1. Introduction
Obesity causes a critical health problem because of an increase in the incidence of metabolic diseases including type 2 diabetes mellitus, atherosclerosis, and hypertension. Energy imbalance between energy uptake and energy consumption leads to the storage of excessive energy in the adipose tissue, resulting in obesity. The expansion of the adipose tissue results from hyperplasia and hypertrophy [
1]. Hyperplasia is induced by the increase of adipocyte numbers due to differentiation (adipogenesis), and hypertrophy is induced by the increase of adipocyte size due to triglyceride (TG) accumulation [
1]. Therefore, the prevention of adipogenesis is a candidate therapeutic approach for the development of anti-obesity agents.
Adipogenesis is mediated by various regulators including transcriptional factors, signaling kinases, and lipid metabolic enzymes [
2]. The molecular and cellular processes of adipogenesis have been characterized, and widely studied, in 3T3-L1 cells. The adipogenesis of 3T3-L1 preadipocyte is composed of two stages, an early stage from 0 to 48 h after adding differentiation medium, and a late stage from day 2 to day 8. In the early stage, growth-arrested confluent 3T3-L1 preadipocytes re-enter the cell cycle simultaneously and go through two rounds of the cell cycle (which is called mitotic clonal expansion (MCE)) [
3]. MCE is a critical step for terminal differentiation into mature adipocytes [
3]. The mitogen-activated protein kinase (MAPK) pathways, including extracellular signal-regulated kinase (ERK) and p38 MAPK, play a positive role in the MCE process during early adipogenesis [
4]. Cyclin and cyclin-dependent kinase (CDK) regulate the progression of MCE, through the regulation of cell division cycles [
3]. The activated MCE proceeds to the late phase of adipogenesis, during which well-programmed transcription cascades stimulate the expression of terminal differentiation-related genes including fatty acid synthase (
FAS), fatty acid binding protein 2
(aP2), and fatty acid translocase/cluster of differentiation 36 (
FAT/CD36) [
5].
The transcriptional cascades of adipogenesis include peroxisome proliferator-activated receptor γ (PPARγ), CCAAT-enhancer-binding protein α, C/EBPβ, C/EBPδ, and sterol regulatory element-binding proteins 1c (SERBP1c). C/EBPβ and C/EBPδ, upregulated at the early stage, stimulate the expression of cell cycle-associated genes and terminal transcriptional factors such as
PPARγ2 and
C/EBPα during MCE process [
5]. Then, PPARγ and C/EBPα stimulate the expression of lipid metabolism genes for the formation of mature adipocytes [
5].
Salvia miltiorrhiza Bunge (Danshen) is a medicinal herb, traditionally used for the treatment of several diseases including cancer, hyperlipidemia, and cerebrovascular disease [
6]. Tanshinone I (Tan I) is one of the major diterpene compounds (a diterpenoid) isolated from
S. miltiorrhiza Bunge and exhibits a wide spectrum of antitumor effects in several cancers including gastric, prostate, and breast cancer [
6]. However, the anti-obesity effects of Tan I have remained unexplored. Thus, in the current study, the protective effects of Tan I against diet-induced obesity (DIO) were investigated in high-fat-diet (HFD)-induced obese mice, and the underlying mechanisms, involved in the anti-obesity effects exerted by Tan I, were characterized in 3T3-L1 cells. Here, for the first time, we revealed that Tan I exerted an anti-obese effect against DIO mice, through the inhibition of early adipogenesis, via suppression of MCE and regulation of the early adipogenic transcription cascade.
2. Materials and Methods
2.1. Reagents
Tanshinone I (≥98% purity) was purchased from ChemFaces (Wuhan, China). Dulbecco’s modified Eagle’s medium (DMEM), bovine calf serum (BCS), fetal bovine serum (FBS), and penicillin/streptomycin were obtained from HyClone (Logan, UT, USA). 3-isobutyl-1-methylxanthine, rosiglitazone, dexamethasone, and insulin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-p38, anti-p42/44 ERK, anti-pAkt, and anti-pAMPK were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against PPARγ, FAS, aP2, Cyclin D2, Cdk2, p21, p27, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The reagent for the measurement of TG levels was from Asan Pharmaceutical Co., Ltd. (Seoul, South Korea).
2.2. Animal Study
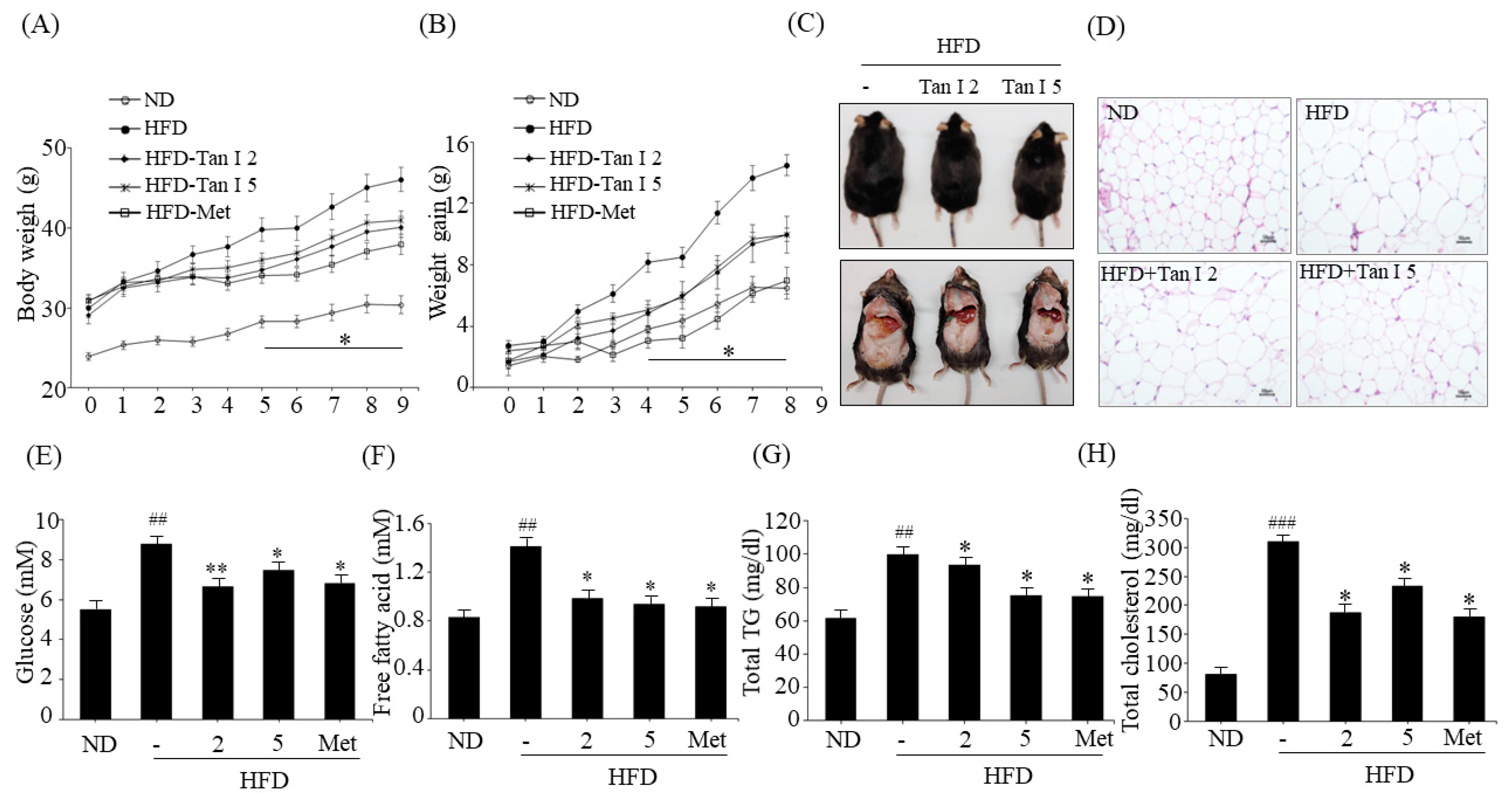
Male 6-week-old C57BL/6 mice were obtained from Jung-Ang Lab Animal, Inc. (Seoul, South Korea). All mice were fed a normal diet (ND) or an HFD for four weeks. Then, the HFD-fed mice were randomly divided into four groups (n = 10/group): HFD (vehicle-treated) group, HFD plus low-dose Tan I (2 mg/kg of body weight) group, HFD plus high-dose Tan I (5 mg/kg of body weight) group, and metformin (200 mg/kg body weight) group as a positive control. AIN93G diet with the high-fat diet containing 60% kcal fat was used, and the control diet contained 10% kcal fat. Tan I was dissolved in DMSO at 1 mg/mL, diluted in distilled water, and administered orally three times a week, for eight weeks. The animal experiments were approved by the Animal Care and Use Committee at Pusan National University in accordance with the established ethical and scientific care procedures (approval number: PNU-2019–2203).
2.3. Glucose and Insulin Tolerance Tests
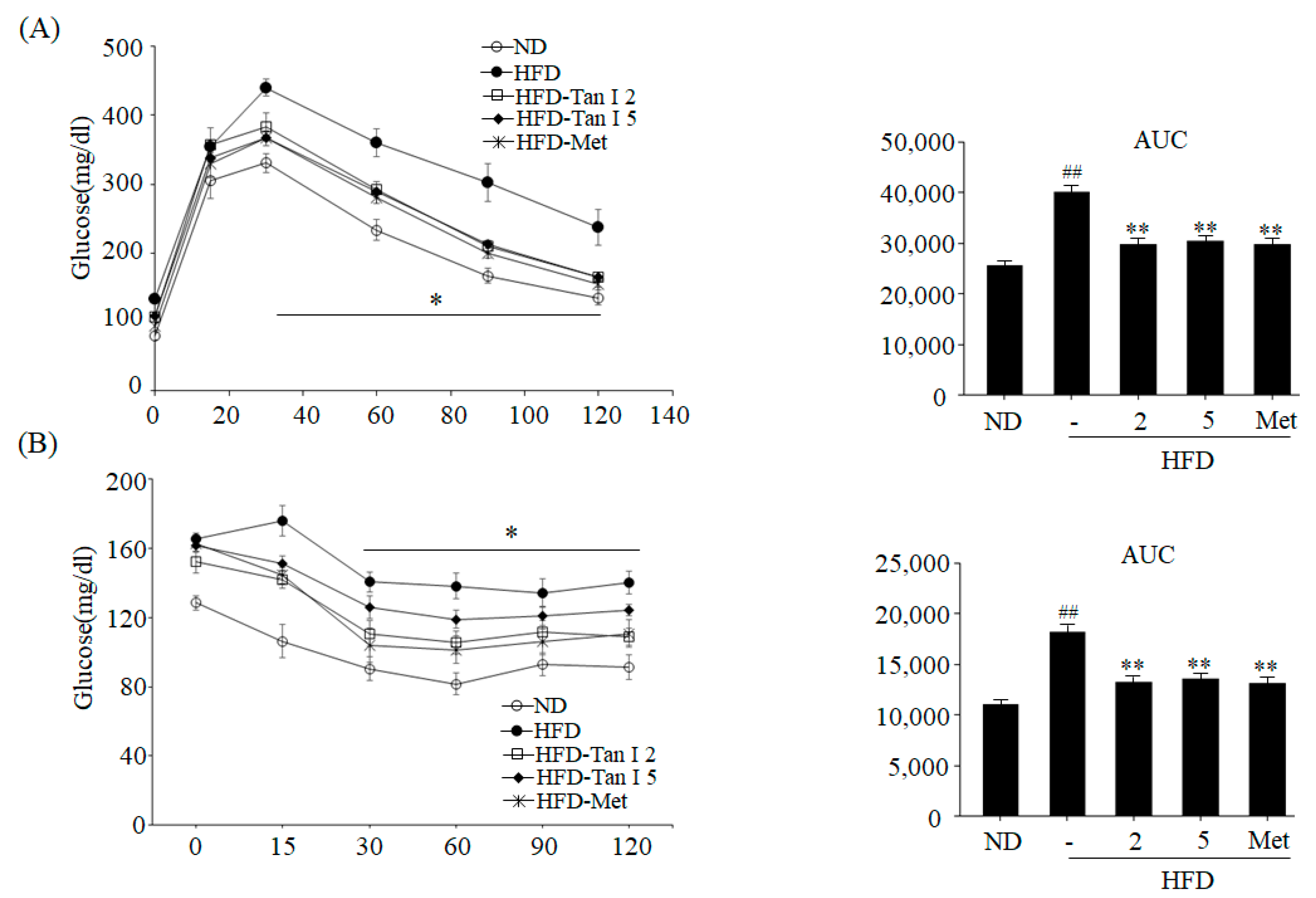
To perform the intraperitoneal glucose tolerance test (IPGTT), mice were fasted for 12 h with access to drink water. All mice were weighed and then injected intraperitoneally with glucose (2 g/kg body weight). Blood glucose levels were measured by placing on the strip of glucose meter at 30, 60, 90, and 120 min thereafter. The insulin tolerance test (ITT) was conducted after mice were fasted for 6 h, and all mice were injected with human insulin (0.75 U/kg body weight) intraperitoneally. Glucose levels were measured using a glucometer at 15, 30, 45, 60, 90, and 120 min.
2.4. Analysis of Serum Biological Parameters
Whole blood was collected in a heparinized tube and centrifuged at 3000 rpm for 20 min at 4 °C. The concentrations of plasma free fatty acid (FA) and total cholesterol level were measured by colorimetric methods using kits (Biomax, Seoul, South Korea), and plasma TG levels were measured by commercial spectrophotometric kits (Asan Pharmaceutical Company, Seoul, South Korea). All plasma samples were aliquoted at −80 °C before the measurements.
2.5. Cell Culture and Adipocyte Differentiation
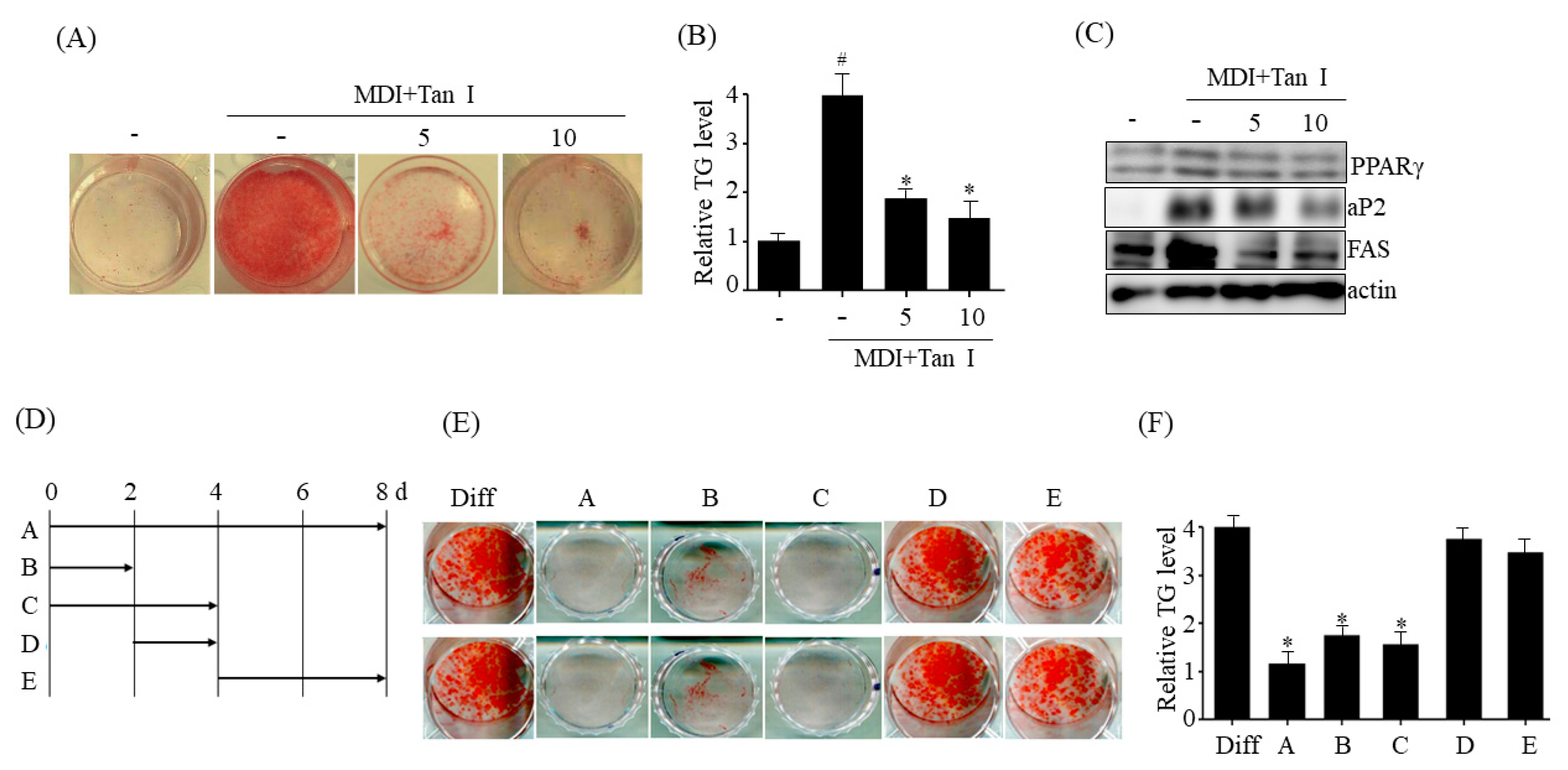
The 3T3-L1 mouse preadipocytes obtained from American Type Culture Collection (Manassas, VA, USA) were maintained in DMEM supplemented with 10% (v/v) BCS and penicillin/streptomycin (100 U/mL/100 mg/mL). Post-confluent 3T3-L1 cells were differentiated in differentiation medium (MDI) containing DMEM, 0.5 mM IBMX, 10% FBS, 5 μg/mL insulin, 2 mM rosiglitazone, and 1 μM dexamethasone as described previously [
7].
2.6. Cell Proliferation Assays
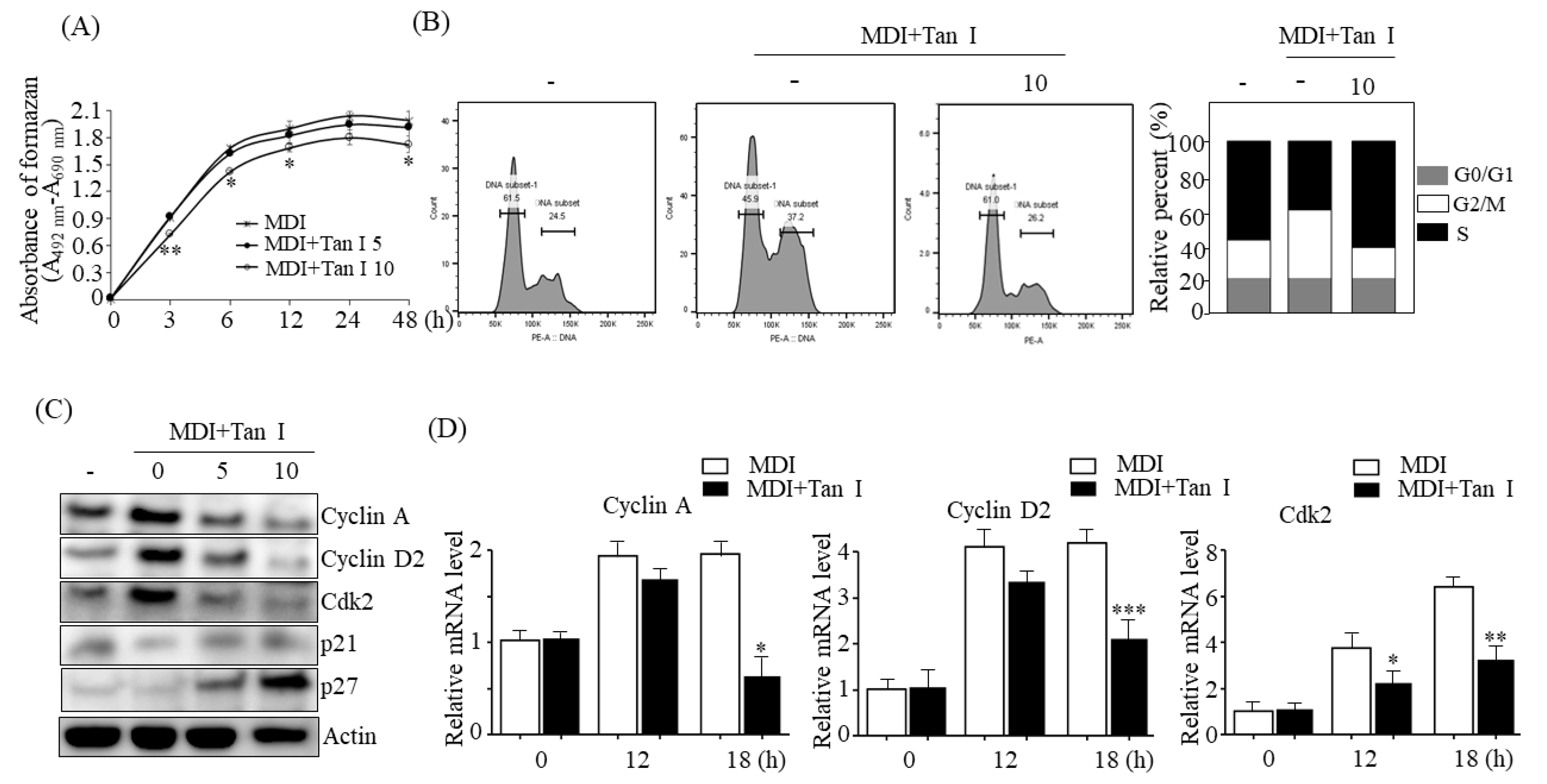
The 3T3-L1 cell proliferation was measured using the Cell Proliferation Kit II (XTT) (Roche Diagnosis GmbH, Penzberg, Germany) according to the manufacturer’s instructions.
2.7. Oil Red O (ORO) Staining
The 3T3-L1 cells were washed with phosphate-buffered saline (PBS) and fixed with 4% formaldehyde for 30 min. After discarding the formalin solution, the cells were washed with PBS and stained with Oil Red O, as described previously [
7].
2.8. Measurement of TG Levels
The 3T3-L1 preadipocytes were washed with PBS, and the harvested cell suspensions were extracted with chloroform:methanol (3:1, v:v) for 1 h. The TG levels in the mixture were measured as described previously [
7].
2.9. Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from 3T3-L1 cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA synthesis was made by TOPscript
TM RT DryMIX (Enzynomics, Daejeon, South Korea), and subjected to qPCR using a SYBR Green master mixture with gene-specific primers (
Table S1).
2.10. Western Blotting
The proteins were prepared from 3T3-L1 cells using Pro-prep
TM protein extraction solution (Intron Biotechnology, Seongnam, South Korea) and assayed, as described previously [
7].
2.11. Cell Cycle Analysis
The 3T3-L1 cells were collected and fixed with 70% ice-cold ethanol for 12 h at 4 ℃. After washing with ice-cold PBS, the cells were stained with 25 μg/mL propidium iodide (PI) (BioLegend, San Diego, CA, USA) and analyzed by FACS (fluorescence activated cell sorter) Canto II (Becton, Dickins and Company, San Jose, CA, USA) according to the manufacturer’s instructions. Analysis of the cell cycle populations was conducted using the BD Pro software (BD Biosciences, San Jose, CA, USA).
2.12. Statistical Analysis
All data were expressed as the mean ± SEM. The statistical analysis was conducted using one-way ANOVA analysis of variance followed by Tukey’s test. Differences were considered statistically significant at p values <0.05.
4. Discussion
It has been reported that some phytochemicals inhibited adipogenesis and thereby exhibited anti-obesity effects [
11]. Tan I, a diterpenoid isolated from
S. miltiorrhiza Bunge, is reported to have antitumor effects in several cancers [
6]. However, the anti-obesity effects of Tan I have not been investigated. Thus, in the current study, we evaluated the anti-obesity properties of Tan I in HFD-induced obese mice and characterized its underlying mechanisms in 3T3-L1 cells. First, we investigated whether Tan I exerted in vivo anti-obesity effects in HFD-induced obese mice. Administration of Tan I to HFD-induced obese mice lowered HFD-induced body weight, weight gain, and white adipocyte size, with no significant change in the food intake. Further, Tan I administration also reduced HFD-induced blood levels of glucose, free fatty acids, total TG, and total cholesterol, indicating that Tan I can prevent the HFD-induced obesity and ameliorate the serum metabolic parameters. Moreover, Tan I improved HFD-induced glucose and insulin tolerance. Based on these results, Tan I has a great potential in the prevention and treatment of obesity and the improvement of glucose and lipid metabolism.
Next, we investigated the molecular mechanisms involved in the protection afforded by Tan I against HFD-induced obesity. Adipocyte hyperplasia contributes to obesity by increasing the numbers of adipocytes, via differentiation of preadipocytes into mature adipocytes (adipogenesis) [
1]. Therefore, inhibition of adipogenesis is a potential target of anti-obesity agents. On the basis of this knowledge, we evaluated whether Tan I inhibited adipogenesis of 3T3-L1 preadipocytes. Our current results revealed that treatment with Tan I significantly inhibited MDI-induced adipogenesis of 3T3-L1 preadipocytes, Furthermore, the Tan I-mediated inhibition of adipogenesis mostly occurred during the early adipogenesis phase, since Tan I treatment at the early adipogenic phase significantly inhibited adipogenesis, almost similar to the effect of a continuous treatment.
During the early adipogenesis phase, incubation of the growth-arrested preadipocytes with MDI causes the preadipocytes to process MCE [
3]. Then, MCE activates early adipogenic transcription factors including C/EBPβ/δ during early adipogenesis, which subsequently stimulate the late adipogenic transcription factors, including PPARγ and C/EBPα, responsible for the induction of terminal adipocyte differentiation [
3]. Therefore, to further investigate whether Tan I inhibited the MCE process during early adipogenesis, we assessed the effects of Tan I on the proliferation and cell cycles of 3T3-L1 cells during this phase. The current data revealed that Tan I treatment inhibited cell proliferation of 3T3-L1 adipocytes and arrested cell-cycle from G1/S into the S and G2/M phases during MCE. This suggested that Tan I inhibited the cell cycle pathway, resulting in the suppression of MCE at the early adipogenesis phase. In addition, our current results revealed that Tan I treatment inhibited the expression of cyclin A, cyclin D2, and Cdk2 during the MDI process and stimulated the expression of p21 and p27, which are negative regulators of the G1/S transition of the cell cycle. The cyclin A- and E-dependent activation of Cdk2, and downregulation of p27 are required for the progression of the cell cycle from G1 to S phase [
3]. During MCE, cyclin D and cyclin E are activated, and the activated cyclin D interacts with Cdk4 and Cdk6, consequently inducing the G1/S transition. Meanwhile, cyclin E interacts with Cdk2 which recruits cyclin A, and the association of Cdk2 and cyclin A are essential for G1/S progression. Therefore, cyclins A and D are essential for the progression of the cell cycle from the G0/G1 phase to the S and G2/M phases. Based on our current results, the downregulation of cyclin A, cyclin D2, Cdk2, and upregulation of p21 and p27 may lead to the Tan I-mediated inhibition of MCE, through the delayed entry of G0/G1 cells into the S phase.
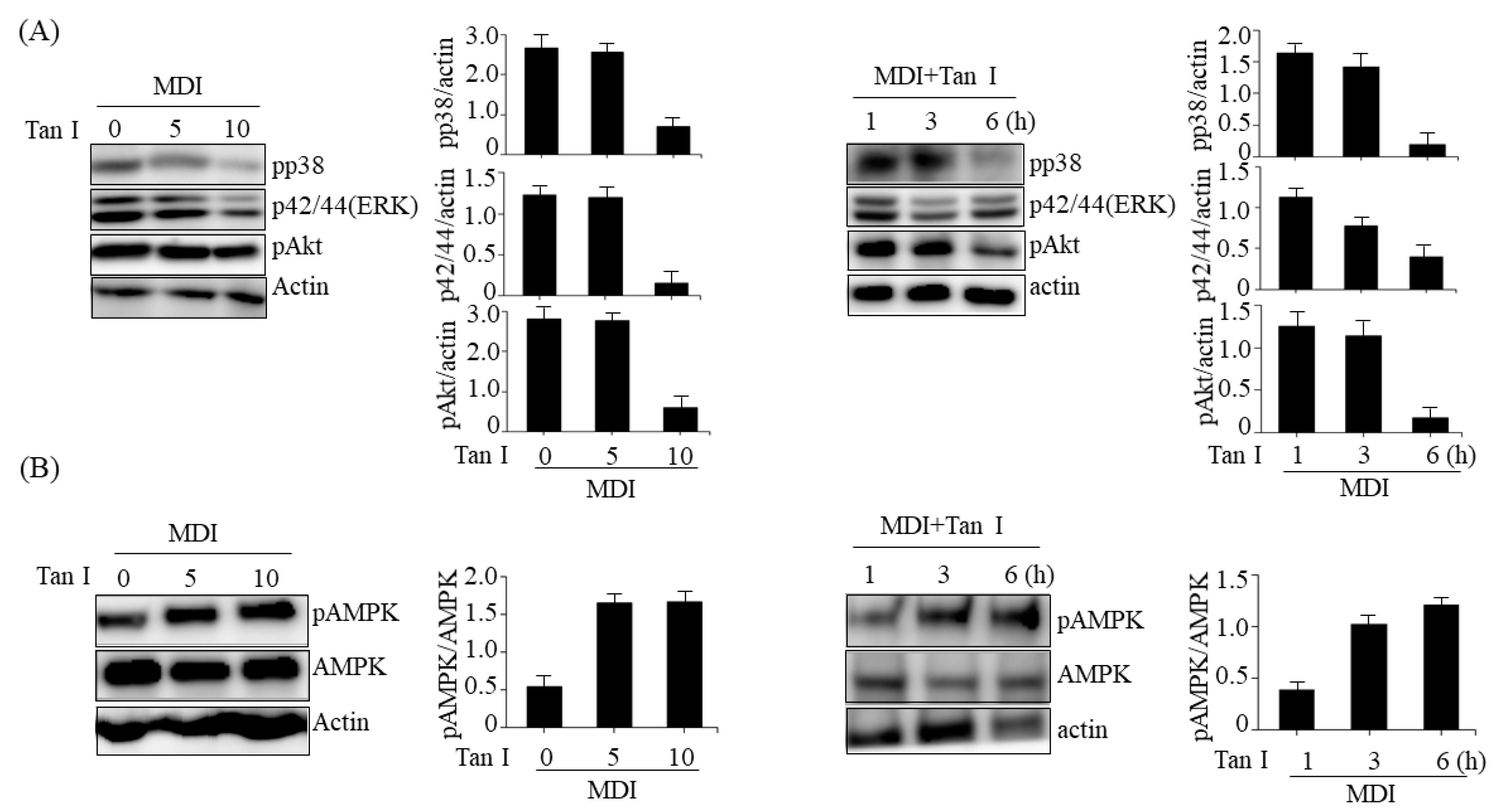
The MAPKs including p38, ERK, and PI3/Akt pathways play important roles in differentiation, proliferation, mitosis, and cell survival [
12]. The MAPKs are activated during the MCE process and stimulate MCE through the regulation of cyclin D and p27 expression [
4]. Furthermore, the MAPKs and PI3/Akt pathways activate C/EBPβ and its target mitogenic signaling molecules [
13]. Therefore, we investigated whether Tan I could affect MAPKs and PI3K/Akt signaling pathways during the MCE process. Our current results revealed that treatment with Tan I reduced the phosphorylation of p38, ERK, and Akt in dose- and time-dependent manners in 3T3-L1 adipocytes treated with MDI, indicating that Tan I may inhibit the cell proliferation during MCE through the suppression of MAPKs and PI3/Akt pathways. Notably, AMPK inhibits MCE through suppression of mammalian target of rapamycin complex 1 (mTORC1) signaling and stimulates Wnt/β-catenin signaling, which results in the repression of PPARγ expression [
8,
14]. Furthermore, AMPK attenuates TG accumulation in adipocytes during adipogenesis via deactivation of acetyl coA carboxylase, fatty acid synthesis enzymes [
15]. Therefore, we further investigated whether Tan I could activate AMPK in 3T3-L1 cells. Treatment with Tan I enhanced the phosphorylation of AMPK in dose- and time-dependent manners in 3T3-L1 cells treated with MDI, which suggested that Tan I-activated AMPK might also contribute to inhibiting MCE.
C/EBPβ plays a critical role in the MCE process through the stimulation of cell cycle genes [
9]. Recently, it was reported that C/EBPβ increases the expression of histone H3K9 demethylase, JMJD2B, which upregulates C/EBPβ target cell cycle genes, including
Cdc25c,
Cdc45l, and
Mcm3, as a cofactor of C/EBPβ via removing inhibitory histone marker H3K9me3 in their promoter regions [
10]. Thus, we considered that the Tan I inhibition of MCE might be regulated epigenetically through the downregulation of C/EBPβ–JMJD2B, and cell cycle genes. Our current data demonstrated that treatment with Tan I reduced the expression of C/EBPβ and its target epigenetic histone demethylase JMJD2B, and thereby repressed the expression of C/EBPβ-regulated cell cycle genes
Cdc25c,
Cdc45l, and
Mcm3 during MCE process. These results indicated that Tan I inhibited MCE epigenetically through the downregulation of C/EBPβ, JMJD2B, and cell cycle genes.
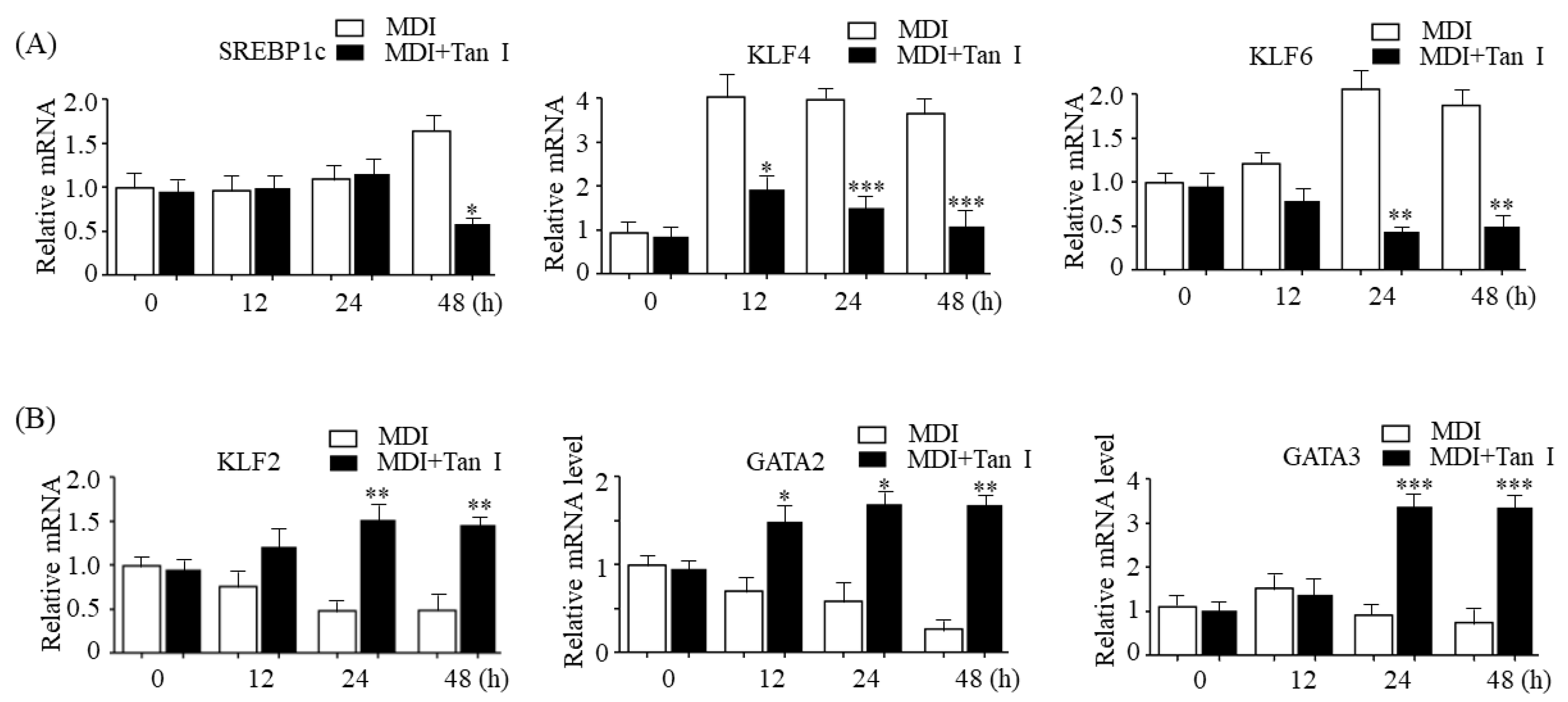
Adipogenesis is tightly controlled by transcription factor cascades, which activate or repress adipogenesis transcription factors of each other [
5]. In the current study, we found that Tan I downregulated early adipogenic activators, SREBP1c, KLF4, and KLF6, whereas it upregulated early adipogenic repressors, KLF2, GATA2, and GATA3, suggesting that Tan I may also inhibit adipogenesis through the regulation of adipogenesis transcription factor cascades during the early adipogenesis process. Besides Tan I, two compounds belonging to the group of tanshinones including tanshinone IIA and cryptotanshinone have been reported to exert anti-adipogenic effects through the downregulation of positive adipogenesis factors including C/EBPα, C/EBPβ, and PPARγ; upregulation of negative adipogenesis factors GATA2, CHOP (C/EBP homologous protein), and TNF-α (tumor necrosis factor); and inhibition of STAT3/ 5 signaling [
16,
17]. The important difference in molecular mechanisms between Tan I and the other two compounds is that Tan I attenuates the MCE process during early adipogenesis and represses the expression of epigenetic regulator, JMJD2B associated with activation of adipogenesis, which may also contribute to the inhibition of adipogenesis of 3T3-L1.