity, Metabolism, and Brain Aging; Mori, N., Mook-Jung, I., Eds.; Springer: Japan, Tokyo, 2015; pp. 123–169. [Google Scholar] [CrossRef]
- Koch, K.; Weldle, N.; Baier, S.; Büchter, C.; Wätjen, W. Hibiscus sabdariffa L. Extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020, 59, 137–150. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crop. Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Moreno-Arriola, E.; El Hafidi, M.; Ortega-Cuellar, D.; Carvajal, K. AMP-Activated protein kinase regulates oxidative metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 transcriptional regulators. PLoS ONE 2016, 11, e0148089. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Song, H.-O.; Cho, J.H. Mitochondrial uncoupling attenuates age-dependent neurodegeneration in C. elegans. Mol. Cells 2017, 40, 864–870. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Krstin, S.; Rohrig, T.; Richling, E.; Wink, M. An anthocyanin-rich extract of acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 1283–1290. [Google Scholar] [CrossRef]
- Yan, F.; Chen, X.A.; Zheng, X. Protective effect of mulberry fruit anthocyanin on human hepatocyte cells (LO2) and Caenorhabditis elegans under hyperglycemic conditions. Food Res. Int. 2017, 102, 213–224. [Google Scholar] [CrossRef]
- Miriyala, S.; Holley, A.K.; St Clair, D.K. Mitochondrial superoxide dismutase—Signals of distinction. Anticancer Agents Med. Chem. 2011, 11, 181–190. [Google Scholar] [CrossRef]
- Unlu, E.S.; Koc, A. Effects of deleting mitochondrial antioxidant genes on life span. Ann. N. Y. Acad. Sci. 2007, 1100, 505–509. [Google Scholar] [CrossRef]
- Oh, S.S.; Sullivan, K.A.; Wilkinson, J.E.; Backus, C.; Hayes, J.M.; Sakowski, S.A.; Feldman, E.L. Neurodegeneration and early lethality in superoxide dismutase 2-deficient mice: A comprehensive analysis of the central and peripheral nervous systems. Neuroscience 2012, 212, 201–213. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Sarasija, S.; Norman, K.R. Measurement of oxygen consumption rates in intact Caenorhabditis elegans. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Desler, C.; Hansen, T.L.; Frederiksen, J.B.; Marcker, M.L.; Singh, K.K.; Juel Rasmussen, L. Is there a link between mitochondrial reserve respiratory capacity and aging? J. Aging Res. 2012, 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 1955, 217, 383–393. [Google Scholar]
- Gerencser, A.A.; Neilson, A.; Choi, S.W.; Edman, U.; Yadava, N.; Oh, R.J.; Ferrick, D.A.; Nicholls, D.G.; Brand, M.D. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal. Chem. 2009, 81, 6868–6878. [Google Scholar] [CrossRef]
- Will, Y.; Hynes, J.; Ogurtsov, V.I.; Papkovsky, D.B. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat. Protoc. 2006, 1, 2563–2572. [Google Scholar] [CrossRef]
- Dilberger, B.; Passon, M.; Asseburg, H.; Silaidos, C.V.; Schmitt, F.; Schmiedl, T.; Schieber, A.; Eckert, G.P. Polyphenols and Metabolites Enhance Survival in Rodents and Nematodes-Impact of Mitochondria. Nutrients 2019, 11, 1886. [Google Scholar] [CrossRef]
- Enerback, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Arsenijevic, D.; Onuma, H.; Pecqueur, C.; Raimbault, S.; Manning, B.S.; Miroux, B.; Couplan, E.; Alves-Guerra, M.C.; Goubern, M.; Surwit, R.; et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A.J.; Grujic, D.; Zhang, C.Y.; Hagen, T.; Boss, O.; Ido, Y.; Szczepanik, A.; Wade, J.; Mootha, V.; Cortright, R.; et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 2000, 275, 16258–16266. [Google Scholar] [CrossRef] [PubMed]
- Iser, W.B.; Kim, D.; Bachman, E.; Wolkow, C. Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans. Mech. Ageing Dev. 2005, 126, 1090–1096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, I.; Hwang, G.J.; Cho, J.H. Uncoupling Protein, UCP-4 May Be Involved in neuronal defects during aging and resistance to pathogens in Caenorhabditis elegans. Mol. Cells 2016, 39, 680–686. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayarathne, S.; Ramalingam, L.; Edwards, H.; Vanapalli, S.A.; Moustaid-Moussa, N. Tart Cherry Increases Lifespan in Caenorhabditis elegans by Altering Metabolic Signaling Pathways. Nutrients 2020, 12, 1482. https://doi.org/10.3390/nu12051482
Jayarathne S, Ramalingam L, Edwards H, Vanapalli SA, Moustaid-Moussa N. Tart Cherry Increases Lifespan in Caenorhabditis elegans by Altering Metabolic Signaling Pathways. Nutrients. 2020; 12(5):1482. https://doi.org/10.3390/nu12051482
Chicago/Turabian StyleJayarathne, Shasika, Latha Ramalingam, Hunter Edwards, Siva A. Vanapalli, and Naima Moustaid-Moussa. 2020. "Tart Cherry Increases Lifespan in Caenorhabditis elegans by Altering Metabolic Signaling Pathways" Nutrients 12, no. 5: 1482. https://doi.org/10.3390/nu12051482
APA StyleJayarathne, S., Ramalingam, L., Edwards, H., Vanapalli, S. A., & Moustaid-Moussa, N. (2020). Tart Cherry Increases Lifespan in Caenorhabditis elegans by Altering Metabolic Signaling Pathways. Nutrients, 12(5), 1482. https://doi.org/10.3390/nu12051482






