Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of RA
2.2. Reagents and Materials
2.3. Cell Culture
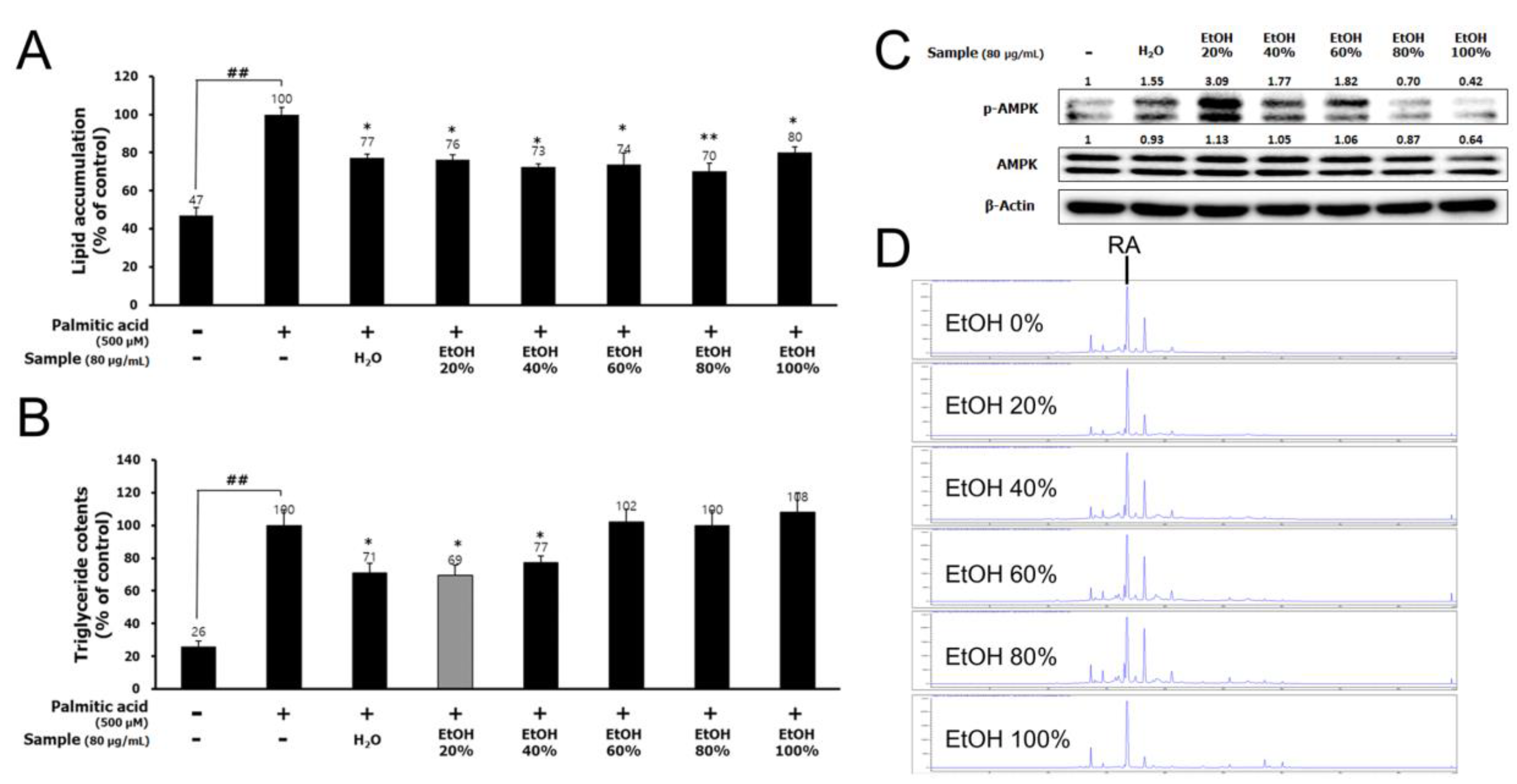
2.4. Chemical Analysis
2.5. Animals and Treatments
2.6. Histopathological Analysis
2.7. Biochemical Analysis
2.8. Determination of the Liver Collagen Content
2.9. Western Blot Analysis
2.10. Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction
2.11. Statistical Analysis
3. Results
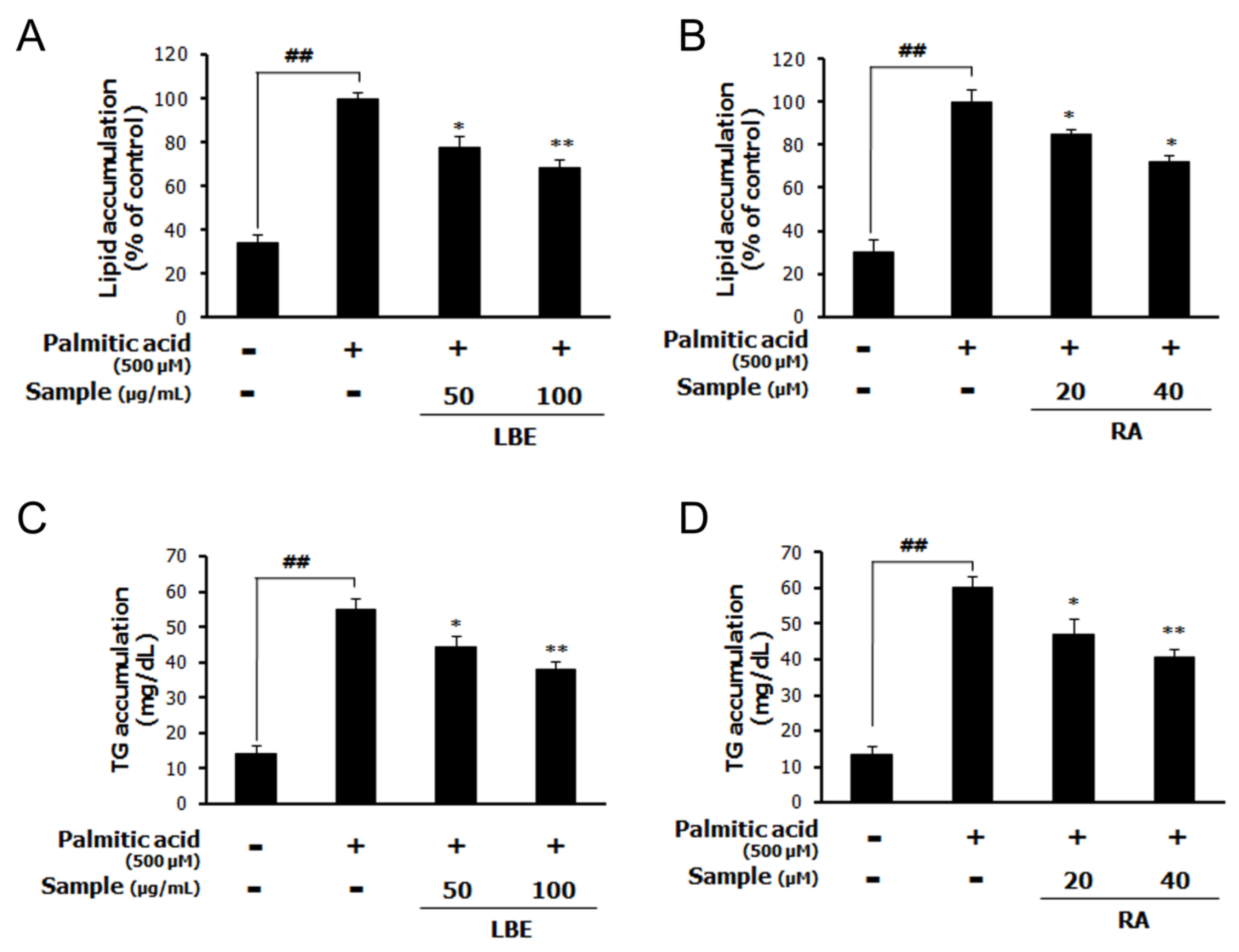
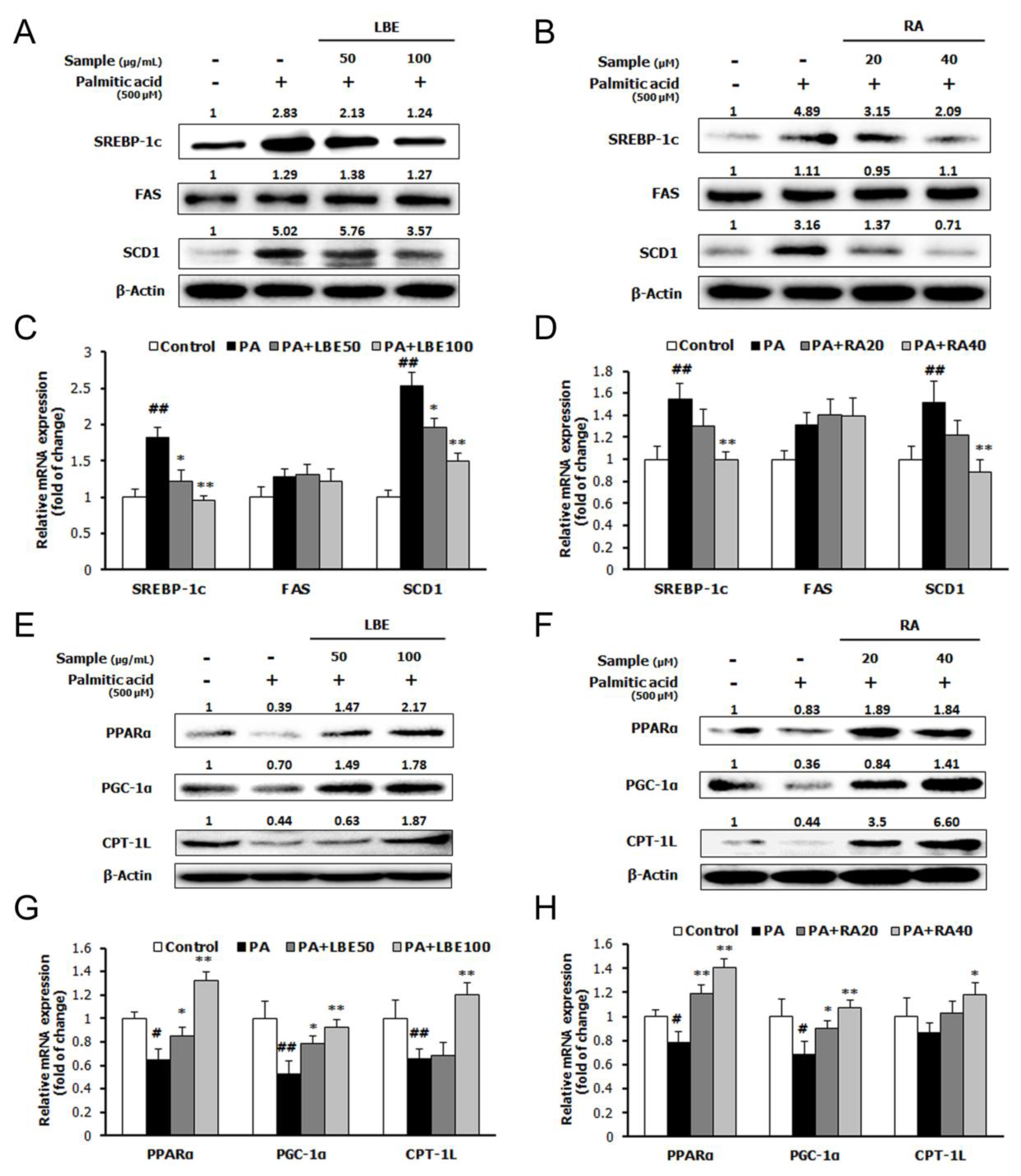
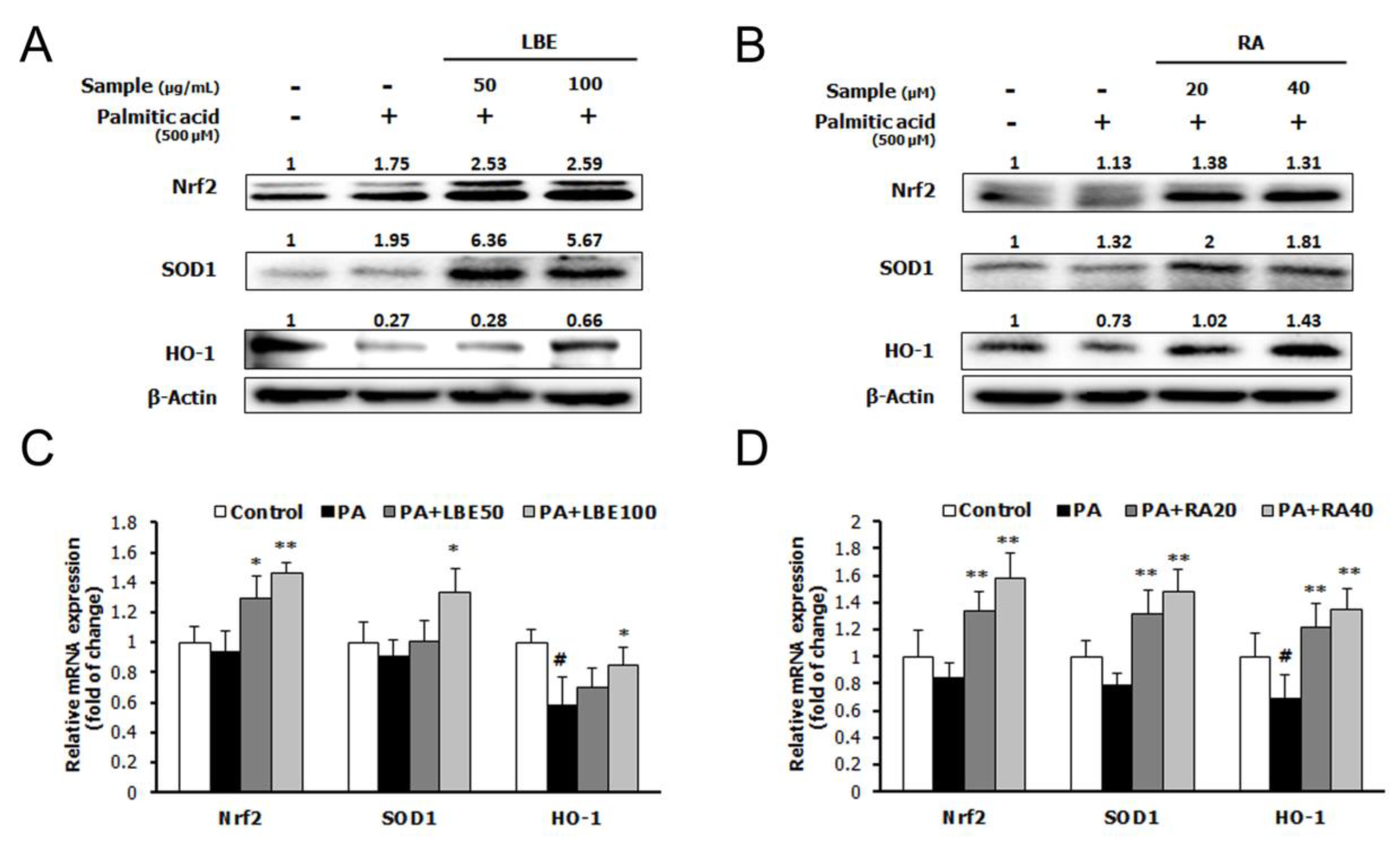
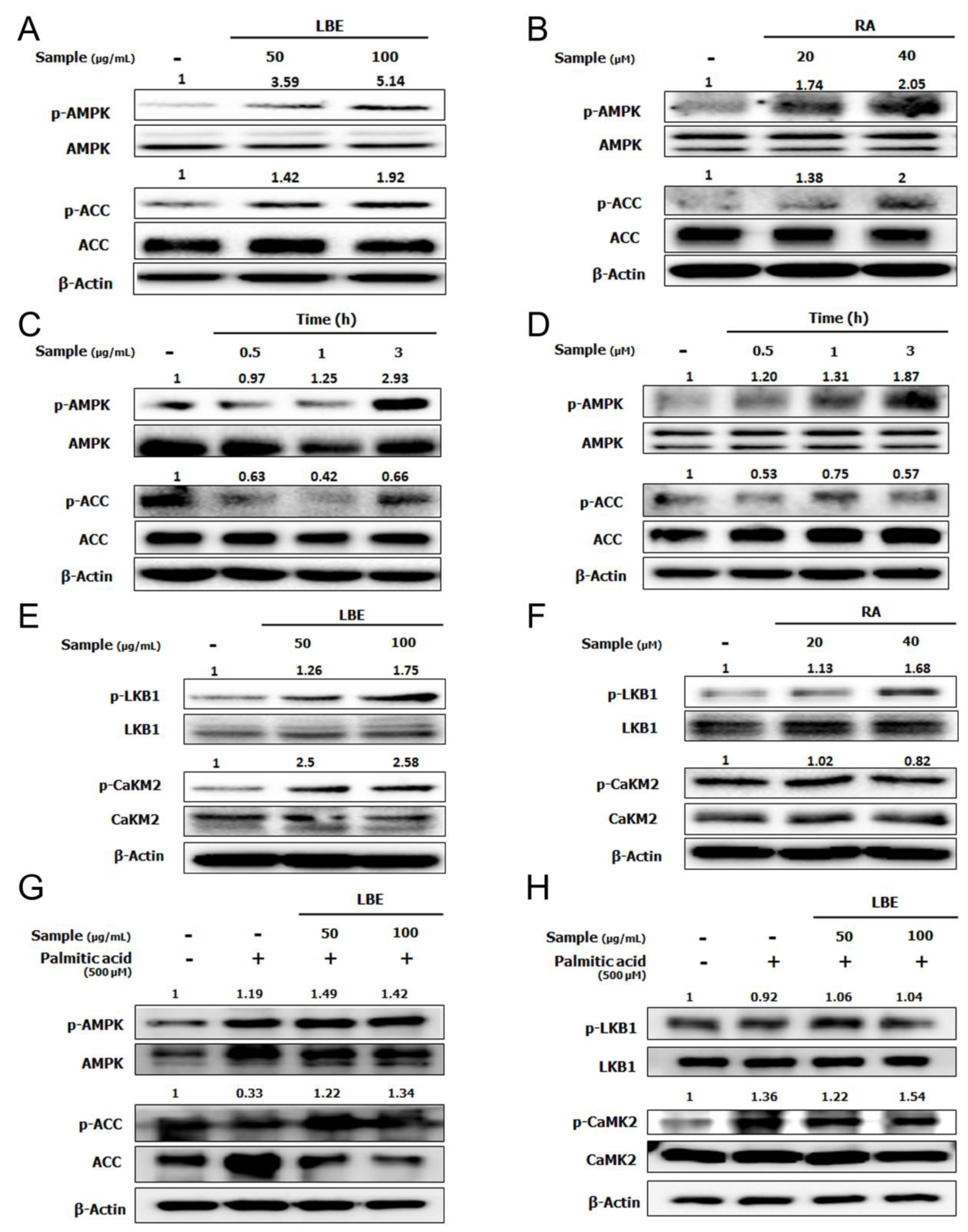
3.1. LBE and RA Regulate Lipid Metabolism and Oxidative Stress in PA-Treated HepG2 Cells
3.2. LBE and RA Increase the Level of Phosphorylated AMPK in HepG2 Cells
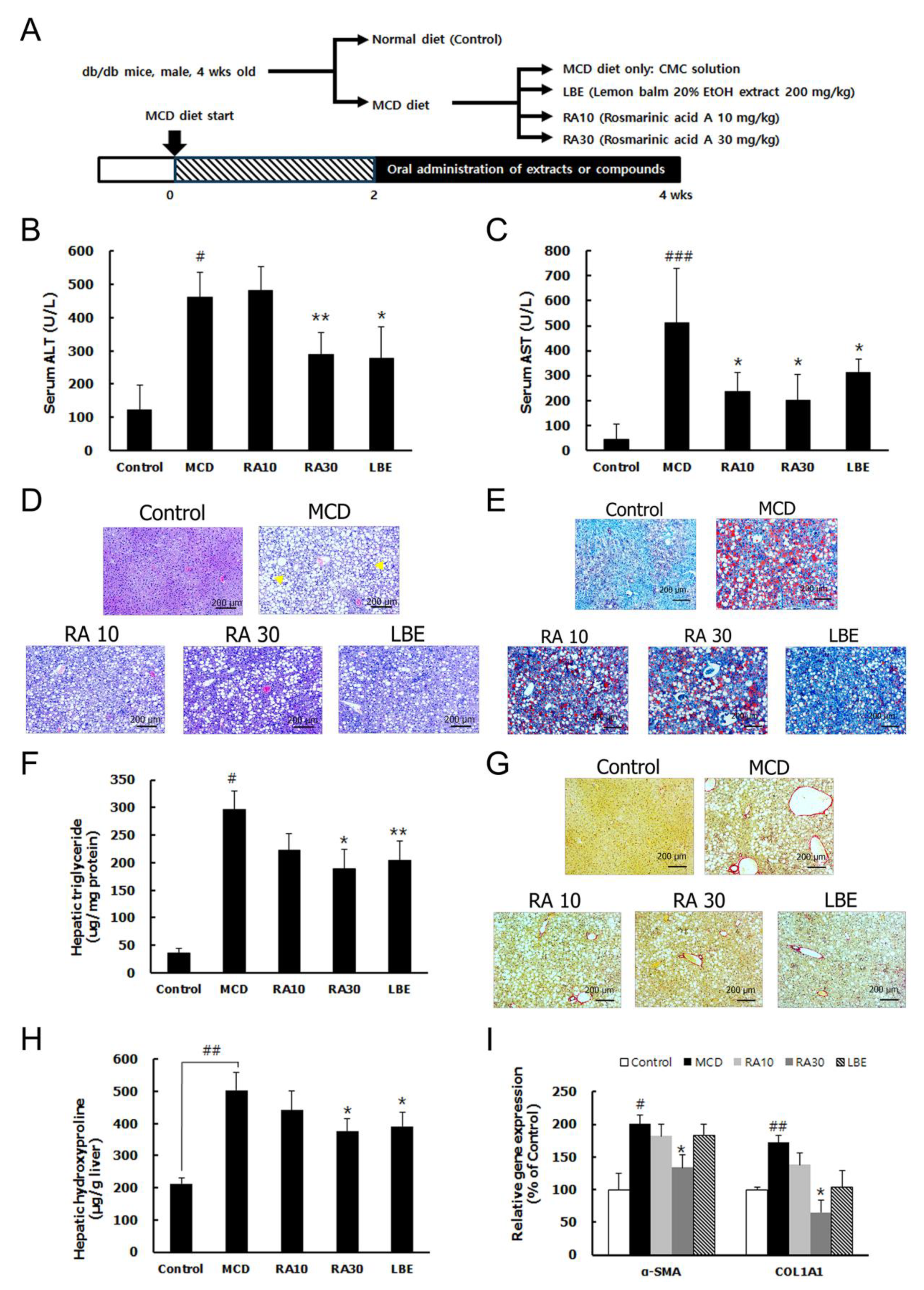
3.3. LBE and RA Reduce Liver Damage by Reducing Lipid Accumulation and Hepatic Fibrosis in MCD Diet-Fed db/db Mice
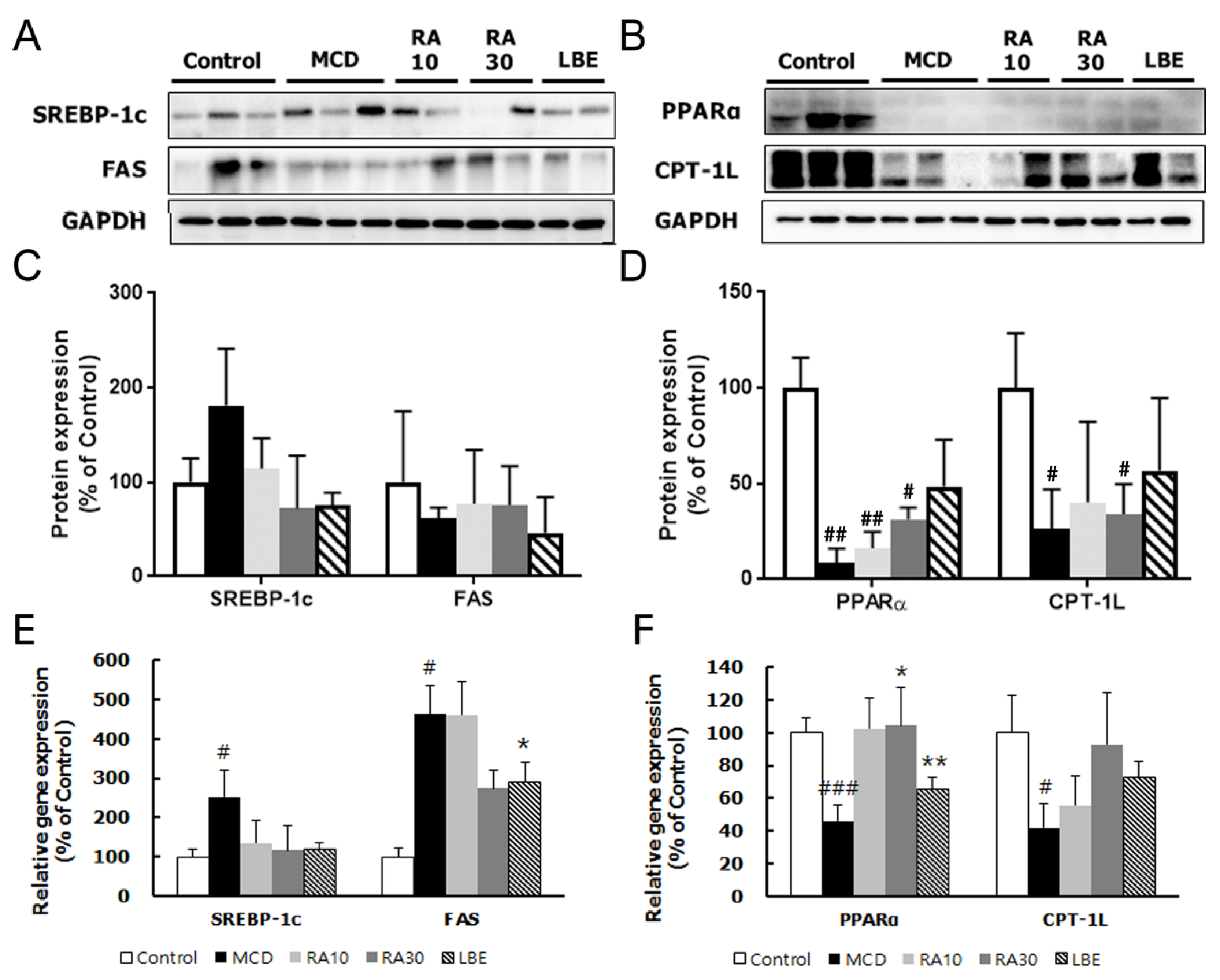
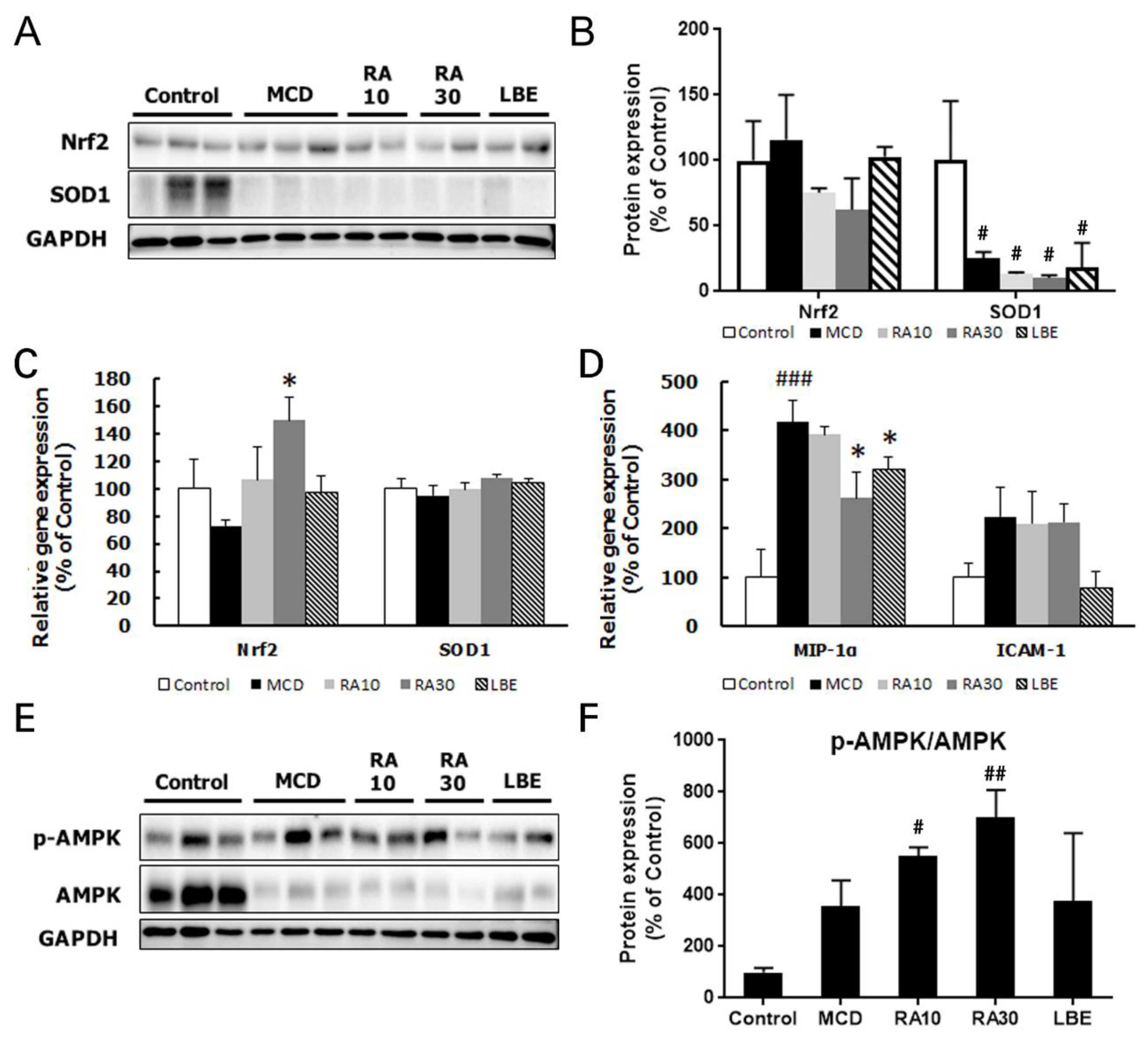
3.4. LBE and RA30 Reduce NASH via Regulation of the AMPK Pathway, Inflammation, and the NRF2 Pathway in MCD Diet-Fed db/db Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | acetyl-CoA carboxylase |
| ALT | alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| AST | aspartate aminotransferase |
| CAMKII | Ca2+/calmodulin-dependent protein kinase II |
| CoA | coenzyme A |
| COL1A1 | collagen type I alpha 1 |
| CPT-1 | carnitine palmitoyl transferase I |
| CRTC | CREB-regulated transcription coactivator 1 |
| FAS | fatty acid synthase |
| HO-1 | heme oxygenase-1 |
| LBE | lemon balm extract extracted by 20% ethanol |
| LKB-1 | liver kinase B1 |
| MCD diet | methionine- and choline-deficient diet |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| NRF2 | nuclear factor erythroid-derived 2-related factor 2 |
| PA | palmitic acid |
| PGC-1 | peroxisome proliferator-activated receptor γ coactivator 1 |
| PPARα | peroxisome proliferator-activated receptor α |
| RA | rosmarinic acid |
| SCD-1 | stearoyl-CoA desaturase-1 |
| α-SMA | α -smooth muscle actin |
| SOD1 | superoxide dismutase 1 |
| SREBP-1 | sterol regulatory element-binding protein-1 |
| TG | triglyceride |
References
- Anderson, N.; Borlak, J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008, 60, 311–357. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. New peroxisome proliferator-activated receptor agonists: Potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin. Pharmacother. 2014, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Weng, S.Y.; Schuppan, D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J. Hepatol. 2013, 58, 619–621. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Tildesley, N.T.; Perry, E.K.; Wesnes, K.A. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol. Biochem. Behav. 2002, 72, 953–964. [Google Scholar] [CrossRef]
- Scholey, A.; Gibbs, A.; Neale, C.; Perry, N.; Ossoukhova, A.; Bilog, V.; Kras, M.; Scholz, C.; Sass, M.; Buchwald-Werner, S. Anti-stress effects of lemon balm-containing foods. Nutrients 2014, 6, 4805–4821. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Jeung, I.C.; Jee, D.; Rho, C.R.; Kang, S. Melissa officinalis L. Extracts Protect Human Retinal Pigment Epithelial Cells against Oxidative Stress-Induced Apoptosis. Int. J. Med. Sci. 2016, 13, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lim, J.; Oh, J.; Shin, S.S.; Yoon, M. The Angiogenesis Inhibitor ALS-L1023 from Lemon-Balm Leaves Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease through Regulating the Visceral Adipose-Tissue Function. Int. J. Mol. Sci. 2017, 18, 846. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lim, J.; Lee, H.; Yoon, S.; Shin, S.S.; Yoon, M. The lemon balm extract ALS-L1023 inhibits obesity and nonalcoholic fatty liver disease in female ovariectomized mice. Food Chem. Toxicol. 2017, 106, 292–305. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. Recent studies on rosmarinic acid and its biological and pharmacological activities. EXCLI J. 2014, 13, 1192–1195. [Google Scholar]
- Govindaraj, J.; Sorimuthu Pillai, S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: Streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2015, 404, 143–159. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, Q.; Liu, M.Y.; Yu, H.Y.; Xu, J.Q.; Wu, J.Q.; Zhang, Y.; Wang, T. Regulation effects of rosemary (Rosmarinus officinalis Linn.) on hepatic lipid metabolism in OA induced NAFLD rats. Food Funct. 2019, 10, 7356–7365. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Iwasa, K.; Nagai, T.; Kobayashi, S.; Nakamura, H.; Yamada, M. Pharmacokinetics, Safety and Tolerability of Melissa officinalis Extract which Contained Rosmarinic Acid in Healthy Individuals: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0126422. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, S.; Chu, E.S.; Go, M.Y.; Lau, R.H.; Zhao, J.; Wu, C.W.; Tong, L.; Zhao, J.; Poon, T.C.; et al. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int. J. Biochem. Cell Biol. 2010, 42, 948–957. [Google Scholar] [CrossRef]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Attia, R.R.; Connaughton, S.; Niesen, M.I.; Ness, G.C.; Elam, M.B.; Hori, R.T.; Cook, G.A.; Park, E.A. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol. Cell. Endocrinol. 2010, 325, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Kohler, U.A.; Zhang, L.; Roenneburg, D.; Werner, S.; Johnson, J.A.; Foley, D.P. Activation of the Nrf2-ARE pathway in hepatocytes protects against steatosis in nutritionally induced non-alcoholic steatohepatitis in mice. Toxicol. Sci. 2014, 142, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Li, G.S.; Jiang, W.L.; Tian, J.W.; Qu, G.W.; Zhu, H.B.; Fu, F.H. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine 2010, 17, 282–288. [Google Scholar] [CrossRef]
- Balachander, G.J.; Subramanian, S.; Ilango, K. Rosmarinic acid attenuates hepatic steatosis by modulating ER stress and autophagy in oleic acid-induced HepG2 cells. RSC Adv. 2018, 8, 26656–26663. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, H.T.D.; Oinonen, P.P.; Darwis, Y.; Laakso, I.; Hiltunen, R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT Food Sci. Technol. 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Wang, X.; Hasegawa, J.; Kitamura, Y.; Wang, Z.; Matsuda, A.; Shinoda, W.; Miura, N.; Kimura, K. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J. Pharmacol. Sci. 2011, 117, 129–138. [Google Scholar] [CrossRef]
- Liu, J.F.; Ma, Y.; Wang, Y.; Du, Z.Y.; Shen, J.K.; Peng, H.L. Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother. Res. 2011, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Petzke, K.J.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, N.; Bahcecioglu, I.H.; Dagli, A.F.; Ozercan, I.H.; Ustundag, B.; Sahin, K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 2008, 23, e465–e470. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Ho, W.S. Anti-oxidative and hepatoprotective effects of lithospermic acid against carbon tetrachloride-induced liver oxidative damage in vitro and in vivo. Oncol. Rep. 2015, 34, 673–680. [Google Scholar] [CrossRef] [PubMed]
| Sample | Solvent | g/100 g ext. |
|---|---|---|
| Lemon balm extract | H2O | 4.476 ± 0.041 |
| 20% EtOH (LBE) | 4.288 ± 0.430 | |
| 40% EtOH | 4.711 ± 0.006 | |
| 60% EtOH | 4.865 ± 0.068 | |
| 80% EtOH | 5.282 ± 0.058 | |
| 100% EtOH | 5.221 ± 0.016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Yoo, G.; Randy, A.; Son, Y.-J.; Hong, C.R.; Kim, S.M.; Nho, C.W. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients 2020, 12, 1166. https://doi.org/10.3390/nu12041166
Kim M, Yoo G, Randy A, Son Y-J, Hong CR, Kim SM, Nho CW. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients. 2020; 12(4):1166. https://doi.org/10.3390/nu12041166
Chicago/Turabian StyleKim, Myungsuk, GyHye Yoo, Ahmad Randy, Yang-Ju Son, Chi Rac Hong, Sang Min Kim, and Chu Won Nho. 2020. "Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis" Nutrients 12, no. 4: 1166. https://doi.org/10.3390/nu12041166
APA StyleKim, M., Yoo, G., Randy, A., Son, Y.-J., Hong, C. R., Kim, S. M., & Nho, C. W. (2020). Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients, 12(4), 1166. https://doi.org/10.3390/nu12041166






