Moderate Weight Loss Modifies Leptin and Ghrelin Synthesis Rhythms but Not the Subjective Sensations of Appetite in Obesity Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
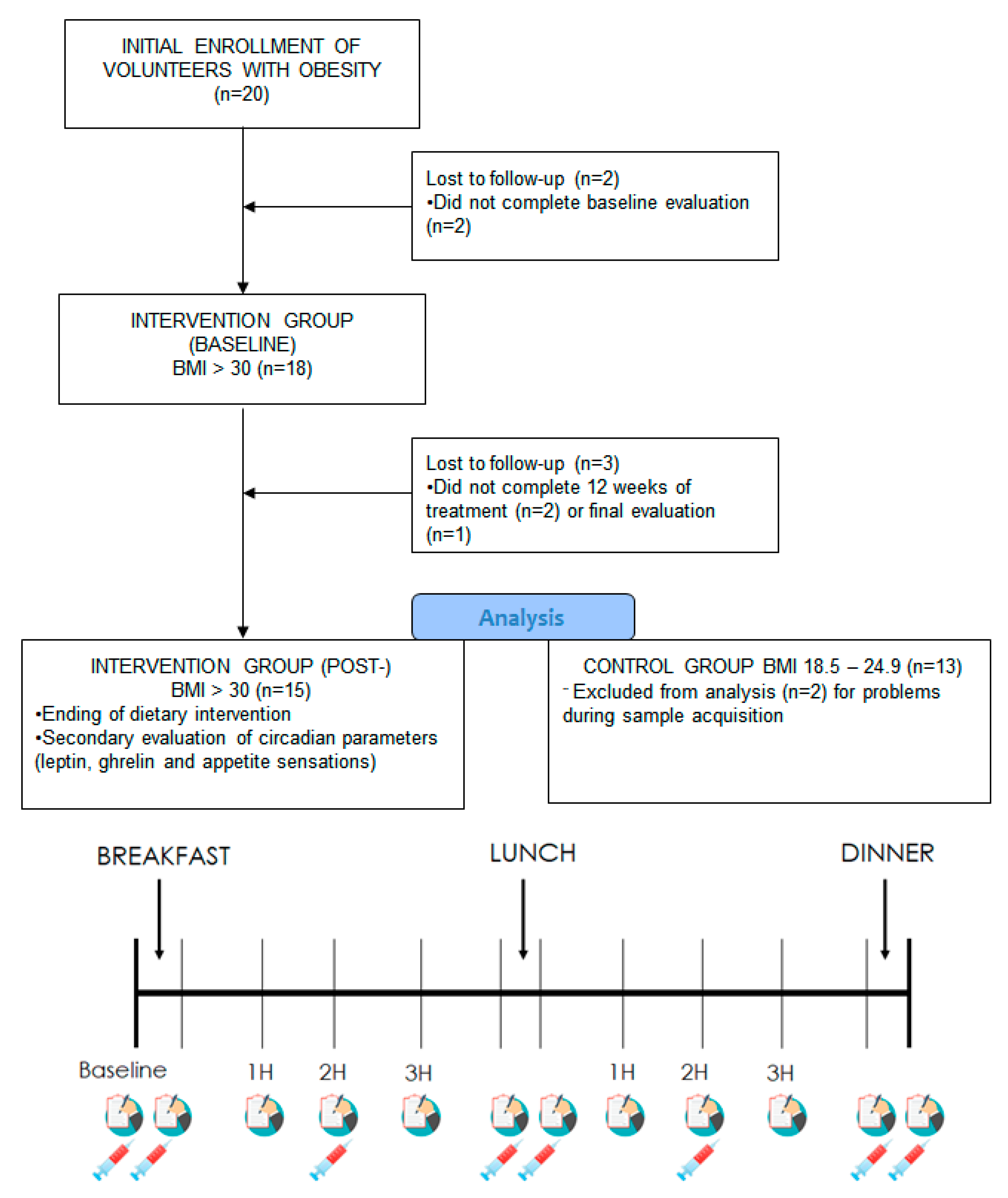
2.2. Study Design
2.3. Hypocaloric Treatment
2.4. Evaluation of Subjective Appetite Sensations
2.5. Clinical Parameters
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Subjects Studied
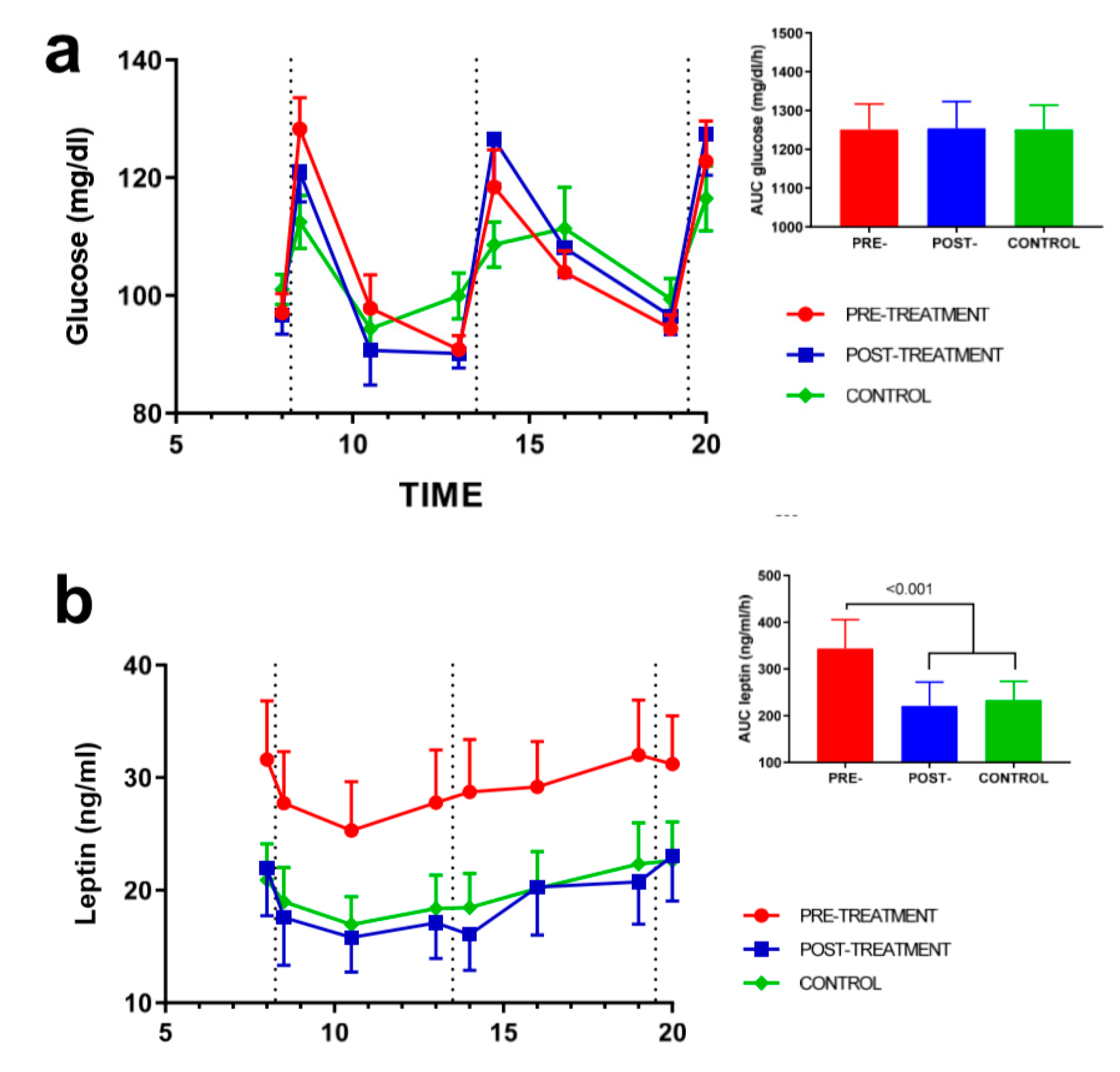
3.2. Effect of Weight Loss on Appetite-Regulating Hormones
3.3. Effect of Weight Loss on Subjective Appetite Sensations
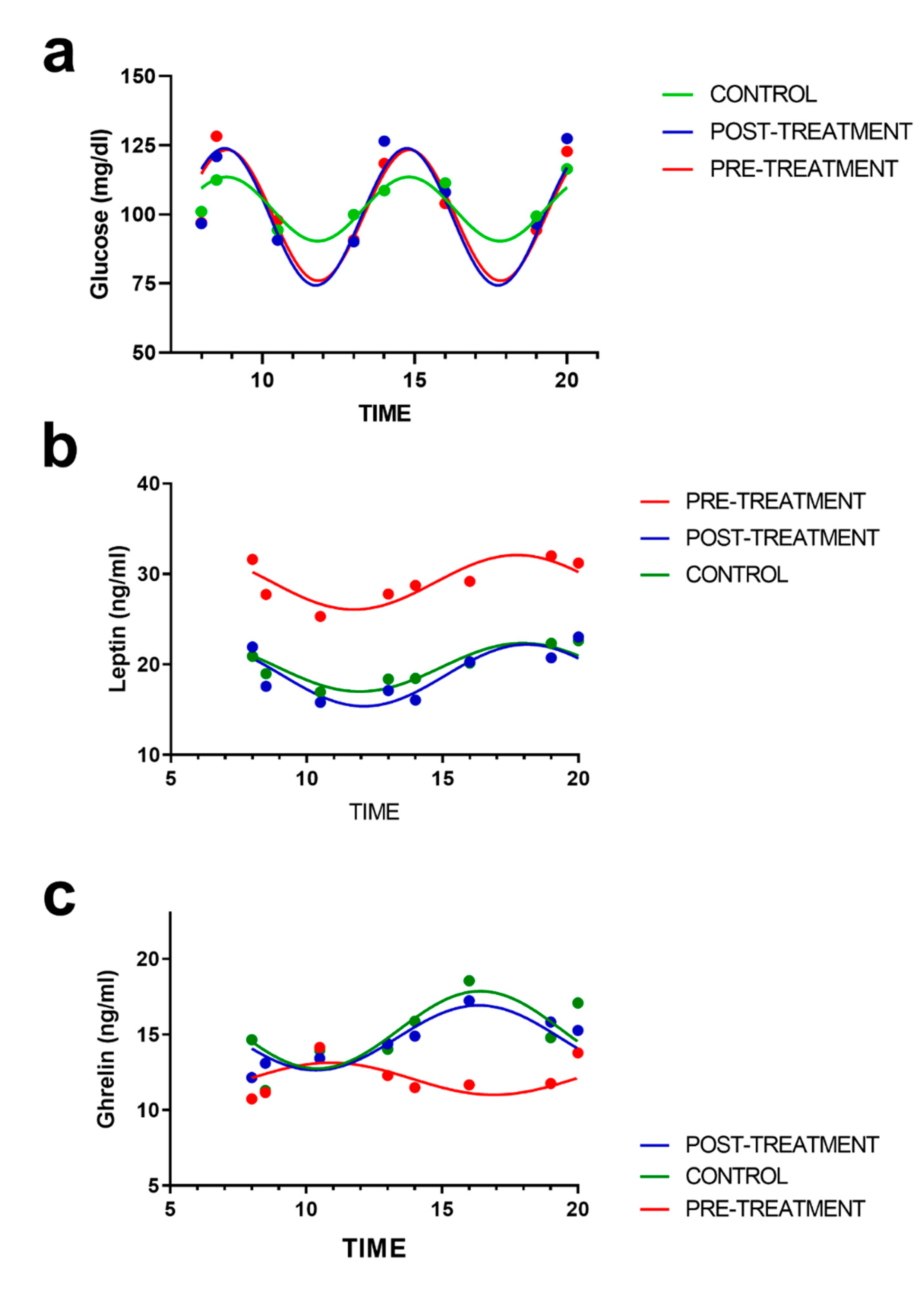
3.4. Rhythmic Analysis of Leptin and Ghrelin and Their Relation to Appetite Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacMahon, S.; Baigent, C.; Duffy, S.; Rodgers, A.; Tominaga, S.; Chambless, L.; De Backer, G.; De Bacquer, D.; Kornitzer, M.; Whincup, P.; et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Action Plan for the Prevention and Control of NCDs 2013–2020; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Schwartz, M.W. Brain pathways controlling food intake and body weight. Exp. Biol. Med. 2001, 226, 978–981. [Google Scholar] [CrossRef]
- Sahu, A. Leptin signaling in the hypothalamus: Emphasis on energy homeostasis and leptin resistance. Front. Neuroendocrinol. 2003, 24, 225–253. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin Enhances Appetite and Increases Food Intake in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Mark, A.L.; Correia, M.L.G.; Rahmouni, K.; Haynes, W.G. Selective leptin resistance: A new concept in leptin physiology with cardiovascular implications. J. Hypertens. 2002, 20, 1245–1250. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; Mantzoros, C.S.; Kuliawat, R.; Hadro, E.; Flier, J.S.; Kahn, B.B. Evidence for leptin binding to proteins in serum of rodents and humans: Modulation with obesity. Diabetes 1996, 45, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- English, P.J.; Ghatei, M.A.; Malik, I.A.; Bloom, S.R.; Wilding, J.P.H. Food Fails to Suppress Ghrelin Levels in Obese Humans. J. Clin. Endocrinol. Metab. 2002, 87, 2984. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Istrate, N.; Wang, L.; Nichols, A.J.; Tozzo, E.; Stricker-Krongrad, A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: Reversal upon weight loss. Int. J. Obes. 2004, 28, 879–885. [Google Scholar] [CrossRef]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909.e20. [Google Scholar] [CrossRef]
- Potter, G.D.M.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Nutrition and the circadian system. Br. J. Nutr. 2016, 116, 434–442. [Google Scholar] [CrossRef]
- Hernandez-Morante, J.J.; Gomez-Santos, C.; Milagro, F.; Campión, J.; Martínez, J.A.; Zamora, S.; Garaulet, M.; Campion, J.; Martinez, J.A.; Zamora, S.; et al. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int. J. Obes. (Lond.) 2009, 33, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, F.; Mir, A.A.; Balazs, K.; Wierer, M.; Dyar, K.A.; Jouffe, C.; Makris, K.; Hawe, J.; Heinig, M.; Filipp, F.V.; et al. Cistromic Reprogramming of the Diurnal Glucocorticoid Hormone Response by High-Fat Diet. Mol. Cell 2019, 76, 531–545.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yin, Y.; Zhang, W. Ghrelin restores the disruption of the circadian clock in steatotic liver. Int. J. Mol. Sci. 2018, 19, 3134. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015, 22, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Galindo Muñoz, J.S.; Morillas-Ruiz, J.M.; Gómez Gallego, M.; Díaz Soler, I.; Barberá Ortega, M.D.C.; Martínez, C.M.; Hernández Morante, J.J. Cognitive Training Therapy Improves the Effect of Hypocaloric Treatment on Subjects with Overweight/Obesity: A Randomised Clinical Trial. Nutrients 2019, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Galindo Muñoz, J.S.; Gómez Gallego, M.; Díaz Soler, I.; Barberá Ortega, M.C.; Martínez Cáceres, C.M.; Hernández Morante, J.J. Effect of a chronotype-adjusted diet on weight loss effectiveness: A randomized clinical trial. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gargallo Fernández Manuel, M.; Breton Lesmes, I.; Basulto Marset, J.; Quiles Izquierdo, J.; Formiguera Sala, X.; Salas-Salvadó, J. Evidence-based nutritional recommendations for the prevention and treatment of overweight and obesity in adults (FESNAD-SEEDO consensus document). The role of diet in obesity treatment (III/III). Nutr. Hosp. 2012, 27, 833–864. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Morante, J.J.; Galindo-Muñoz, J.S.; Barberá-Ortega, M.C. Development and validation of a smartphone application to analyze subjective appetite variables. Nutr. Hosp. 2016, 33, 415–421. [Google Scholar]
- Gibbons, C.; Caudwell, P.; Finlayson, G.; King, N.; Blundell, J. Validation of a new hand-held electronic data capture method for continuous monitoring of subjective appetite sensations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 57. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef]
- Galindo Muñoz, J.S.; Jiménez Rodríguez, D.; Hernández Morante, J.J.; Muñoz, J.S.G.; Rodríguez, D.J.; Morante, J.J.H. Diurnal rhythms of plasma GLP-1 levels in normal and overweight/obese subjects: Lack of effect of weight loss. J. Physiol. Biochem. 2015, 71, 17–28. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Rubio, M.A.; Barbany, M.; Moreno, B.; Grupo Colaborativo de la SEEDO. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. 2007, 128, 184–196. [Google Scholar]
- Mayr, S.; Buchner, A.; Erdfelder, E.; Faul, F. A short tutorial of GPower. Tutor. Quant. Methods Psychol. 2007, 3, 51–59. [Google Scholar] [CrossRef]
- Gierke, C.L.; Cornelissen, G. Chronomics analysis toolkit (CATkit). Biol. Rhythm Res. 2016, 47, 163–181. [Google Scholar] [CrossRef]
- Patton, D.F.; Mistlberger, R.E. Circadian adaptations to meal timing: Neuroendocrine mechanisms. Front. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Perfetto, F.; Tarquini, R.; Cornélissen, G.; Mello, G.; Tempestini, A.; Gaudiano, P.; Mancuso, F.; Halberg, F. Circadian phase difference of leptin in android versus gynoid obesity. Peptides 2004, 25, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Kanabrocki, E.L.; Hermida, R.C.; Wright, M.; Young, R.M.I.; Bremner, F.W.; Third, J.L.H.C.; Ryan, M.D.; Ayala, D.E.; Johnson, M.; Nemchausky, B.A.; et al. Circadian variation of serum leptin in healthy and diabetic men. Chronobiol. Int. 2001, 18, 273–283. [Google Scholar] [CrossRef]
- Thompson, H.J.; Sedlacek, S.M.; Wolfe, P.; Paul, D.; Lakoski, S.G.; Playdon, M.C.; McGinley, J.N.; Matthews, S.B. Impact of weight loss on plasma leptin and adiponectin in overweight-to-obese post menopausal breast cancer survivors. Nutrients 2015, 7, 5156–5176. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Goyenechea, E.; Abete, I.; Lage, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J. Clin. Endocrinol. Metab. 2010, 95, 5037–5044. [Google Scholar] [CrossRef]
- Strohacker, K.; McCaffery, J.M.; Maclean, P.S.; Wing, R.R. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: A review of current literature. Int. J. Obes. 2014, 38, 388–396. [Google Scholar] [CrossRef]
- Costa Justus, J.F.; Ligocki Campos, A.C.; Figueroa, A.L.C.; Gomis, R.; Santo, M.A.; Fávero, G.M.; Milléo, F.Q.; Vieira, E. Early Effect of Bariatric Surgery on the Circadian Rhythms of Adipokines in Morbidly Obese Women. Metab. Syndr. Relat. Disord. 2016, 14, 16–22. [Google Scholar] [CrossRef]
- Raffaelli, M.; Iaconelli, A.; Nanni, G.; Guidone, C.; Callari, C.; Fernandez Real, J.M.; Bellantone, R.; Mingrone, G. Effects of biliopancreatic diversion on diurnal leptin, insulin and free fatty acid levels. Br. J. Surg. 2015, 102, 682–690. [Google Scholar] [CrossRef]
- Mäntele, S.; Otway, D.T.; Middleton, B.; Bretschneider, S.; Wright, J.; Robertson, M.D.; Skene, D.J.; Johnston, J.D. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS ONE 2012, 7, e37123. [Google Scholar] [CrossRef]
- Sofer, S.; Eliraz, A.; Kaplan, S.; Voet, H.; Fink, G.; Kima, T.; Madar, Z. Changes in daily leptin, ghrelin and adiponectin profiles following a diet with carbohydrates eaten at dinner in obese subjects. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Morris, A. Mechanisms of leptin resistance revealed. Nat. Rev. Endocrinol. 2018, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; De Sousa, G.; Roth, C.L. Obestatin and ghrelin levels in obese children and adolescents before and after reduction of overweight. Clin. Endocrinol. (Oxf.) 2008, 68, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.F.; Passos, E.P.; Moulin, C.C. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, B.; Yilmaz, H.; Kebapçilar, L.; Kirbiyik, H.; Alacacioǧlu, A.; Bozkaya, G.; Pamuk, G.; Demirpence, M. The effect of orlistat and weight loss diet on plasma ghrelin and obestatin. J. Res. Med. Sci. 2018, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Weigle, D.S.; Scott Frayo, R.; Breen, P.A.; Ma, M.K.; Patchen Dellinger, E.; Purnell, J.Q. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef]
- Ott, V.; Friedrich, M.; Zemlin, J.; Lehnert, H.; Schultes, B.; Born, J.; Hallschmid, M. Meal anticipation potentiates postprandial ghrelin suppression in humans. Psychoneuroendocrinology 2012, 37, 1096–1100. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Briggs, D.I.; Lockie, S.H.; Benzler, J.; Wu, Q.; Stark, R.; Reichenbach, A.; Hoy, A.J.; Lemus, M.B.; Coleman, H.A.; Parkington, H.C.; et al. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology 2014, 155, 2411–2422. [Google Scholar] [CrossRef]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic inflammation and obesity: A mechanistic review. Arch. Pharmacal Res. 2019, 42, 383–392. [Google Scholar] [CrossRef]
- Farias, G.; Netto, B.D.M.; Boritza, K.; Bettini, S.C.; Vilela, R.M.; Dâmaso, A.R. Impact of Weight Loss on Inflammation State and Endothelial Markers Among Individuals with Extreme Obesity After Gastric Bypass Surgery: A 2-Year Follow-up Study. Obes. Surg. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Lantieri, L.; Leto, G.; Plescia, F.; Di Majo, D. Nutrition, obesity and hormones. J. Biol. Res. 2018, 91, 108–118. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Neuhuber, W.L. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann. N. Y. Acad. Sci. 2019, 1454, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Song, S.E.; Oh, J.U.; Hwang, M.; Cho, H.W.; Bae, J.H.; Im, S.S.; Kim, J.I.; Song, D.K. Orexin A-induced inhibition of leptin expression and secretion in adipocytes reducing plasma leptin levels and hypothalamic leptin resistance. Pflug. Arch. Eur. J. Physiol. 2019, 471, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Münzberg, H.; Morrison, C.D. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 2017, 152, 1728–1738. [Google Scholar] [CrossRef]
- Gómez-Abellán, P.; Hernández-Morante, J.J.; Luján, J.A.; Madrid, J.A.; Garaulet, M. Clock genes are implicated in the human metabolic syndrome. Int. J. Obes. 2008, 32, 121–128. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]




| Control (n = 13) | Intervention (n = 18) | p (t,χ2) | |
|---|---|---|---|
| Age (years) | 44 ± 7 | 47 ± 9 | 0.222 |
| Sex (n of women) | 9 | 13 | 0.583 |
| Weight (kg) | 63.8 ± 8.1 | 85.4 ± 13.2 | <0.001 |
| BMI (kg/m2) | 22.46 ± 2.11 | 31.14 ± 3.50 | <0.001 |
| Waist (cm) | 83.0 ± 5.7115 | 102.6 ± 10.6 | <0.001 |
| Body fat (%) | 26.08 ± 5.44 | 37.09 ± 5.93 | <0.001 |
| SBP (mmHg) | 120 ± 13 | 126 ± 16 | 0.309 |
| DBP (mmHg) | 71 ± 9 | 75 ± 8 | 0.237 |
| Fasting glucose (mg/dL) | 101 ± 9 | 97 ± 13 | 0.382 |
| Triglycerides (mg/dL) | 103.2 ± 33.5307 | 114.1 ± 27.6 | 0.413 |
| cHDL (mg/dL) | 61.6 ± 23.7 | 49.4 ± 10.8 | 0.146 |
| Baseline (n = 18) | Post-Intervention (n = 15) | Change | p | |
|---|---|---|---|---|
| Weight (kg) | 85.4 ± 13.2 | 76.9 ± 11.0 | −8.5 | <0.001 |
| BMI (kg/m2) | 31.14 ± 3.50 | 28.04 ± 2.89 | −3.10 | <0.001 |
| Waist (cm) | 102.6 ± 10.6 | 92.7 ± 9.9 | −9.9 | <0.001 |
| Body fat (%) | 37.09 ± 5.93 | 32.42 ± 5.83 | −4.67 | <0.001 |
| SBP (mmHg) | 126 ± 16 | 120 ± 12 | −6 | 0.146 |
| DBP (mmHg) | 75 ± 8 | 70 ± 8 | −5 | 0.008 |
| Fasting glucose(mg/dL) | 97.1 ± 14.7 | 96.7 ± 12.8 | −0.4 | 0.886 |
| Triglycerides (mg/dL) | 114.1 ± 27.6 | 90.0 ± 21.8 | −24.1 | 0.034 |
| cHDL (mg/dL) | 49.4 ± 10.8 | 60.8 ± 16.6 | +11.4 | 0.108 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández Morante, J.J.; Díaz Soler, I.; Muñoz, J.S.G.; Sánchez, H.P.; Barberá Ortega, M.d.C.; Martínez, C.M.; Morillas Ruiz, J.M. Moderate Weight Loss Modifies Leptin and Ghrelin Synthesis Rhythms but Not the Subjective Sensations of Appetite in Obesity Patients. Nutrients 2020, 12, 916. https://doi.org/10.3390/nu12040916
Hernández Morante JJ, Díaz Soler I, Muñoz JSG, Sánchez HP, Barberá Ortega MdC, Martínez CM, Morillas Ruiz JM. Moderate Weight Loss Modifies Leptin and Ghrelin Synthesis Rhythms but Not the Subjective Sensations of Appetite in Obesity Patients. Nutrients. 2020; 12(4):916. https://doi.org/10.3390/nu12040916
Chicago/Turabian StyleHernández Morante, Juan José, Inmaculada Díaz Soler, Joaquín S. Galindo Muñoz, Horacio Pérez Sánchez, Mª del Carmen Barberá Ortega, Carlos Manuel Martínez, and Juana Mª Morillas Ruiz. 2020. "Moderate Weight Loss Modifies Leptin and Ghrelin Synthesis Rhythms but Not the Subjective Sensations of Appetite in Obesity Patients" Nutrients 12, no. 4: 916. https://doi.org/10.3390/nu12040916
APA StyleHernández Morante, J. J., Díaz Soler, I., Muñoz, J. S. G., Sánchez, H. P., Barberá Ortega, M. d. C., Martínez, C. M., & Morillas Ruiz, J. M. (2020). Moderate Weight Loss Modifies Leptin and Ghrelin Synthesis Rhythms but Not the Subjective Sensations of Appetite in Obesity Patients. Nutrients, 12(4), 916. https://doi.org/10.3390/nu12040916






