Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea?
Abstract
1. Introduction
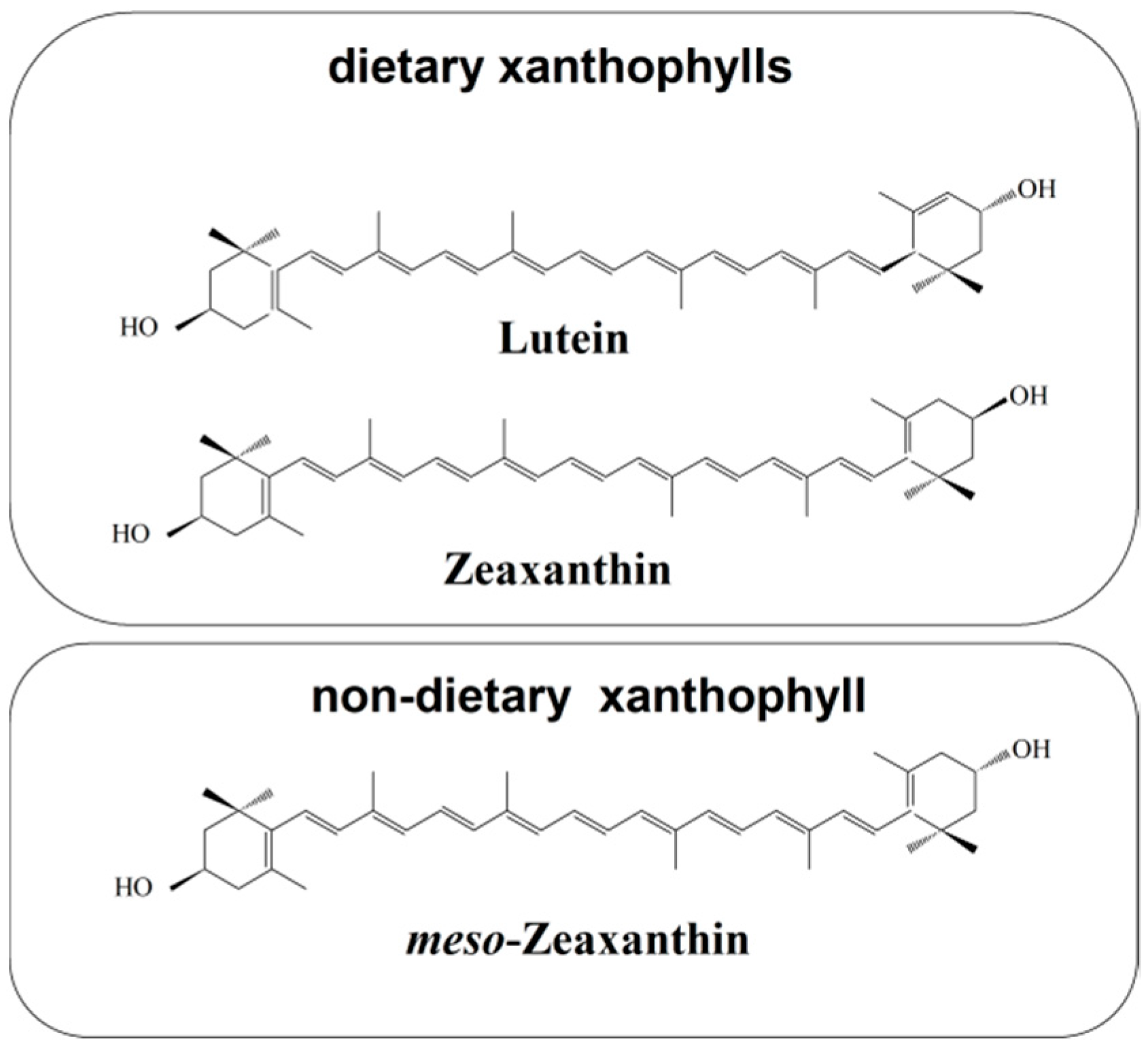
2. Zeaxanthin and Human Nutrition
2.1. Dietary Sources of Zeaxanthin
2.2. Bioavailability of Zeaxanthin from the food Matrix
3. Zeaxanthin in the Human Retina
3.1. Mechanisms of Selective Transport
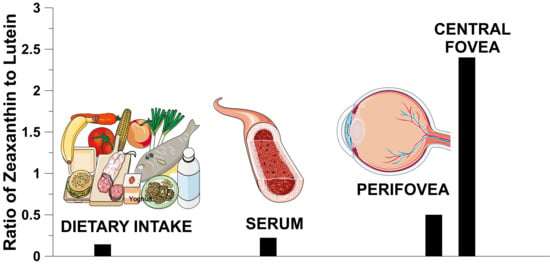
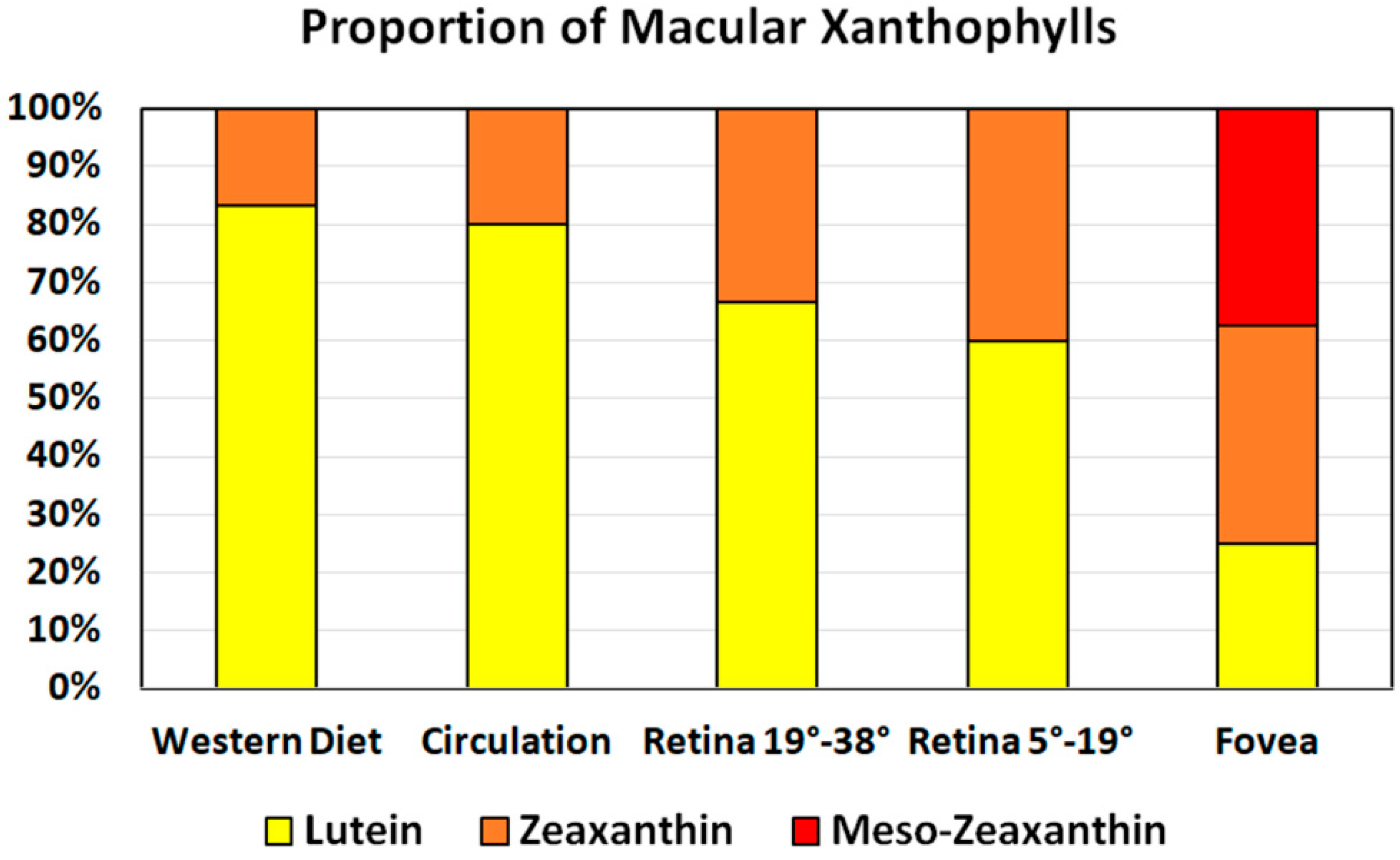
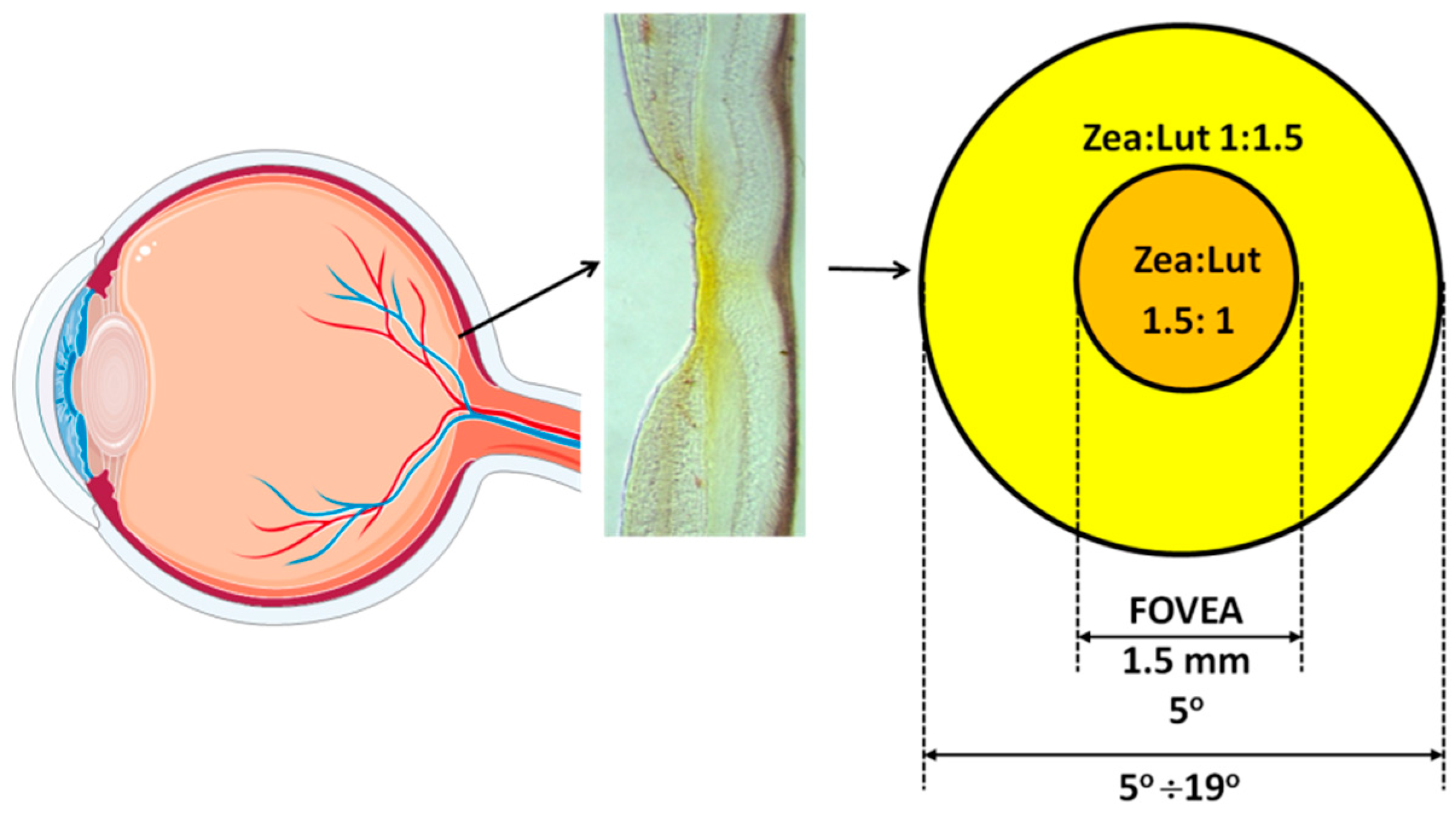
3.2. Spatial Distribution
3.3. Retinal Layers Distribution
4. Antioxidant Properties of Zeaxanthin
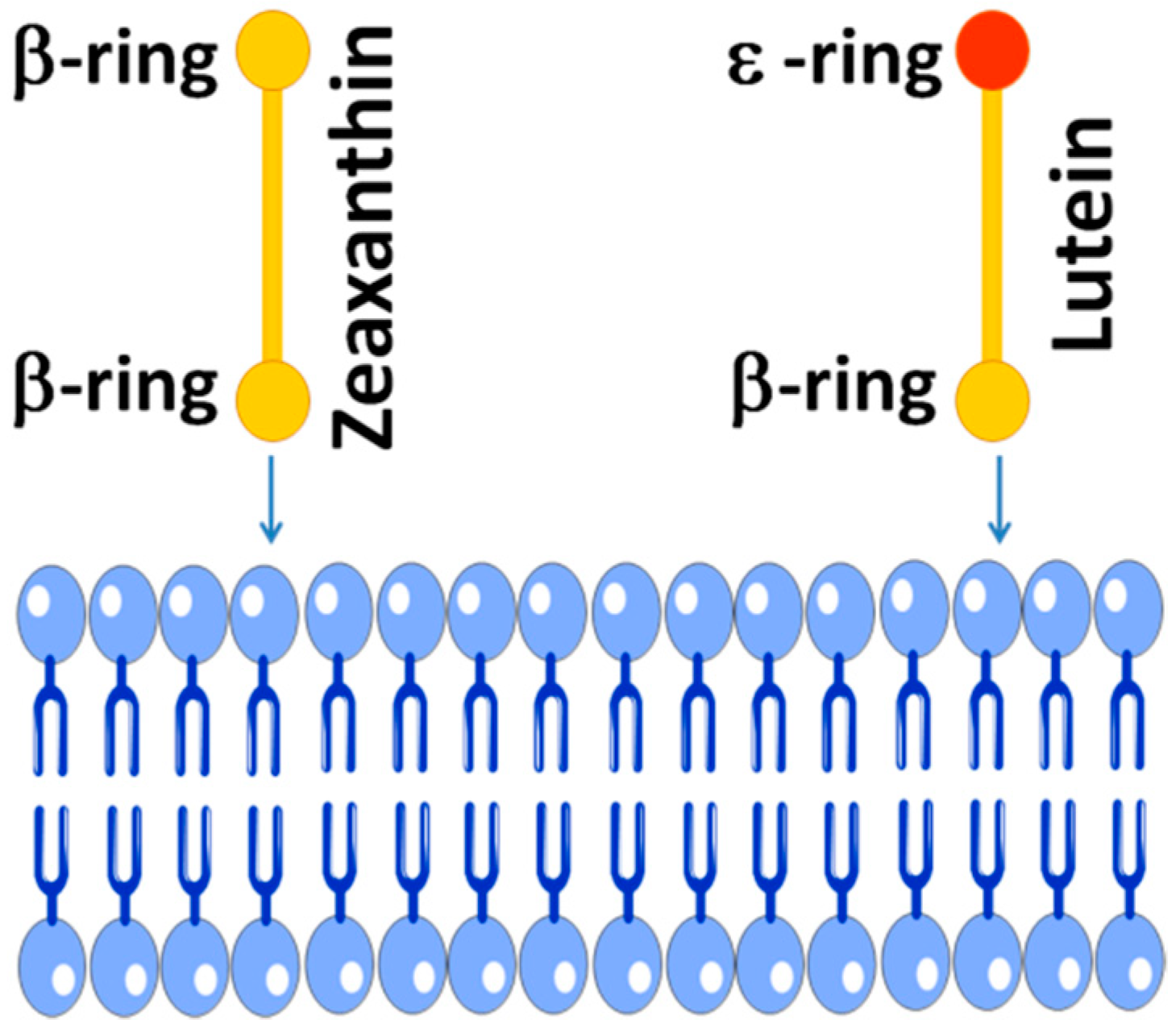
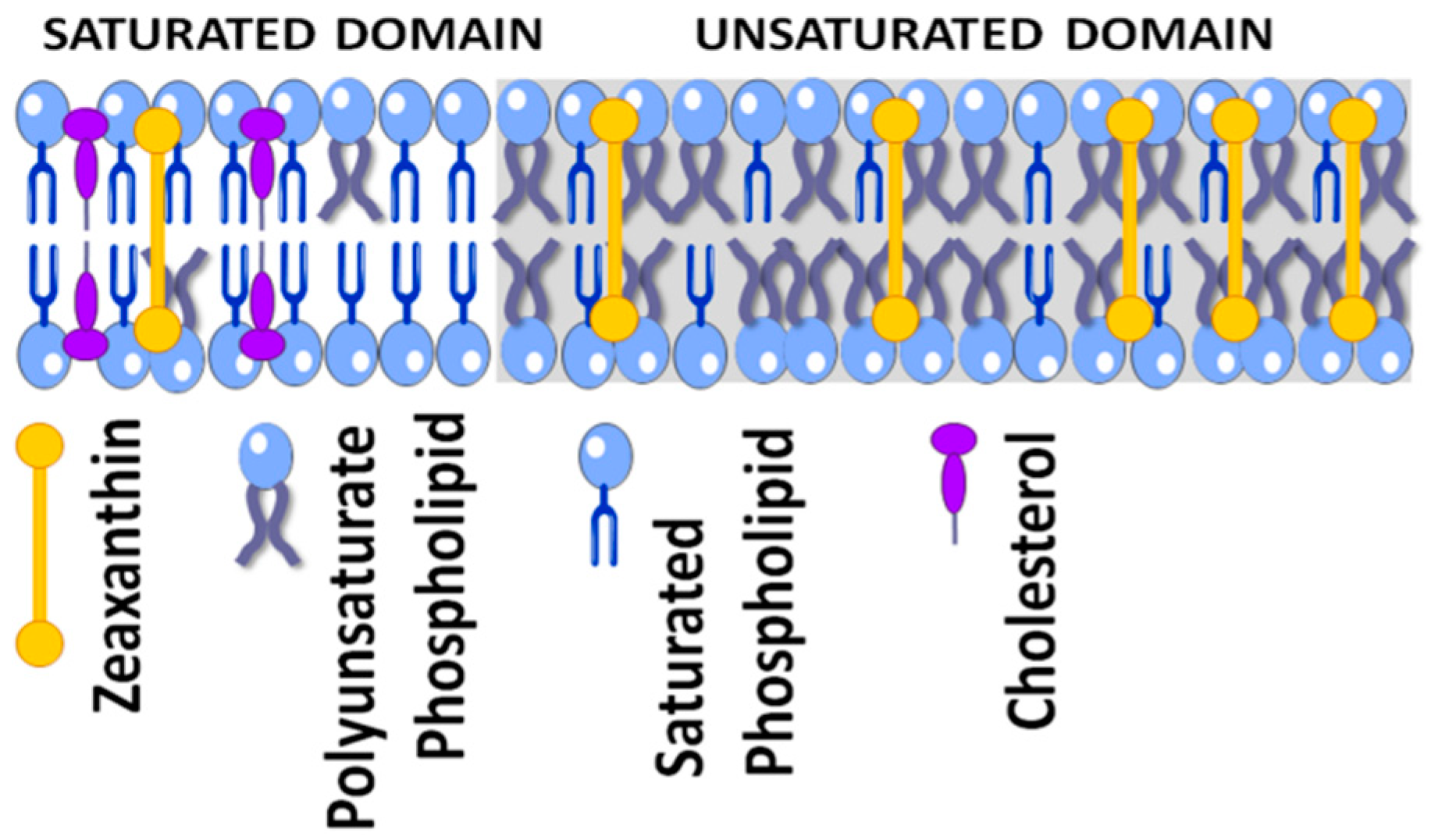
4.1. Physical Antioxidant Actions
4.2. Chemical Antioxidant Actions
5. Zeaxanthin Supplementation
5.1. Zeaxanthin Supplements and AREDS Formulation
5.2. Effects of Zeaxanthin Supplementation on MPOD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chung, H.-Y.; Ferreira, A.L.A.; Epstein, S.; Paiva, S.A.R.; Castaneda-Sceppa, C.; Johnson, E.J. Site-specific concentrations of carotenoids in adipose tissue: Relations with dietary and serum carotenoid concentrations in healthy adults. Am. J. Clin. Nutr. 2009, 90, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla, B.; Granado, F.; Southon, S.; Wright, A.J.A.; Blanco, I.; Gil-Martinez, E.; van den Berg, H.; Corridan, B.; Roussel, A.-M.; Chopra, M.; et al. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br. J. Nutr. 2001, 85, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef] [PubMed]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health. Aging 2004, 8, 156–162. [Google Scholar] [PubMed]
- Whitehead, A.J.; Mares, J.A.; Danis, R.P. Macular pigment: A review of current knowledge. Arch. Ophthalmol. 2006, 124, 1038–1045. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Shyam, R.; Li, B.; Vachali, P.; Subhani, Y.K.; Nelson, K.; Bernstein, P.S. Developmentally regulated production of meso-zeaxanthin in chicken retinal pigment epithelium/choroid and retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1853–1861. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Cao, Y.; Howard, A.N.; Alvarez-Calderon, F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr. Metab. 2007, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Ophthalmol. Vis. Sci. 2005, 46, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.R.; Feeney-Burns, L.; Peterson, L.H.; Klein, M.L.; Neuringer, M. Diet-related macular anomalies in monkeys. Investig. Ophthalmol. Vis. Sci. 1980, 19, 857–863. [Google Scholar] [PubMed]
- Li, B.; Vachali, P.; Bernstein, P.S. Human ocular carotenoid-binding proteins. Photochem. Photobiol. Sci. 2010, 9, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.J.; Snodderly, D.M.; Krinsky, N.I.; Russett, M.D.; Adler, A.J. Biological control of primate macular pigment. Biochemical and densitometric studies. Investig. Ophthalmol. Vis. Sci. 1991, 32, 257–267. [Google Scholar] [PubMed]
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011, 50, 2541–2549. [Google Scholar] [CrossRef]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef]
- Hammond, B.R.; Johnson, E.J.; Russell, R.M.; Krinsky, N.I.; Yeum, K.J.; Edwards, R.B.; Snodderly, D.M. Dietary modification of human macular pigment density. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1795–1801. [Google Scholar]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Guerra, L.H.; Ruiz, C.A. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J. Nutr. 2003, 133, 992–998. [Google Scholar] [CrossRef]
- Hammond, B.R.; Wooten, B.R.; Snodderly, D.M. Individual variations in the spatial profile of human macular pigment. J. Opt. Soc. Am. A JOSAA 1997, 14, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Zeimer, M.B.; Krömer, I.; Spital, G.; Lommatzsch, A.; Pauleikhoff, D. Macular telangiectasia: Patterns of distribution of macular pigment and response to supplementation. Retina (Philadelphia, PA) 2010, 30, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Snodderly, D.M. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 1995, 62, 1448S–1461S. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R.; Goli, M.B.; Lusby, W.R. Separation and quantitation of carotenoids in foods. Meth. Enzymol. 1992, 213, 347–359. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R.; Goli, M.B.; Lusby, W.R.; Daitch, C.E. Separation and quantification of carotenoids in human plasma. Meth. Enzymol. 1992, 213, 205–219. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Brown, P.K.; Delori, F.C.; Auran, J.D. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 660–673. [Google Scholar]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Sommerburg, O.; Siems, W.G.; van Kuijk, F.J. Localization of carotenoids in different eye tissues. Biofactors 2000, 11, 3–6. [Google Scholar] [CrossRef]
- Rapp, L.M.; Maple, S.S.; Choi, J.H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1200–1209. [Google Scholar] [PubMed]
- Bernstein, P.S.; Khachik, F.; Carvalho, L.S.; Muir, G.J.; Zhao, D.-Y.; Katz, N.B. Identification and Quantitation of Carotenoids and their Metabolites in the Tissues of the Human Eye. Exp. Eye Res. 2001, 72, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Koo, E.; Neuringer, M.; SanGiovanni, J.P. Macular xanthophylls, lipoprotein-related genes, and age-related macular degeneration1234. Am. J. Clin. Nutr. 2014, 100, 336S–346S. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Davis, M.D.; Ferris, F.L.; Gensler, G.R.; Kurinij, N.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007, 125, 671–679. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Neuringer, M. The promise of molecular genetics for investigating the influence of macular xanthophyllys on advanced age-related macular degeneration. Carotenoids Retinal Dis. 2013, 93–128. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Delori, F.C.; Richer, S.; van Kuijk, F.J.M.; Wenzel, A.J. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vis. Res. 2010, 50, 716–728. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vis. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Bhosale, P.; Zhao, D.Y.; Bernstein, P.S. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Investig. Ophthalmol. Vis. Sci. 2007, 48, 543–549. [Google Scholar] [CrossRef]
- Handelman, G.J.; Dratz, E.A.; Reay, C.C.; van Kuijk, J.G. Carotenoids in the human macula and whole retina. Investig. Ophthalmol. Vis. Sci. 1988, 29, 850–855. [Google Scholar] [PubMed]
- Beatty, S.; Boulton, M.; Henson, D.; Koh, H.; Murray, I. Macular pigment and age related macular degeneration. Br. J. Ophthalmol. 1999, 83, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Siems, W.G.; Hurst, J.S.; Lewis, J.W.; Kliger, D.S.; Kuijk, F.J.G.M. van Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr. Eye Res. 1999, 19, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; van Kuijk, F.J.G.M.; Chakravarthy, U. Macular pigment and age-related macular degeneration: Longitudinal data and better techniques of measurement are needed. Investig. Ophthalmol. Vis. Sci. 2008, 49, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Chucair, A.J.; Rotstein, N.P.; SanGiovanni, J.P.; During, A.; Chew, E.Y.; Politi, L.E. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: Relation with docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5168–5177. [Google Scholar] [CrossRef]
- Barker, F.M.; Snodderly, D.M.; Johnson, E.J.; Schalch, W.; Koepcke, W.; Gerss, J.; Neuringer, M. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3934–3942. [Google Scholar] [CrossRef]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Snellen, E.L.M.; Verbeek, A.L.M.; Van Den Hoogen, G.W.P.; Cruysberg, J.R.M.; Hoyng, C.B. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol. Scand. 2002, 80, 368–371. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L.; Gensler, G.E.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin a, e, and c intake with age-related macular degeneration in a case-control study: AREDS report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef]
- Cho, E.; Hankinson, S.E.; Rosner, B.; Willett, W.C.; Colditz, G.A. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am. J. Clin. Nutr. 2008, 87, 1837–1843. [Google Scholar] [CrossRef]
- Tan, J.S.L.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The blue mountains eye study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Eye disease case-control study group. Antioxidant status and neovascular age-related macular degeneration. Arch. Ophthalmol. 1993, 111, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A.; Kilburn, M.D. The macular pigment: A possible role in protection from age-related macular degeneration. Adv. Pharmacol. 1997, 38, 537–556. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; Klein, B.E.; Bowen, P.; Stacewicz-Sapuntzakis, M.; Palta, M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch. Ophthalmol. 1995, 113, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Nolan, J.M.; Stringham, J.M.; Beatty, S.; Snodderly, D.M. Spatial profile of macular pigment and its relationship to foveal architecture. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2134–2142. [Google Scholar] [CrossRef]
- Delori, F.C.; Goger, D.G.; Keilhauer, C.; Salvetti, P.; Staurenghi, G. Bimodal spatial distribution of macular pigment: Evidence of a gender relationship. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2006, 23, 521–538. [Google Scholar] [CrossRef]
- Berendschot, T.T.J.M.; van Norren, D. Macular pigment shows ringlike structures. Investig. Ophthalmol. Vis. Sci. 2006, 47, 709–714. [Google Scholar] [CrossRef][Green Version]
- Werner, J.S.; Donnelly, S.K.; Kliegl, R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vis. Res. 1987, 27, 257–268. [Google Scholar] [CrossRef]
- Pease, P.L.; Adams, A.J.; Nuccio, E. Optical density of human macular pigment. Vis. Res. 1987, 27, 705–710. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fuld, K. Interocular differences in macular pigment density. Investig. Ophthalmol. Vis. Sci. 1992, 33, 350–355. [Google Scholar] [PubMed]
- Sharifzadeh, M.; Zhao, D.-Y.; Bernstein, P.S.; Gellermann, W. Resonance Raman imaging of macular pigment distributions in the human retina. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2008, 25, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, M.; Bernstein, P.S.; Gellermann, W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2006, 23, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Bernstein, P.S.; Garland, D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1802–1811. [Google Scholar]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Gruszecki, W.I.; Sielewiesiuk, J. Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: A spin-label study. Biochim. Biophys. Acta 1992, 1105, 97–108. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Can macular xanthophylls replace cholesterol in formation of the liquid-ordered phase in lipid-bilayer membranes? Acta Biochim. Pol. 2012, 59, 109–114. [Google Scholar] [CrossRef]
- Wisniewska, A.; Widomska, J.; Subczynski, W.K. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006, 53, 475–484. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006, 41, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, A.; Subczynski, W.K. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med. 2006, 40, 1820–1826. [Google Scholar] [CrossRef]
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Keunen, J.E.E.; Bird, A.C.; Kuijk, F.J.G.M. van Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Nolan, J.; Kavanagh, H.; O’Donovan, O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch. Biochem. Biophys. 2004, 430, 70–76. [Google Scholar] [CrossRef]
- Nebeling, L.C.; Forman, M.R.; Graubard, B.I.; Snyder, R.A. Changes in carotenoid intake in the United States: The 1987 and 1992 National Health Interview Surveys. J. Am. Diet Assoc. 1997, 97, 991–996. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Dixon, Z.; Chen, Y.; Llerena, C.M. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp. Eye Res. 2000, 71, 239–245. [Google Scholar] [CrossRef]
- Curran-Celentano, J.; Hammond, B.R.; Ciulla, T.A.; Cooper, D.A.; Pratt, L.M.; Danis, R.B. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am. J. Clin. Nutr. 2001, 74, 796–802. [Google Scholar] [CrossRef]
- Li, S.; Liu, N.; Lin, L.; Sun, E.-D.; Li, J.-D.; Li, P.-K. Macular pigment and serum zeaxanthin levels with Goji berry supplement in early age-related macular degeneration. Int. J. Ophthalmol. 2018, 11, 970–975. [Google Scholar] [CrossRef]
- Wen, X.; Hempel, J.; Schweiggert, R.M.; Ni, Y.; Carle, R. Carotenoids and carotenoid esters of red and yellow physalis (physalis alkekengi L. and P. pubescens L.) fruits and calyces. J. Agric. Food Chem. 2017, 65, 6140–6151. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.; Breithaupt, D.E. Identification and quantification of zeaxanthin esters in plants using liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2003, 51, 7044–7049. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.-P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focşan, M.; Rugină, D.O.; Pintea, A. Sea buckthorn oil as a valuable source of bioaccessible xanthophylls. Nutrients 2020, 12, 76. [Google Scholar] [CrossRef]
- Handelman, G.J.; Nightingale, Z.D.; Lichtenstein, A.H.; Schaefer, E.J.; Blumberg, J.B. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am. J. Clin. Nutr. 1999, 70, 247–251. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Johnson, E.J. Lutein. Encyclopedia of Dietary Supplements. Available online: https://www.crcpress.com/Encyclopedia-of-Dietary-Supplements/Coates-Betz-Blackman-Cragg-Levine-Moss-White/p/book/9781439819289 (accessed on 30 March 2020).
- Castenmiller, J.J.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and zeaxanthin—Food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment zeaxanthin—A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Hen Egg Carotenoids (Lutein and Zeaxanthin) and Nutritional Impacts on Human Health: A review: CyTA—Journal of Food: Vol 15, No 3. Available online: https://www.tandfonline.com/doi/full/10.1080/19476337.2016.1266033 (accessed on 4 March 2020).
- Bowen, P.E.; Herbst-Espinosa, S.M.; Hussain, E.A.; Stacewicz-Sapuntzakis, M. Esterification does not impair lutein bioavailability in humans. J. Nutr. 2002, 132, 3668–3673. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, C.; Li, Y.; Leung, K.S.-Y.; Jiang, Z.-H.; Zhao, Z. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus Lycii). Plant Foods Hum. Nutr. 2005, 60, 161–164. [Google Scholar] [CrossRef]
- O’Connell, O.F.; Ryan, L.; O’Brien, N.M. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Weller, P.; Wolters, M.; Hahn, A. Comparison of plasma responses in human subjects after the ingestion of 3R,3R’-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R’-zeaxanthin using chiral high-performance liquid chromatography. Br. J. Nutr. 2004, 91, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Weller, P.; Wolters, M.; Hahn, A. Plasma response to a single dose of dietary beta-cryptoxanthin esters from papaya (Carica papaya L.) or non-esterified beta-cryptoxanthin in adult human subjects: A comparative study. Br. J. Nutr. 2003, 90, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wingerath, T.; Stahl, W.; Sies, H. β-cryptoxanthin selectively increases in human chylomicrons upon ingestion of tangerine concentrate rich in β-cryptoxanthin esters. Arch. Biochem. Biophys. 1995, 324, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-Y.; Rasmussen, H.M.; Johnson, E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Milanowska, J.; Gruszecki, W.I. Heat-induced and light-induced isomerization of the xanthophyll pigment zeaxanthin. J. Photochem. Photobiol. B Biol. 2005, 80, 178–186. [Google Scholar] [CrossRef]
- Updike, A.A.; Schwartz, S.J. Thermal processing of vegetables increases cis isomers of lutein and zeaxanthin. J. Agric. Food Chem. 2003, 51, 6184–6190. [Google Scholar] [CrossRef]
- Boileau, T.W.-M.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. (Maywood) 2002, 227, 914–919. [Google Scholar] [CrossRef]
- All-trans β-Carotene Appears to Be More Bioavailable than 9-cis or 13-cis β-Carotene in Gerbils Given Single Oral Doses of Each Isomer | The Journal of Nutrition | Oxford Academic. Available online: https://academic.oup.com/jn/article/132/9/2700/4687746 (accessed on 4 March 2020).
- Ben-Amotz, A.; Levy, Y. Bioavailability of a natural isomer mixture compared with synthetic all-trans beta-carotene in human serum. Am. J. Clin. Nutr. 1996, 63, 729–734. [Google Scholar] [CrossRef]
- Hempel, J.; Fischer, A.; Fischer, M.; Högel, J.; Bosy-Westphal, A.; Carle, R.; Schweiggert, R.M. Effect of aggregation form on bioavailability of zeaxanthin in humans: A randomised cross-over study. Br. J. Nutr. 2017, 118, 698–706. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Chung, W.Y.; Wang, J.; Richelle, M.; Bucheli, P. Enhanced bioavailability of zeaxanthin in a milk-based formulation of wolfberry (Gou Qi Zi; Fructus barbarum L.). Br. J. Nutr. 2006, 96, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, F.; Cortés, C.; Esteve, M.J.; Frígola, A. Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. J. Agric. Food Chem. 2005, 53, 9519–9525. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Castón, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Effect of domestic cooking methods on egg yolk xanthophylls. J. Agric. Food Chem. 2012, 60, 12547–12552. [Google Scholar] [CrossRef]
- Rosenthal, J.M.; Kim, J.; de Monastario, F.; Thompson, D.J.S.; Bone, R.A.; Landrum, J.T.; de Moura, F.F.; Khachik, F.; Chen, H.; Schleicher, R.L.; et al. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5227–5233. [Google Scholar] [CrossRef]
- Wang, Y.; Roger Illingworth, D.; Connor, S.L.; Barton Duell, P.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef]
- Berg, H. van den Carotenoid interactions. Nutr. Rev. 1999, 57, 1–10. [Google Scholar] [CrossRef]
- Micozzi, M.S.; Brown, E.D.; Edwards, B.K.; Bieri, J.G.; Taylor, P.R.; Khachik, F.; Beecher, G.R.; Smith, J.C. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am. J. Clin. Nutr. 1992, 55, 1120–1125. [Google Scholar] [CrossRef]
- Borel, P. Carotenoids in biological emulsions: Solubility, surface-to-core distribution, and release from lipid droplets. J. Lipid Res. 1996, 37, 250–261. [Google Scholar]
- Perera, C.O.; Yen, G.M. Functional properties of carotenoids in human health. Int. J. Food Prop. 2007, 10, 201–230. [Google Scholar] [CrossRef]
- Tyssandier, V.; Lyan, B.; Borel, P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2001, 1533, 285–292. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Mechanisms of carotenoid intestinal absorption: Where do we stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Harrison, E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Duell, P.B.; Kean, R.; Wang, Y. The prime role of HDL to transport lutein into the retina: Evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4226–4231. [Google Scholar] [CrossRef]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019, 63, 1801046. [Google Scholar] [CrossRef]
- Tserentsoodol, N.; Sztein, J.; Campos, M.; Gordiyenko, N.V.; Fariss, R.N.; Lee, J.W.; Fliesler, S.J.; Rodriguez, I.R. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 2006, 12, 1306–1318. [Google Scholar]
- Shyam, R.; Vachali, P.; Gorusupudi, A.; Nelson, K.; Bernstein, P.S. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch. Biochem. Biophys. 2017, 634, 21–28. [Google Scholar] [CrossRef]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Horvath, M.P.; George, E.W.; Tran, Q.T.; Baumgardner, K.; Zharov, G.; Lee, S.; Sharifzadeh, H.; Shihab, S.; Mattinson, T.; Li, B.; et al. Structure of the lutein-binding domain of human StARD3 at 1.74 Å resolution and model of a complex with lutein. Acta Cryst. F 2016, 72, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Balashov, N.A.; Tsong, E.D.; Rando, R.R. Retinal tubulin binds macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1997, 38, 167–175. [Google Scholar] [PubMed]
- Makuch, K.; Markiewicz, M.; Pasenkiewicz-Gierula, M. Asymmetric spontaneous intercalation of lutein into a phospholipid bilayer, a computational study. Comput. Struct. Biotechnol. J. 2019, 17, 516–526. [Google Scholar] [CrossRef]
- Sanabria, J.C.; Bass, J.; Spors, F.; Gierhart, D.L.; Davey, P.G. Measurement of carotenoids in perifovea using the macular pigment reflectometer. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Prog. Retin. Eye Res. 2017, 60, 120–143. [Google Scholar] [CrossRef]
- Sauer, L.; Andersen, K.M.; Li, B.; Gensure, R.H.; Hammer, M.; Bernstein, P.S. Fluorescence lifetime imaging ophthalmoscopy (FLIO) of macular pigment. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3094–3103. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Handelman, G.J.; Adler, A.J. Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys. Investig. Ophthalmol. Vis. Sci. 1991, 32, 268–279. [Google Scholar]
- Gass, J.D. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: Hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch. Ophthalmol. 1999, 117, 821–823. [Google Scholar] [CrossRef]
- Powner, M.B.; Gillies, M.C.; Tretiach, M.; Scott, A.; Guymer, R.H.; Hageman, G.S.; Fruttiger, M. Perifoveal müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology 2010, 117, 2407–2416. [Google Scholar] [CrossRef]
- Powner, M.B.; Gillies, M.C.; Zhu, M.; Vevis, K.; Hunyor, A.P.; Fruttiger, M. Loss of Müller’s cells and photoreceptors in macular telangiectasia type 2. Ophthalmology 2013, 120, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Distler, C.; Dreher, Z. Glia cells of the monkey retina--II. Müller cells. Vis. Res. 1996, 36, 2381–2394. [Google Scholar] [CrossRef]
- Trieschmann, M.; van Kuijk, F.J.G.M.; Alexander, R.; Hermans, P.; Luthert, P.; Bird, A.C.; Pauleikhoff, D. Macular pigment in the human retina: Histological evaluation of localization and distribution. Eye 2008, 22, 132–137. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids 1998, 33, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Kim, S.R.; Nakanishi, K.; Itagaki, Y.; Sparrow, J.R. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp. Eye Res. 2006, 82, 828–839. [Google Scholar] [CrossRef]
- Mozzo, M.; Dall’Osto, L.; Hienerwadel, R.; Bassi, R.; Croce, R.D. Photoprotection in the antenna complexes of photosystem II: Role of individual xanthophylls in chlorophyll triplet quenching. J. Boil. Chem. 2008. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Holt, N.E.; Kaligotla, S.; Fuciman, M.; Cazzaniga, S.; Carbonera, D.; Frank, H.A.; Alric, J.; Bassi, R. Zeaxanthin protects plant photosynthesis by modulating chlorophyll triplet yield in specific light-harvesting antenna subunits. J. Biol. Chem. 2012, 287, 41820–41834. [Google Scholar] [CrossRef]
- Maeda, T.; Golczak, M.; Maeda, A. Retinal photodamage mediated by all-trans-retinal. Photochem. Photobiol. 2012, 88, 1309–1319. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef]
- Różanowska, M.; Handzel, K.; Boulton, M.E.; Różanowski, B. Cytotoxicity of All-Trans-Retinal Increases Upon Photodegradation. Photochem. Photobiol. 2012, 88, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104. [Google Scholar] [CrossRef]
- Liebler, D.C. Antioxidant reactions of carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Fiedor, J.; Fiedor, L.; Haessner, R.; Scheer, H. Cyclic endoperoxides of beta-carotene, potential pro-oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta 2005, 1709, 1–4. [Google Scholar] [CrossRef]
- Nishino, A.; Yasui, H.; Maoka, T. Reaction and Scavenging Mechanism of β-Carotene and Zeaxanthin with Reactive Oxygen Species. J. Oleo Sci. 2017, 66, 77–84. [Google Scholar] [CrossRef]
- Bhosale, P.; Bernstein, P.S. Quantitative measurement of 3’-oxolutein from human retina by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry. Anal. Biochem. 2005, 345, 296–301. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-L.; Havaux, M. Chemical Quenching of Singlet Oxygen by Carotenoids in Plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef]
- Trevithick, J.R.; Trevithick–Sutton, C.C.; Dzialoszynski, T.; Collins, M.; Kolenko, M.; Ajami, Z. Radical Scavenging by Lutein and Related Carotenoids May Contribute to Risk Reduction of AMD and Cataract. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3855. [Google Scholar]
- Berdeaux, O.; Acar, N. Very-long-chain polyunsaturated fatty acids in the retina: Analysis and clinical relevance in physiological and pathological conditions. OCL 2011, 18, 284–290. [Google Scholar] [CrossRef][Green Version]
- Fliesler, S.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Agbaga, M.-P.; Mandal, M.N.A.; Anderson, R.E. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J. Lipid Res. 2010, 51, 1624–1642. [Google Scholar] [CrossRef]
- Agbaga, M.-P.; Brush, R.S.; Mandal, M.N.A.; Henry, K.; Elliott, M.H.; Anderson, R.E. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci. USA 2008, 105, 12843–12848. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Agrón, E.; Meleth, A.D.; Reed, G.F.; Sperduto, R.D.; Clemons, T.E.; Chew, E.Y. Age-Related Eye Disease Study Research Group {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2009, 90, 1601–1607. [Google Scholar] [CrossRef]
- Parekh, N.; Voland, R.P.; Moeller, S.M.; Blodi, B.A.; Ritenbaugh, C.; Chappell, R.J.; Wallace, R.B.; Mares, J.A. CAREDS Research Study Group Association between dietary fat intake and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): An ancillary study of the Women’s Health Initiative. Arch. Ophthalmol. 2009, 127, 1483–1493. [Google Scholar] [CrossRef]
- Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial | Geriatrics | JAMA | JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/1684847 (accessed on 30 March 2020).
- Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 2001, 119, 1439–1452. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Agrón, E.; Clemons, T.E.; Ferris, F.L.; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Klein, R.; et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch. Ophthalmol. 2008, 126, 1274–1279. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Agrón, E.; Clemons, T.E.; Chew, E.Y. Omega-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch. Ophthalmol. 2009, 127, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L.; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R.; et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar] [CrossRef] [PubMed]
- AREDS2 Research Group; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, S.; Sanchez, S. Influence of carbon and nitrogen sources on Flavobacteriumgrowth and zeaxanthin biosynthesis. J. Ind. Microbiol. Biotechnol. 1999, 23, 697–700. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Bule, M.V.; Chavan, P.; Singhal, R.S.; Kamat, M.Y. Development of efficient supercritical carbon dioxide extraction methodology for zeaxanthin from dried biomass of Paracoccus zeaxanthinifaciens. Sep. Purif. Technol. 2010, 71, 173–177. [Google Scholar] [CrossRef]
- Masetto, A.; Flores-Cotera, L.B.; Díaz, C.; Langley, E.; Sanchez, S. Application of a complete factorial design for the production of zeaxanthin by Flavobacterium sp. J. Biosci. Bioeng. 2001, 92, 55–58. [Google Scholar] [CrossRef]
- Soukup, M.; Widmer, E.; Lukáč, T. Technical Procedures for the Syntheses of Carotenoids and Related Compounds from 6-Oxo-isophorone: Syntheses of (3R,3′R)-Zeaxanthin. Part II. Helv. Chim. Acta 1990, 73, 868–873. [Google Scholar] [CrossRef]
- Ernst, H. Recent advances in industrial carotenoid synthesis. Pure Appl. Chem. 2002, 74, 2213–2226. [Google Scholar] [CrossRef]
- Englert, G.; Noack, K.; Broger, E.A.; Glinz, E.; Vecchi, M.; Zell, R. Synthesis, Isolation, and Full Spectroscopic Characterization of Eleven (Z)-Isomers of (3R,3′R)-Zeaxanthin. Helv. Chim. Acta 1991, 74, 969–982. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Paredes-Lopez, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-4200-3171-3. [Google Scholar]
- Mitchell, D. The Complete Book of Nutritional Healing: The Top 100 Medicinal Foods and Supplements and the Diseases They Treat; St. Martin’s Publishing Group: New York, NY, USA, 2008; ISBN 978-1-4299-4854-8. [Google Scholar]
- Madhavan, J.; Chandrasekharan, S.; Priya, M.; Godavarthi, A. Modulatory effect of carotenoid supplement constituting lutein and zeaxanthin (10:1) on anti-oxidant enzymes and macular pigments level in rats. Pharmacogn. Mag. 2018, 14, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Schalch, W.; Cohn, W.; Barker, F.M.; Köpcke, W.; Mellerio, J.; Bird, A.C.; Robson, A.G.; Fitzke, F.F.; van Kuijk, F.J.G.M. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—The LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch. Biochem. Biophys. 2007, 458, 128–135. [Google Scholar] [CrossRef]
- Stringham, J.M.; Stringham, N.T. Serum and retinal responses to three different doses of macular carotenoids over 12 weeks of supplementation. Exp. Eye Res. 2016, 151, 1–8. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef]
- Vu, H.T.V.; Robman, L.; McCarty, C.A.; Taylor, H.R.; Hodge, A. Does dietary lutein and zeaxanthin increase the risk of age related macular degeneration? The melbourne visual impairment project. Br. J. Ophthalmol. 2006, 90, 389–390. [Google Scholar] [CrossRef]
- Delcourt, C.; Carrière, I.; Delage, M.; Barberger-Gateau, P.; Schalch, W. POLA Study Group Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA Study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2329–2335. [Google Scholar] [CrossRef]
- Gale, C.R.; Hall, N.F.; Phillips, D.I.W.; Martyn, C.N. Lutein and zeaxanthin status and risk of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2461–2465. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A.; Joa, H.; Kilburn, M.D.; Moore, L.L.; Sprague, K.E. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp. Eye Res. 1997, 65, 57–62. [Google Scholar] [CrossRef]
- Schweitzer, D.; Lang, G.E.; Beuermann, B.; Remsch, H.; Hammer, M.; Thamm, E.; Spraul, C.W.; Lang, G.K. Objective determination of optical density of xanthophyll after supplementation of lutein. Ophthalmologe 2002, 99, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-M.; Dou, H.-L.; Huang, F.-F.; Xu, X.-R.; Zou, Z.-Y.; Lin, X.-M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Gorusupudi, A.; Wegner, K.; Sharifzadeh, M.; Gellermann, W.; Bernstein, P.S. Macular pigment distribution responses to high-dose zeaxanthin supplementation in patients with macular telangiectasia type 2 (MacTel). Retina 2017, 37, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Thum, L.A.; Naash, M.I. Oxygen-induced retinopathy in the rat. Vitamins C and E as potential therapies. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1836–1845. [Google Scholar] [PubMed]
- Katz, M.L.; Eldred, G.E. Failure of vitamin E to protect the retina against damage resulting from bright cyclic light exposure. Investig. Ophthalmol. Vis. Sci. 1989, 30, 29–36. [Google Scholar] [PubMed]
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Chen, Z.-H.; Cynshi, O.; Jishage, K.-I.; Niki, E.; Ohira, A. Acceleration of age-related changes in the retina in alpha-tocopherol transfer protein null mice fed a Vitamin E-deficient diet. Investig. Ophthalmol. Vis. Sci. 2007, 48, 396–404. [Google Scholar] [CrossRef]
- Farnsworth, C.C.; Stone, W.L.; Dratz, E.A. Effects of vitamin E and selenium deficiency on the fatty acid composition of rat retinal tissues. Biochim. Biophys. Acta 1979, 552, 281–293. [Google Scholar] [CrossRef]
- Infante, J.P. Vitamin E and selenium participation in fatty acid desaturation A proposal for an enzymatic function of these nutrients. Mol. Cell Biochem. 1986, 69, 93–108. [Google Scholar] [CrossRef]
- Kannan, R.; Tang, D.; Mackic, J.B.; Zlokovic, B.V.; Fernandez-Checa, J.C. A simple technique to determine glutathione (GSH) levels and synthesis in ocular tissues as GSH-bimane adduct: Application to normal and galactosemic guinea-pigs. Exp. Eye Res. 1993, 56, 45–50. [Google Scholar] [CrossRef]
- Gosbell, A.D.; Stefanovic, N.; Scurr, L.L.; Pete, J.; Kola, I.; Favilla, I.; Haan, J.B. de Retinal light damage: structural and functional effects of the antioxidant glutathione peroxidase-1. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2613–2622. [Google Scholar] [CrossRef]
- Agardh, C.-D.; Gustavsson, C.; Hagert, P.; Nilsson, M.; Agardh, E. Expression of antioxidant enzymes in rat retinal ischemia followed by reperfusion. Metab. Clin. Exp. 2006, 55, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N.; Amin, R.H.; Puklin, J.E. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am. J. Ophthalmol. 1999, 127, 694–709. [Google Scholar] [CrossRef]
- Bilgihan, A.; Bilgihan, K.; Yis, O.; Sezer, C.; Akyol, G.; Hasanreisoglu, B. Effects of topical vitamin E on corneal superoxide dismutase, glutathione peroxidase activities and polymorphonuclear leucocyte infiltration after photorefractive keratectomy. Acta Ophthalmol. Scand. 2003, 81, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Havaux, M.; Triantaphylidès, C.; Ksas, B.; Pascal, A.A.; Robert, B.; Davison, P.A.; Ruban, A.V.; Horton, P. Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J. Biol. Chem. 2007, 282, 22605–22618. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Sielewiesiuk, J. Spin-label studies on phosphatidylcholine-polar carotenoid membranes: Effects of alkyl-chain length and unsaturation. Biochim. Biophys. Acta 1993, 1150, 173–181. [Google Scholar] [CrossRef]
| Food | Zeaxanthin Content (μg/100 g) |
|---|---|
| Goji berry | 280,000 |
| Red Chinese lantern fruit | 84,700 |
| Orange pepper | 5580 |
| Sea buckthorn | 1930 |
| Egg yolk (raw) | 762 |
| Corn | 105 |
| Orange juice | 26 |
| Peach | 39 |
| Spinach | 75 |
| Kale | 62 |
| Papaya | 6 |
| AREDS1 | |
|---|---|
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| β-carotene | 15 mg |
| Zinc | 80 mg |
| Copper | 2 mg |
| AREDS2 | |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Zinc | 25 mg |
| Copper | 2 mg |
| Zeaxanthin | 2 mg |
| Lutein | 10 mg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widomska, J.; SanGiovanni, J.P.; Subczynski, W.K. Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients 2020, 12, 1333. https://doi.org/10.3390/nu12051333
Widomska J, SanGiovanni JP, Subczynski WK. Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients. 2020; 12(5):1333. https://doi.org/10.3390/nu12051333
Chicago/Turabian StyleWidomska, Justyna, John Paul SanGiovanni, and Witold K. Subczynski. 2020. "Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea?" Nutrients 12, no. 5: 1333. https://doi.org/10.3390/nu12051333
APA StyleWidomska, J., SanGiovanni, J. P., & Subczynski, W. K. (2020). Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients, 12(5), 1333. https://doi.org/10.3390/nu12051333