A Critical Review on the Role of Food and Nutrition in the Energy Balance
Abstract
1. Introduction
- (i)
- The energy balance in humans;
- (ii)
- Energy intake from food;
- (iii)
- Energy expenditure due to food intake;
- -
- the role of nutrients;
- -
- the role of foods;
- -
- the role of diet plans;
- (iv)
- The impact of the gut microbiota on the human energy balance.
2. Methods
3. The Energy Balance in Humans
4. Energy Intake from Food
5. Energy Expenditure Due to Food Intake
5.1. The Role of Nutrients
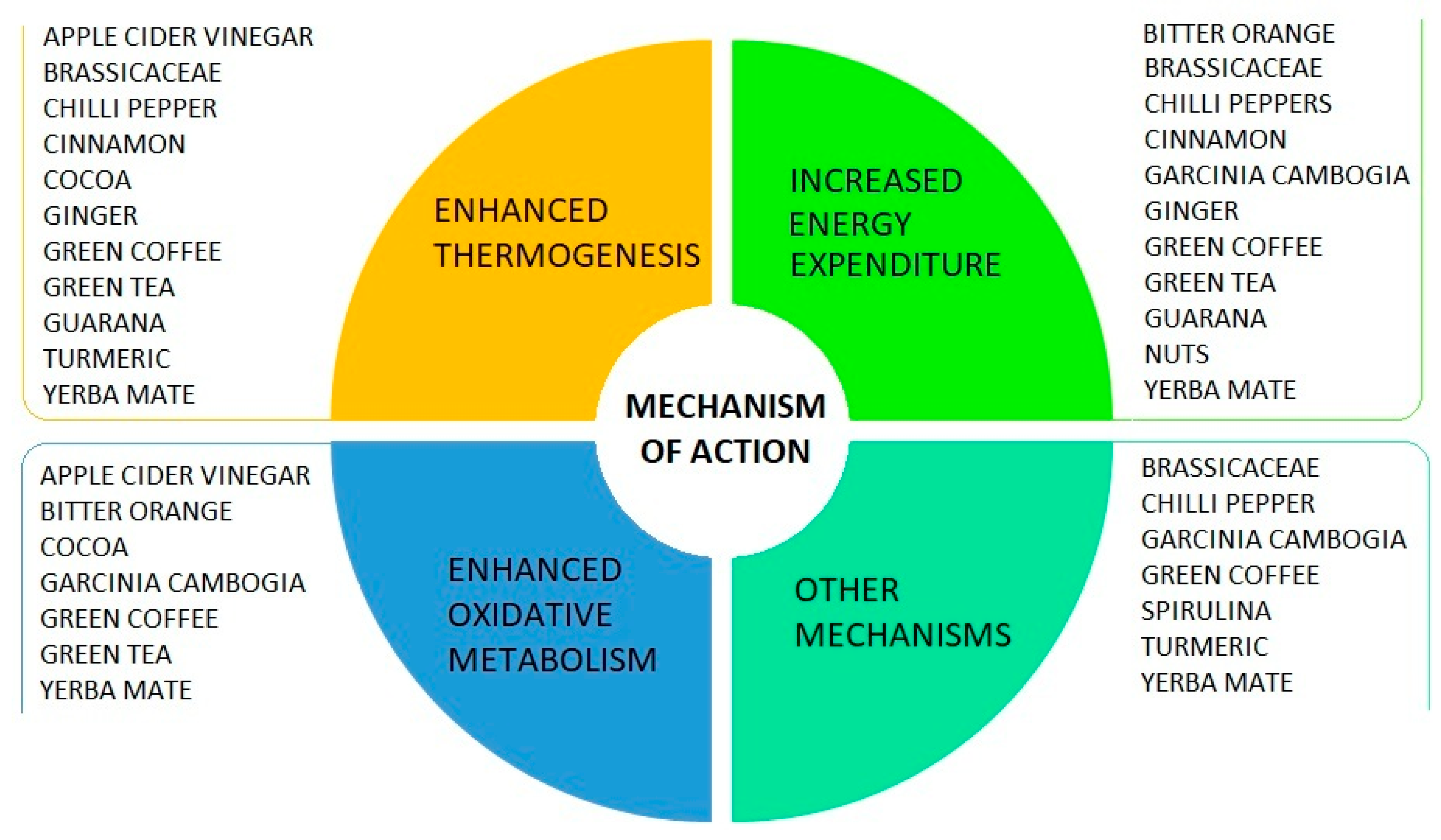
5.2. The Role of Foods
5.2.1. Green Coffee
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.2. Green Tea (Camellia sinensis)
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.3. Cocoa and Dark Chocolate
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.4. Yerba Mate (Ilex paraguariensis)
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.5. Bitter Orange (Citrus aurantium)
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.6. Ginger
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.7. Curcuma Longa
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.8. Cinnamon
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.9. Chili Pepper (Capsicum Species)
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.10. Garcinia cambogia
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.11. Guarana (Paullinia cupana)
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.12. Brassicaceae
Available Evidence
Molecular Mechanisms of Action
Toxicity and Reactivity
5.2.13. Nuts
5.2.14. Apple Cider Vinegar
5.2.15. Spirulina
5.2.16. Foods without Scientific Evidence to Date
5.3. The Role of Diet Plans
6. The Impact of the Gut Microbiota on the Human Energy Balance
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shader, R.I. Troublesome news, fake news, biased or incomplete news. Clin. Ther. 2018, 40, 1429–1434. [Google Scholar] [CrossRef]
- Casazza, K.; Fontaine, K.R.; Astrup, A.; Birch, L.L.; Brown, A.W.; Bohan Brown, M.M.; Durant, N.; Dutton, G.; Foster, E.M.; Heymsfield, S.B.; et al. Myths, presumptions, and facts about obesity. N. Engl. J. Med. 2013, 368, 446–454. [Google Scholar] [CrossRef]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef] [PubMed]
- L’alimentazione. Available online: https://www.issalute.it/index.php/falsi-miti-e-bufale/l-alimentazione?limitstart=0 (accessed on 12 October 2019).
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. Reviewers of the AACE/ACE obesity clinical practice guidelines. American association of clinical endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22, 842–884. [Google Scholar] [CrossRef] [PubMed]
- de Keersmaecker, J.; Roets, A. ‘Fake news’: Incorrect, but hard to correct: The role of cognitive ability on the impact of false information on social impressions. Intelligence 2017, 65, 107–110. [Google Scholar] [CrossRef]
- Hopkins, M.; Blundell, J.E. Energy balance, body composition, sedentariness and appetite regulation: Pathways to obesity. Clin. Sci. 2016, 130, 1615–1628. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Carneiro, I.P.; Elliott, S.A.; Siervo, M.; Padwal, R.; Bertoli, S.; Battezzati, A.; Prado, C.M. Is obesity associated with altered energy expenditure? Adv. Nutr. 2016, 7, 476–487. [Google Scholar] [CrossRef]
- Yoo, S. Dynamic energy balance and obesity prevention. J. Obes. Metab. Syndr. 2018, 27, 203–212. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Casa, D.J.; Belval, L.N. Metabolism, bioenergetics and thermal physiology: Influences of the human intestinal microbiota. Nutr. Res. Rev. 2019, 32, 1–13. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Quatela, A.; Callister, R.; Patterson, A.; MacDonald-Wicks, L. The energy content and composition of meals consumed after an overnight fast and their effects on diet induced thermogenesis: A systematic review, meta-analyses and meta-regressions. Nutrients 2016, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef]
- Müller, M.J.; Bosy-Westphal, A. Adaptive thermogenesis with weight loss in humans. Obesity 2013, 21, 218–228. [Google Scholar] [CrossRef]
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Leibel, R.L. Adaptive thermogenesis in humans. Int. J. Obes. 2005 2010, 34, S47–S55. [Google Scholar] [CrossRef]
- Saito, M.; Yoneshiro, T.; Matsushita, M. Activation and recruitment of brown adipose tissue by cold exposure and food ingredients in humans. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 537–547. [Google Scholar] [CrossRef]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly)phenols, brown adipose tissue activation, and energy expenditure: A narrative review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The browning of white adipose tissue: Some burning issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef]
- Müller, M.J.; Enderle, J.; Bosy-Westphal, A. Changes in energy expenditure with weight gain and weight loss in humans. Curr. Obes. Rep. 2016, 5, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Oliviero, T.; Fogliano, V.; Pellegrini, N. Role of the food matrix and digestion on calculation of the actual energy content of food. Nutr. Rev. 2018, 76, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Petersen, K.S.; Lamendella, R.; Shearer, G.C.; Murray-Kolb, L.E.; Proctor, D.N.; Kris-Etherton, P.M. Tree nut consumption and adipose tissue mass: Mechanisms of action. Curr. Dev. Nutr. 2018, 2, nzy069. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Nuts and CVD. Br. J. Nutr. 2015, 11, S111–S120. [Google Scholar] [CrossRef]
- Novotny, J.A.; Gebauer, S.K.; Baer, D.J. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am. J. Clin. Nutr. 2012, 96, 296–301. [Google Scholar] [CrossRef]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts consumed by healthy adults provide less available energy than predicted by the Atwater factors. J. Nutr. 2016, 146, 9–13. [Google Scholar] [CrossRef]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Measured energy value of pistachios in the human diet. Br. J. Nutr. 2012, 107, 120–125. [Google Scholar] [CrossRef]
- Baer, D.J.; Novotny, J.A. Metabolizable energy from cashew nuts is less than that predicted by atwater factors. Nutrients 2018, 11, 33. [Google Scholar] [CrossRef]
- Capuano, E.; Pellegrini, N.; Ntone, E.; Nikiforidis, C.V. In vitro lipid digestion in raw and roasted hazelnut particles and oil bodies. Food Funct. 2018, 9, 2508–2516. [Google Scholar] [CrossRef]
- Ellis, P.R.; Kendall, C.W.C.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J.A. Role of Cell Walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Parker, M.L.; Grundy, M.M.L.; Grassby, T.; Smeriglio, A.; Bisignano, C.; Raciti, R.; Trombetta, D.; Baer, D.J.; Wilde, P.J. Understanding the effect of particle size and processing on almond lipid bioaccessibility through microstructural analysis: From mastication to faecal collection. Nutrients 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Traoret, C.J.; Lokko, P.; Cruz, A.C.R.F.; Oliveira, C.G.; Costa, N.M.B.; Bressan, J.; Alfenas, R.C.G.; Mattes, R.D. Peanut digestion and energy balance. Int. J. Obes. 2008, 32, 322–328. [Google Scholar] [CrossRef]
- Levine, J.A. Measurement of energy expenditure. Public Health Nutr. 2005, 8, 1123–1132. [Google Scholar] [CrossRef]
- Levine, J.A.; Schleusner, S.J.; Jensen, M.D. Energy expenditure of nonexercise activity. Am. J. Clin. Nutr. 2000, 72, 1451–1454. [Google Scholar] [CrossRef]
- Ho, K.K.Y. Diet-induced thermogenesis: Fake friend or foe? J. Endocrinol. 2018, 238, R185–R191. [Google Scholar] [CrossRef]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The thermic effect of food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef]
- Camastra, S.; Bonora, E.; Del Prato, S.; Rett, K.; Weck, M.; Ferrannini, E. Effect of obesity and insulin resistance on resting and glucose-induced thermogenesis in man. EGIR (European Group for the Study of Insulin Resistance). Int. J. Obes. 1999, 23, 1307–1313. [Google Scholar] [CrossRef]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695. [Google Scholar] [CrossRef]
- Bo, S.; Broglio, F.; Settanni, F.; Parasiliti Caprino, M.; Ianniello, A.; Mengozzi, G.; De Francesco, A.; Fadda, M.; Fedele, D.; Guggino, A.; et al. Effects of meal timing on changes in circulating epinephrine, norepinephrine, and acylated ghrelin concentrations: A pilot study. Nutr. Diabetes 2017, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Normand, S.; Sothier, M.; Peyrat, J.; Louche-Pelissier, C.; Laville, M. Is advice for breakfast consumption justified? Results from a short-term dietary and metabolic experiment in young healthy men. Br. J. Nutr. 2000, 84, 337–344. [Google Scholar] [CrossRef]
- Soenen, S.; Martens, E.A.P.; Hochstenbach-Waelen, A.; Lemmens, S.G.T.; Westerterp-Plantenga, M.S. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J. Nutr. 2013, 143, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Sakane, N.; Moritani, T. Metabolic responses to high-fat or low-fat meals and association with sympathetic nervous system activity in healthy young men. J. Nutr. Sci. Vitaminol. 2005, 51, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Raben, A.; Agerholm-Larsen, L.; Flint, A.; Holst, J.J.; Astrup, A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am. J. Clin. Nutr. 2003, 77, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ravn, A.M.; Gregersen, N.T.; Christensen, R.; Rasmussen, L.G.; Hels, O.; Belza, A.; Raben, A.; Larsen, T.M.; Toubro, S.; Astrup, A. Thermic effect of a meal and appetite in adults: An individual participant data meta-analysis of meal-test trials. Food Nutr. Res. 2013, 57, 19676. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.; Godin, J.P.; Moille, S.E.; Nielsen-Moennoz, C.; Groulx, K.; Oguey-Araymon, S.; Praplan, F.; Beaumont, M.; Sauser, J.; Monnard, I.; et al. Effects of protein quantity and type on diet induced thermogenesis in overweight adults: A randomized controlled trial. Clin. Nutr. 2019, 38, 1570–1580. [Google Scholar] [CrossRef]
- Clegg, M.E.; Golsorkhi, M.; Henry, C.J. Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans. Eur. J. Nutr. 2013, 52, 1579–1585. [Google Scholar] [CrossRef]
- Kasai, M.; Nosaka, N.; Maki, H.; Suzuki, Y.; Takeuchi, H.; Aoyama, T.; Ohra, A.; Harada, Y.; Okazaki, M.; Kondo, K. Comparison of diet-induced thermogenesis of foods containing medium-versus long-chain triacylglycerols. J. Nutr. Sci. Vitaminol. 2002, 48, 536–540. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Gómez-Flores, A.; Salas-Salvadó, J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin. Nutr. 2009, 28, 39–45. [Google Scholar] [CrossRef]
- Zamora Navarro, S.; Pérez-Llamas, F. Errors and myths in feeding and nutrition: Impact on the problems of obesity. Nutr. Hosp. 2013, 28, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.C.; Santos, T.F.; Mamede, A.M.G.N.; Oliveira, T.C.; Souza, A.M.; Freitas-Silva, O.; Oliveira, E.M.M. Coffea arabica and c. canephora discrimination in roasted and ground coffee from reference material candidates by real-time PCR. Food Res. Int. 2019, 115, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, A.A. Bioactive micronutrients in coffee: Recent analytical approaches for characterization and quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, K.; Wayne, D.; Leija, H.A.L.; Bloor, I.; Morris, D.E.; Law, J.; Budge, H.; Sacks, H.; Symonds, M.E.; Sottile, V. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Sci. Rep. 2019, 9, 9104. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Geissler, C.A.; Horton, T.; Collins, A.; Miller, D.S. Normal caffeine consumption: Influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am. J. Clin. Nutr. 1989, 49, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Koot, P.; Deurenberg, P. Comparison of changes in energy expenditure and body temperatures after caffeine consumption. Ann. Nutr. Metab. 1995, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Belza, A.; Toubro, S.; Astrup, A. The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur. J. Clin. Nutr. 2009, 63, 57–64. [Google Scholar] [CrossRef]
- Gorji, Z.; Varkaneh, H.K.; Talaei, S.; Nazary-Vannani, A.; Clark, C.C.T.; Fatahi, S.; Rahmani, J.; Salamat, S.; Zhang, Y. The effect of green-coffee extract supplementation on obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 63, 153018. [Google Scholar] [CrossRef]
- Ghadieh, H.E.; Smiley, Z.N.; Kopfman, M.W.; Najjar, M.G.; Hake, M.J.; Najjar, S.M. Chlorogenic acid/chromium supplement rescues diet-induced insulin resistance and obesity in mice. Nutr. Metab. 2015, 12, 19. [Google Scholar] [CrossRef]
- Acar-Tek, N.; Aǧagündüz, D.; Ayhan, B. Effect of green coffee consumption on resting energy expenditure, blood pressure, and body temperature in healthy women: A pilot study. J. Am. Coll. Nutr. 2018, 37, 691–700. [Google Scholar] [CrossRef]
- Dulloo, A.G. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Badmaev, V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O'Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.; Kim, H.A.; Oketch-Rabah, H.; Marles, R.J.; Roe, A.L.; Calderón, A.I. Safety of guarana seed as a dietary ingredient: A review. J. Agric. Food Chem. 2019, 67, 11281–11287. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Türközü, D.; Tek, N.A. A minireview of effects of green tea on energy expenditure. Crit. Rev. Food Sci. Nutr. 2017, 57, 254–258. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H.; Sheridan, Z.P. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J. Food Drug Anal. 2018, 26, 1–13. [Google Scholar] [CrossRef]
- Seo, D.B.; Jeong, H.W.; Kim, Y.J.; Kim, S.; Kim, J.; Lee, J.H.; Joo, K.; Choi, J.K.; Shin, S.S.; Lee, S.J. Fermented green tea extract exhibits hypolipidaemic effects through the inhibition of pancreatic lipase and promotion of energy expenditure. Br. J. Nutr. 2017, 117, 177–186. [Google Scholar] [CrossRef]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu Ngoc, V.T.; et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.H.R.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, T.M.; Whelan, A.M.; Killian, L.; Doucette, S.; Kirk, S.; Foy, E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst. Rev. 2012, 12, CD008650. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Cooper, K.A.; Campos-Giménez, E.; Jiménez Alvarez, D.; Rytz, A.; Nagy, K.; Williamson, G. Predictive relationship between polyphenol and nonfat cocoa solids content of chocolate. J. Agric. Food Chem. 2008, 56, 260–265. [Google Scholar] [CrossRef]
- Baggott, M.J.; Childs, E.; Hart, A.B.; de Bruin, E.; Palmer, A.A.; Wilkinson, J.E.; de Wit, H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology 2013, 228, 109–118. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Kaltsatou, A.; Cicchella, A. Effect of Cocoa products and its polyphenolic constituents on exercise performance and exercise-induced muscle damage and inflammation: A review of clinical trials. Nutrients 2019, 11, 1471. [Google Scholar] [CrossRef]
- Hatano, T.; Miyatake, H.; Natsume, M.; Osakabe, N.; Takizawa, T.; Ito, H.; Yoshida, T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry 2002, 59, 749–758. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Lévèques, A.; Giuffrida, F.; Romanov-Michailidis, F.; Viton, F.; Barron, D.; Duenas-Paton, M.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Williamson, G.; et al. Elucidation of (-)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free Radic. Biol. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Matsui, N.; Ito, R.; Nishimura, E.; Yoshikawa, M.; Kato, M.; Kamei, M.; Shibata, H.; Matsumoto, I.; Abe, K.; Hashizume, S. Ingested Cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition 2005, 21, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Kord-Varkaneh, H.; Ghaedi, E.; Nazary-Vanani, A.; Mohammadi, H.; Shab-Bidar, S. Does Cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Strat, K.M.; Rowley, T.J.; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilson, A.P. Mechanisms by which Cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-molecular-weight Cocoa procyanidins possess enhanced insulin-enhancing and insulin mimetic activities in human primary skeletal muscle cells compared to smaller procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Cacao liquor procyanidins prevent postprandial hyperglycaemia by increasing glucagon-like peptide-1 activity and AMP-activated protein kinase in mice. J. Nutr. Sci. 2019, 8, e2. [Google Scholar] [CrossRef]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch. Biochem. Biophys. 2012, 527, 95–104. [Google Scholar] [CrossRef]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health benefits of methylxanthines in cacao and chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Lindgren, E. The effect of alkylxanthines and other phosphodiesterase inhibitors on adenosine-receptor mediated decrease in lipolysis and cyclic AMP accumulation in rat fat cells. Acta Pharmacol. Toxicol. 1984, 54, 64–71. [Google Scholar] [CrossRef]
- Harpaz, E.; Tamir, S.; Weinstein, A.; Weinstein, Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Sam, J.F.; Gray, P.; Robin, L.P. Cadmium and lead in cocoa powder and chocolate products in the US market. Food Addit. Contam. Part B Surveill. 2018, 11, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Lo Dico, G.M.; Galvano, F.; Dugo, G.; D’ascenzi, C.; Macaluso, A.; Vella, A.; Giangrosso, G.; Cammilleri, G.; Ferrantelli, V. Toxic metal levels in cocoa powder and chocolate by ICP-MS method after microwave-assisted digestion. Food Chem. 2018, 245, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Bracesco, N.; Sanchez, A.G.; Contreras, V.; Menini, T.; Gugliucci, A. Recent advances on ilex paraguariensis research: Minireview. J. Ethnopharmacol. 2011, 136, 378–384. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Martinet, A.; Hostettmann, K.; Schutz, Y. Thermogenic effects of commercially available plant preparations aimed at treating human obesity. Phytomedicine 1999, 6, 231–238. [Google Scholar] [CrossRef]
- Alkhatib, A. Yerba maté (Illex Paraguariensis) ingestion augments fat oxidation and energy expenditure during exercise at various submaximal intensities. Nutr. Metab. 2014, 11, 42. [Google Scholar] [CrossRef]
- Choi, M.S.; Park, H.J.; Kim, S.R.; Kim, D.Y.; Jung, U.J. Long-term dietary supplementation with yerba mate ameliorates diet-induced obesity and metabolic disorders in mice by regulating energy expenditure and lipid metabolism. J. Med. Food 2017, 20, 1168–1175. [Google Scholar] [CrossRef]
- Arçari, D.P.; Santos, J.C.; Gambero, A.; Ribeiro, M.L. The in vitro and in vivo effects of yerba mate (Ilex paraguariensis) extract on adipogenesis. Food Chem. 2013, 141, 809–815. [Google Scholar] [CrossRef]
- Wood Dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; Villena, A.J.; Lima Ribeiro, M. Effects of polyphenols on thermogenesis and mitochondrial biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef]
- de Andrade, F.; de Albuquerque, C.A.C.; Maraschin, M.; da Silva, E.L. Safety assessment of yerba mate (Ilex paraguariensis) dried extract: Results of acute and 90 days subchronic toxicity studies in rats and rabbits. Food Chem. Toxicol. 2012, 50, 328–334. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, W.R.; Lourenço, B.H.L.B.; Reis, M.d.P.; Donadel, G.; Marques, M.A.A.; Cardozo Junior, E.L.; Jacomassi, E.; Belettini, S.T.; Lívero, F.A.D.R.; Gasparotto Junior, A.; et al. Evaluation of reproductive toxicology of aqueous extract of yerba mate (Ilex paraguariensis a. st.-hil.), a traditional south american beverage. J. Med. Food 2019, 22, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Di Ferdinando, D.; Bagchi, M.; Bagchi, D. Citrus aurantium as a thermogenic, weight-reduction replacement for ephedra: An overview. J. Med. 2002, 33, 247–264. [Google Scholar] [PubMed]
- Stohs, S.J. Safety, Efficacy, and mechanistic studies regarding Citrus aurantium (Bitter Orange) extract and p-Synephrine. Phytother. Res. PTR 2017, 31, 1463–1474. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New dietary supplements for obesity: What we currently know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Keith, S.C.; Keith, P.L.; Miller, H.; Kaats, G.R. Effects of p-Synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int. J. Med. Sci. 2011, 8, 295–301. [Google Scholar] [CrossRef]
- Gougeon, R.; Harrigan, K.; Tremblay, J.F.; Hedrei, P.; Lamarche, M.; Morais, J.A. Increase in the Thermic effect of food in women by adrenergic amines extracted from Citrus aurantium. Obes. Res. 2005, 13, 1187–1194. [Google Scholar] [CrossRef]
- Stohs, S.J.; Preuss, H.G.; Shara, M. A Review of the human clinical studies involving Citrus aurantium (Bitter Orange) extract and its primary protoalkaloid p-Synephrine. Int. J. Med. Sci. 2012, 9, 527–538. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Bechke, E.; Williamson, C.; Green, Z.; Bailey, P.; McLester, J.; McLester, C. Citrus Aurantium and caffeine complex versus placebo on biomarkers of metabolism: A double blind crossover design. J. Int. Soc. Sports Nutr. 2019, 16, 4. [Google Scholar] [CrossRef]
- Guo, J.; He, Z.; Wu, S.; Zeng, M.; Chen, J. Binding of aromatic compounds with soy protein isolate in an aqueous model: Effect of pH. J. Food Biochem. 2019, 43, e12817. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.L.; Jung, Y.; Ahn, K.S.; Kwak, H.J.; Um, J.Y. Bitter orange (Citrus aurantium Linné) improves obesity by regulating adipogenesis and thermogenesis through AMPK activation. Nutrients 2019, 11, 1988. [Google Scholar] [CrossRef] [PubMed]
- Bakhiya, N.; Ziegenhagen, R.; Hirsch-Ernst, K.I.; Dusemund, B.; Richter, K.; Schultrich, K.; Pevny, S.; Schäfer, B.; Lampen, A. Phytochemical compounds in sport nutrition: Synephrine and hydroxycitric acid (HCA) as examples for evaluation of possible health risks. Mol. Nutr. Food Res. 2017, 61, 1601020. [Google Scholar] [CrossRef] [PubMed]
- Ginger|plant. Encycl. Br.. 2019. Available online: https://www.britannica.com/plant/ginger (accessed on 30 October 2019).
- Mansour, M.S.; Ni, Y.M.; Roberts, A.L.; Kelleman, M.; Roychoudhury, A.; St-Onge, M.P. Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men: A pilot study. Metabolism 2012, 61, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, N.T.; Belza, A.; Jensen, M.G.; Ritz, C.; Bitz, C.; Hels, O.; Frandsen, E.; Mela, D.J.; Astrup, A. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br. J. Nutr. 2013, 109, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Nammi, S.; Sreemantula, S.; Roufogalis, B.D. Protective effects of ethanolic extract of zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin. Pharmacol. Toxicol. 2009, 104, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.S.; Jung, S.; Son, H.Y.; Park, S.; Kang, B.; Kim, S.Y.; Kim, I.H.; Kim, C.T.; Kim, Y. Ginger extract ameliorates obesity and inflammation via regulating microrna-21/132 expression and AMPK activation in white adipose tissue. Nutrients 2018, 10, 1567. [Google Scholar] [CrossRef]
- Sayed, S.; Ahmed, M.; El-Shehawi, A.; Alkafafy, M.; Al-Otaibi, S.; El-Sawy, H.; Farouk, S.; El-Shazly, S. ginger water reduces body weight gain and improves energy expenditure in rats. Foods 2020, 9, 38. [Google Scholar] [CrossRef]
- Misawa, K.; Hashizume, K.; Yamamoto, M.; Minegishi, Y.; Hase, T.; Shimotoyodome, A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J. Nutr. Biochem. 2015, 26, 1058–1067. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger prevents obesity through regulation of energy metabolism and activation of browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of mitochondrial biogenesis via activation of AMPK-PGC1ɑ signaling pathway by ginger (Zingiber Officinale Roscoe) extract, and its major active component 6-gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-gingerol, a functional polyphenol of ginger, promotes browning through an AMPK-dependent pathway in 3T3-L1 adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef] [PubMed]
- Stanisiere, J.; Mousset, P.Y.; Lafay, S. How safe is ginger rhizome for decreasing nausea and vomiting in women during early pregnancy? Foods 2018, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Turmeric|Description, History,&Uses. Encycl. Br. 2019. Available online: https://www.britannica.com/plant/turmeric (accessed on 30 October 2019).
- Hay, E.; Lucariello, A.; Contieri, M.; Esposito, T.; De Luca, A.; Guerra, G.; Perna, A. Therapeutic effects of turmeric in several diseases: An overview. Chem. Biol. Interact. 2019, 310, 108729. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, D.; Yu, N.; An, T.; Miao, J.; Mo, F.; Gu, Y.; Zhang, D.; Gao, S.; Jiang, G. Curcumin improves glycolipid metabolism through regulating peroxisome proliferator activated receptor γ signalling pathway in high-fat diet-induced obese mice and 3T3-L1 adipocytes. R. Soc. Open Sci. 2017, 4, 170917. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, J.; Song, B.; Xiao, X.; Zhang, B.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating srebp pathway. Toxicol. Appl. Pharmacol. 2016, 304, 99–109. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Dal Ben, S.; Voinovich, D.; Dall’Acqua, S. Untargeted UPLC-MS metabolomics reveals multiple changes of urine composition in healthy adult volunteers after consumption of Curcuma Longa L. Extract. Food Res. Int. Ott. Ont 2020, 127, 108730. [Google Scholar] [CrossRef]
- Zingg, J.-M.; Hasan, S.T.; Nakagawa, K.; Canepa, E.; Ricciarelli, R.; Villacorta, L.; Azzi, A.; Meydani, M. Modulation of CAMP levels by high-fat diet and curcumin and regulatory effects on CD36/FAT scavenger receptor/fatty acids transporter gene expression. BioFactors Oxf. Engl. 2017, 43, 42–53. [Google Scholar] [CrossRef]
- Qiu, P.; Man, S.; Li, J.; Liu, J.; Zhang, L.; Yu, P.; Gao, W. Overdose intake of curcumin initiates the unbalanced state of bodies. J. Agric. Food Chem. 2016, 64, 2765–2771. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma Longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. PTR 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aceñero, M.J.; Ortega Medina, L.; Maroto, M. Herbal drugs: Friend or foe? J. Clin. Exp. Hepatol. 2019, 9, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Cinnamaldehyde. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/637511 (accessed on 30 October 2019).
- Michlig, S.; Merlini, J.M.; Beaumont, M.; Ledda, M.; Tavenard, A.; Mukherjee, R.; Camacho, S.; le Coutre, J. Effects of TRP channel agonist ingestion on metabolism and autonomic nervous system in a randomized clinical trial of healthy subjects. Sci. Rep. 2016, 6, 20795. [Google Scholar] [CrossRef] [PubMed]
- Hochkogler, C.M.; Hoi, J.K.; Lieder, B.; Müller, N.; Hans, J.; Widder, S.; Ley, J.P.; Somoza, V. Cinnamyl isobutyrate decreases plasma glucose levels and total energy intake from a standardized breakfast: A randomized, crossover intervention. Mol. Nutr. Food Res. 2018, 62, 1701038. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Anilakumar, K.R. Cold adaptive thermogenesis following consumption of certain pungent spice principles: A validation study. J. Therm. Biol. 2017, 64, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.G.O.; Boechat, S.K.; Romão, J.S.; Pazos-Moura, C.C.; Oliveira, K.J. Treatment with cinnamaldehyde reduces the visceral adiposity and regulates lipid metabolism, autophagy and endoplasmic reticulum stress in the liver of a rat model of early obesity. J. Nutr. Biochem. 2020, 77, 108321. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, H.; Tomar, V.; Sakharkar, M.K.; Dass, S.K.; Chandra, R. Cinnamon attenuates adiposity and affects the expression of metabolic genes in diet-induced obesity model of zebrafish. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2930–2939. [Google Scholar] [CrossRef]
- Pandit, C.; Latha, S.S.; Rani, T.U.; Anilakumar, K.R. Pepper and cinnamon improve cold induced cognitive impairment via increasing non-shivering thermogenesis; a study. Int. J. Hyperth. 2018, 35, 518–527. [Google Scholar] [CrossRef]
- Jiang, J.; Emont, M.P.; Jun, H.; Qiao, X.; Liao, J.; Kim, D.I.; Wu, J. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism 2017, 77, 58–64. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Wu, J.; Su, T.; Chao, X.-J.; Liu, B.; Fu, X.; Chan, C.L.; Lau, R.H.Y.; Tse, A.K.W.; Han, Q.B.; et al. Cinnamon induces browning in subcutaneous adipocytes. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Clapham, D.E.; Julius, D.; Montell, C.; Schultz, G. International union of pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 2005, 57, 427–450. [Google Scholar] [CrossRef] [PubMed]
- Woehrlin, F.; Fry, H.; Abraham, K.; Preiss-Weigert, A. Quantification of flavoring constituents in cinnamon: High variation of coumarin in cassia bark from the german retail market and in authentic samples from Indonesia. J. Agric. Food Chem. 2010, 58, 10568–10575. [Google Scholar] [CrossRef] [PubMed]
- Brancheau, D.; Patel, B.; Zughaib, M. Do cinnamon supplements cause acute hepatitis? Am. J. Case Rep. 2015, 16, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, J.A.; Casella, S.J.; MacKenzie, T.A.; Curtis, K.M. The effect of cinnamon on A1c among adolescents with type 1 diabetes. Diabetes Care 2007, 30, 813–816. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardena, R.; Pigera, S.; Wathurapatha, W.S.; Weeratunga, H.D.; Premakumara, G.A.S.; Katulanda, P.; Constantine, G.R.; Galappaththy, P. Evaluation of pharmacodynamic properties and safety of cinnamomum zeylanicum (Ceylon Cinnamon) in healthy adults: A phase i clinical trial. BMC Complement. Altern. Med. 2017, 17, 550. [Google Scholar] [CrossRef]
- Maji, A.K.; Banerji, P. Phytochemistry and gastrointestinal benefits of the medicinal spice, Capsicum annuum L. (chilli): A review. J. Complement. Integr. Med. 2016, 13, 97–122. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37, BSR20170286. [Google Scholar] [CrossRef]
- Varghese, S.; Kubatka, P.; Rodrigo, L.; Gazdikova, K.; Caprnda, M.; Fedotova, J.; Zulli, A.; Kruzliak, P.; Büsselberg, D. Chili pepper as a body weight-loss food. Int. J. Food Sci. Nutr. 2017, 68, 392–401. [Google Scholar] [CrossRef]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The Effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef]
- Zsiborás, C.; Mátics, R.; Hegyi, P.; Balaskó, M.; Pétervári, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1419–1427. [Google Scholar] [CrossRef]
- Janssens, P.L.H.R.; Hursel, R.; Martens, E.A.P.; Westerterp-Plantenga, M.S. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS ONE 2013, 8, e67786. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Saito, M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 625–631. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 21 April 2020).
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef]
- Fassina, P.; Scherer Adami, F.; Terezinha Zani, V.; Kasper Machado, I.C.; Garavaglia, J.; Quevedo Grave, M.T.; Ramos, R.; Morelo Dal Bosco, S. The effect of Garcinia cambogia as coadjuvant in the weight loss process. Nutr. Hosp. 2015, 32, 2400–2408. [Google Scholar] [CrossRef]
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia cambogia for weight loss. Am. J. Health. Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef]
- Kriketos, A.D.; Thompson, H.R.; Greene, H.; Hill, J.O. (-)-Hydroxycitric acid does not affect energy expenditure and substrate oxidation in adult males in a post-absorptive state. Int. J. Obes. 1999, 23, 867–873. [Google Scholar] [CrossRef]
- Vasques, C.A.R.; Rossetto, S.; Halmenschlager, G.; Linden, R.; Heckler, E.; Fernandez, M.S.P.; Alonso, J.L.L. Evaluation of the pharmacotherapeutic efficacy of Garcinia cambogia plus Amorphophallus konjac for the treatment of obesity. Phytother. Res. 2008, 22, 1135–1140. [Google Scholar] [CrossRef]
- Kovacs, E.M.; Westerterp-Plantenga, M.S.; Saris, W.H. The effects of 2-week ingestion of (--)-hydroxycitrate and (--)-hydroxycitrate combined with medium-chain triglycerides on satiety, fat oxidation, energy expenditure and body weight. Int. J. Obes. 2001, 25, 1087–1094. [Google Scholar] [CrossRef]
- Payab, M.; Hasani-Ranjbar, S.; Shahbal, N.; Qorbani, M.; Aletaha, A.; Haghi-Aminjan, H.; Soltani, A.; Khatami, F.; Nikfar, S.; Hassani, S.; et al. Effect of the herbal medicines in obesity and metabolic syndrome: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019, 97, e8825. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Yao, Y.; Yang, Z.; Ma, H. (-)-Hydroxycitric acid suppresses lipid droplet accumulation and accelerates energy metabolism via activation of the adiponectin-AMPK signaling pathway in broiler chickens. J. Agric. Food Chem. 2019, 67, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Li, L.; Peng, M.; Ma, H. (-)-Hydroxycitric acid nourishes protein synthesis via altering metabolic directions of amino acids in male rats. Phytother. Res. 2016, 30, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Shuster, J.; Leeuwenburgh, C. Investigations of botanicals on food intake, satiety, weight loss, and oxidative stress: A study protocol of a double-blind, placebo-controlled, crossover study. Zhong Xi Yi Jie He Xue Bao 2011, 9, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Corey, R.; Werner, K.T.; Singer, A.; Moss, A.; Smith, M.; Noelting, J.; Rakela, J. acute liver failure associated with Garcinia cambogia use. Ann. Hepatol. 2016, 15, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rosado, J.; Snipelisky, D.; Matcha, G.; Stancampiano, F. Acute hepatitis induced by pure Garcinia Cambogia. J. Clin. Gastroenterol. 2015, 49, 449–450. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Bodzin, A.S.; Reino, D.C.; Wang, H.L.; Busuttil, R.W. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J. Gastroenterol. 2016, 22, 10071–10076. [Google Scholar] [CrossRef]
- Allen, S.F.; Godley, R.W.; Evron, J.M.; Heider, A.; Nicklas, J.M.; Thomas, M.P. Acute necrotizing eosinophilic myocarditis in a patient taking Garcinia cambogia extract successfully treated with high-dose corticosteroids. Can. J. Cardiol. 2014, 30, e13–e15. [Google Scholar] [CrossRef]
- Lobb, A. Hepatoxicity associated with weight-loss supplements: A case for better post-marketing surveillance. World J. Gastroenterol. WJG 2009, 15, 1786–1787. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Panizzon, G.P.; Aguiar, B.A.A.; Simionato, A.S.; Cardozo-Filho, L.; Andrade, G.; de Oliveira, A.G.; Guedes, T.A.; Mello, J.C. Guaraná (Paullinia cupana) seeds: Selective supercritical extraction of phenolic compounds. Food Chem. 2016, 212, 703–711. [Google Scholar] [CrossRef]
- Smith, N.; Atroch, A.L. Guaraná’s journey from regional tonic to aphrodisiac and global energy drink. Evid. Based Complement. Altern. Med. ECAM 2010, 7, 279–282. [Google Scholar] [CrossRef]
- Bérubé-Parent, S.; Pelletier, C.; Doré, J.; Tremblay, A. Effects of encapsulated green tea and guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in Men. Br. J. Nutr. 2005, 94, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Boozer, C.N.; Nasser, J.A.; Heymsfield, S.B.; Wang, V.; Chen, G.; Solomon, J.L. An herbal supplement containing ma huang-guarana for weight loss: A randomized, double-blind trial. Int. J. Obes. 2001, 25, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, R.C.; Vargas, A.R.; de Miranda Ramos, V.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; da Boit Martinello, K.; Silveira, A.K.; Gomes, H.M.; Rabelo, T.K.; et al. Guarana supplementation attenuated obesity, insulin resistance, and adipokines dysregulation induced by a standardized human western diet via brown adipose tissue activation. Phytother. Res. PTR 2019, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.D.S.; Numata, E.D.P.; Mesquita, L.M.S.; Dias, P.H.; Vilegas, W.; Gambero, A.; Ribeiro, M.L. Modulatory effects of guarana (Paullinia cupana) on adipogenesis. Nutrients 2017, 9, 635. [Google Scholar] [CrossRef]
- Ciszowski, K.; Biedroń, W.; Gomólka, E. Acute caffeine poisoning resulting in atrial fibrillation after guarana extract overdose. Przegl. Lek. 2014, 71, 495–498. [Google Scholar]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate Metabolism, Functionality and Breeding for the Improvement of Brassicaceae Vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Martins, T.; Colaço, B.; Venâncio, C.; Pires, M.J.; Oliveira, P.A.; Rosa, E.; Antunes, L.M. Potential effects of sulforaphane to fight obesity. J. Sci. Food Agric. 2018, 98, 2837–2844. [Google Scholar] [CrossRef]
- Glade, M.J.; Meguid, M.M. A Glance at… Broccoli, glucoraphanin, and sulforaphane. Nutrition 2015, 31, 1175–1178. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Yao, A.; Shen, Y.; Wang, A.; Chen, S.; Zhang, H.; Chen, F.; Chen, Z.; Wei, H.; Zou, Z.; Shan, Y.; et al. Sulforaphane induces apoptosis in adipocytes via Akt/P70s6k1/Bad inhibition and ERK activation. Biochem. Biophys. Res. Commun. 2015, 465, 696–701. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, M.H.; Jeong, J.K.; Park, Y.G.; Lee, Y.J.; Seol, J.W.; Park, S.Y. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2012, 426, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane improves lipid metabolism by enhancing mitochondrial function and biogenesis in vivo and in vitro. Mol. Nutr. Food Res. 2019, 63, 1800795. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Ota, T. Glucoraphanin: A broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte 2018, 7, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef]
- Xu, J.; Kulkarni, S.R.; Donepudi, A.C.; More, V.R.; Slitt, A.L. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 2012, 61, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Rojas-Rueda, D.; Basora, J.; Ros, E.; Salas-Salvadó, J. Nut intake and adiposity: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2013, 97, 1346–1355. [Google Scholar] [CrossRef]
- Agebratt, C.; Ström, E.; Romu, T.; Dahlqvist-Leinhard, O.; Borga, M.; Leandersson, P.; Nystrom, F.H. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PLoS ONE 2016, 11, e0147149. [Google Scholar] [CrossRef]
- Duarte Moreira Alves, R.; Boroni Moreira, A.P.; Silva Macedo, V.; Brunoro Costa, N.M.; Gonçalves Alfenas, R.d.C.; Bressan, J. High-oleic peanuts increase diet-induced thermogenesis in overweight and obese men. Nutr. Hosp. 2014, 29, 1024–1032. [Google Scholar] [CrossRef]
- Gheflati, A.; Bashiri, R.; Ghadiri-Anari, A.; Reza, J.Z.; Kord, M.T.; Nadjarzadeh, A. The effect of apple vinegar consumption on glycemic indices, blood pressure, oxidative stress, and homocysteine in patients with type 2 diabetes and dyslipidemia: A randomized controlled clinical trial. Clin. Nutr. ESPEN 2019, 33, 132–138. [Google Scholar] [CrossRef]
- Siddiqui, F.J.; Assam, P.N.; de Souza, N.N.; Sultana, R.; Dalan, R.; Chan, E.S. Diabetes control: Is vinegar a promising candidate to help achieve targets? J. Evid.-Based Integr. Med. 2018, 23, 2156587217753004. [Google Scholar] [CrossRef] [PubMed]
- Bouderbala, H.; Kaddouri, H.; Kheroua, O.; Saidi, D. Anti-obesogenic effect of apple cider vinegar in rats subjected to a high fat diet. Ann. Cardiol. Angeiol. 2016, 65, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.K.S.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Nachvak, S.M.; Rouhani, M.H. Effects of spirulina supplementation on obesity: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two classes of pigments, carotenoids and c-phycocyanin, in Spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Hamedifard, Z.; Milajerdi, A.; Reiner, Ž.; Taghizadeh, M.; Kolahdooz, F.; Asemi, Z. The effects of Spirulina on glycemic control and serum lipoproteins in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 2609–2621. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity energetics: Body weight regulation and the effects of diet composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef]
- Martens, E.A.; Gonnissen, H.K.; Gatta-Cherifi, B.; Janssens, P.L.; Westerterp-Plantenga, M.S. Maintenance of energy expenditure on high-protein vs. high-carbohydrate diets at a constant body weight may prevent a positive energy balance. Clin. Nutr. 2015, 34, 968–975. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: Randomized trial. BMJ 2018, 363, k4583. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Foster-Schubert, K.E.; Overduin, J. Ghrelin and energy balance: Focus on current controversies. Curr. Drug Targets 2005, 6, 153–169. [Google Scholar] [CrossRef]
- Mihalache, L.; Gherasim, A.; Niţă, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of ghrelin in energy balance and body weight homeostasis. Hormones (Athens) 2016, 15, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. A review of the carbohydrate-insulin model of obesity. Eur. J. Clin. Nutr. 2017, 71, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Friedman, M.I. Increasing adiposity: Consequence or cause of overeating? JAMA 2014, 311, 2167–2168. [Google Scholar] [CrossRef]
- Taubes, G. The Science of obesity: What do we really know about what makes us fat? An essay by Gary Taubes. BMJ 2013, 346, f1050. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J.; Speakman, J.R. Do Low-carbohydrate diets increase energy expenditure? Int. J. Obes. 2019, 43, 2350–2354. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef]
- Duca, F.A.; Lam, T.K.T. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes. Metab. 2014, 16, 68–76. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Bohan, R.; Tianyu, X.; Tiantian, Z.; Ruonan, F.; Hongtao, H.; Qiong, W.; Chao, S. Gut microbiota: A potential manipulator for host adipose tissue and energy metabolism. J. Nutr. Biochem. 2019, 64, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Nguyen, P.; Ballèvre, O.; Krempf, M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc. Nutr. Soc. 2003, 62, 87–93. [Google Scholar] [CrossRef]
- Kocełak, P.; Zak-Gołąb, A.; Zahorska-Markiewicz, B.; Aptekorz, M.; Zientara, M.; Martirosian, G.; Chudek, J.; Olszanecka-Glinianowicz, M. Resting energy expenditure and gut microbiota in obese and normal weight subjects. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2816–2821. [Google Scholar]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Hanatani, S.; Motoshima, H.; Takaki, Y.; Kawasaki, S.; Igata, M.; Matsumura, T.; Kondo, T.; Senokuchi, T.; Ishii, N.; Kawashima, J.; et al. Acetate Alters expression of genes involved in beige adipogenesis in 3T3-L1 cells and obese KK-Ay mice. J. Clin. Biochem. Nutr. 2016, 59, 207–214. [Google Scholar] [CrossRef]
- Okla, M.; Wang, W.; Kang, I.; Pashaj, A.; Carr, T.; Chung, S. Activation of Toll-like Receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. J. Biol. Chem. 2015, 290, 26476–26490. [Google Scholar] [CrossRef]
- Cao, W.; Huang, H.; Xia, T.; Liu, C.; Muhammad, S.; Sun, C. Homeobox A5 promotes white adipose tissue browning through inhibition of the Tenascin C/Toll-Like Receptor 4/Nuclear Factor Kappa B inflammatory signaling in mice. Front. Immunol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Roager, H.M.; Larsen, T.M.; Poulsen, S.K.; Licht, T.R.; Bahl, M.I.; Zohar, Y.; Astrup, A. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 2005 2018, 42, 580–583. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; de Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Kjølbæk, L.; Pulgar, E.M.G.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schött, H.F.; Krautbauer, S.; Liebisch, G.; Boberska, J.; et al. A multi-omics approach to unraveling the microbiome-mediated effects of arabinoxylan oligosaccharides in overweight humans. mSystems 2019, 4, e00209–e00219. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, S.; Fadda, M.; Fedele, D.; Pellegrini, M.; Ghigo, E.; Pellegrini, N. A Critical Review on the Role of Food and Nutrition in the Energy Balance. Nutrients 2020, 12, 1161. https://doi.org/10.3390/nu12041161
Bo S, Fadda M, Fedele D, Pellegrini M, Ghigo E, Pellegrini N. A Critical Review on the Role of Food and Nutrition in the Energy Balance. Nutrients. 2020; 12(4):1161. https://doi.org/10.3390/nu12041161
Chicago/Turabian StyleBo, Simona, Maurizio Fadda, Debora Fedele, Marianna Pellegrini, Ezio Ghigo, and Nicoletta Pellegrini. 2020. "A Critical Review on the Role of Food and Nutrition in the Energy Balance" Nutrients 12, no. 4: 1161. https://doi.org/10.3390/nu12041161
APA StyleBo, S., Fadda, M., Fedele, D., Pellegrini, M., Ghigo, E., & Pellegrini, N. (2020). A Critical Review on the Role of Food and Nutrition in the Energy Balance. Nutrients, 12(4), 1161. https://doi.org/10.3390/nu12041161






