The Combination Effect of Aspalathin and Phenylpyruvic Acid-2-O-β-d-glucoside from Rooibos against Hyperglycemia-Induced Cardiac Damage: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatment Conditions
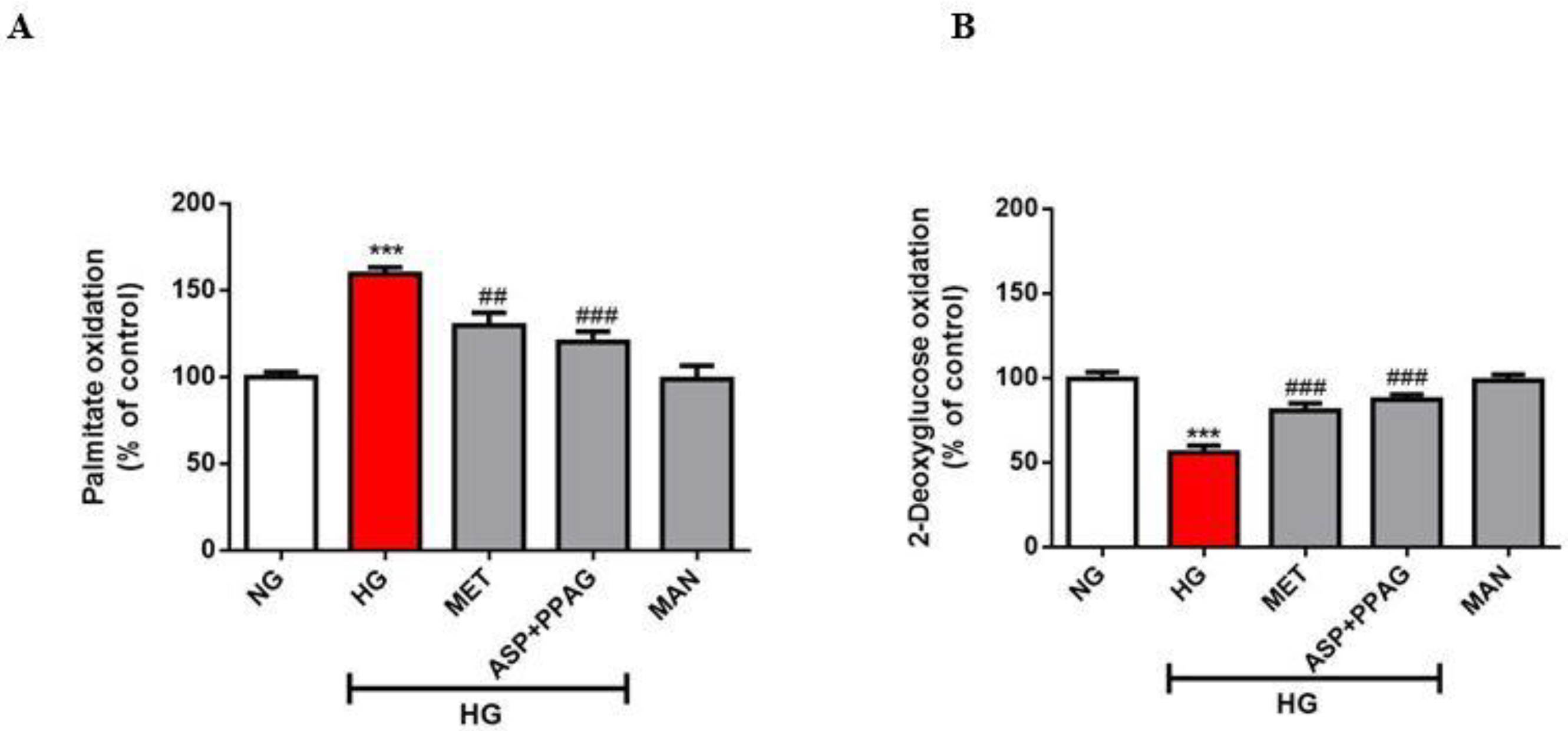
2.3. FFA and Glucose Oxidation Assays
2.4. Determination of Mitochondrial Membrane Potential (ΔΨm)
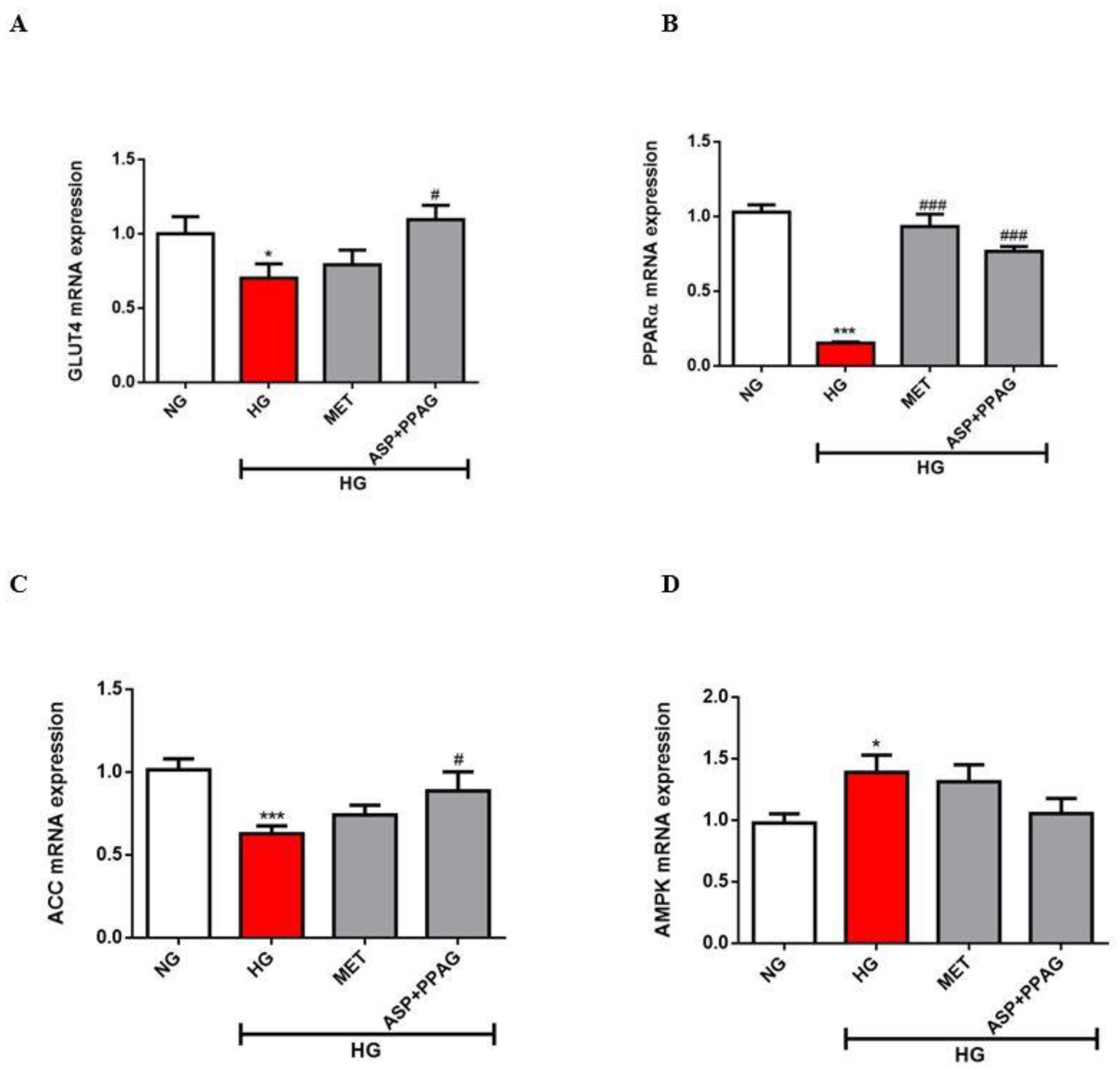
2.5. mRNA Expression Analysis
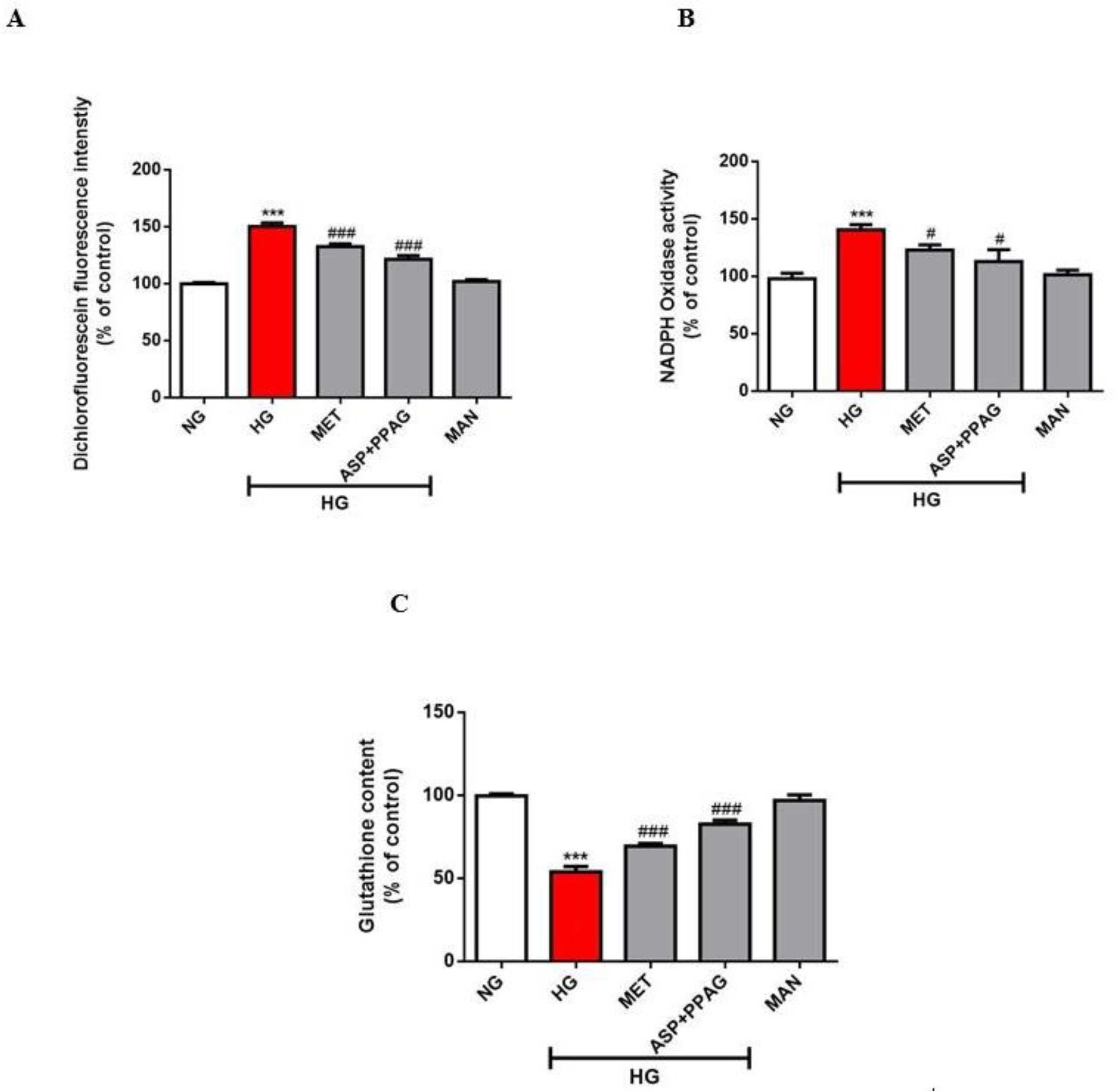
2.6. Oxidative Stress Assessment
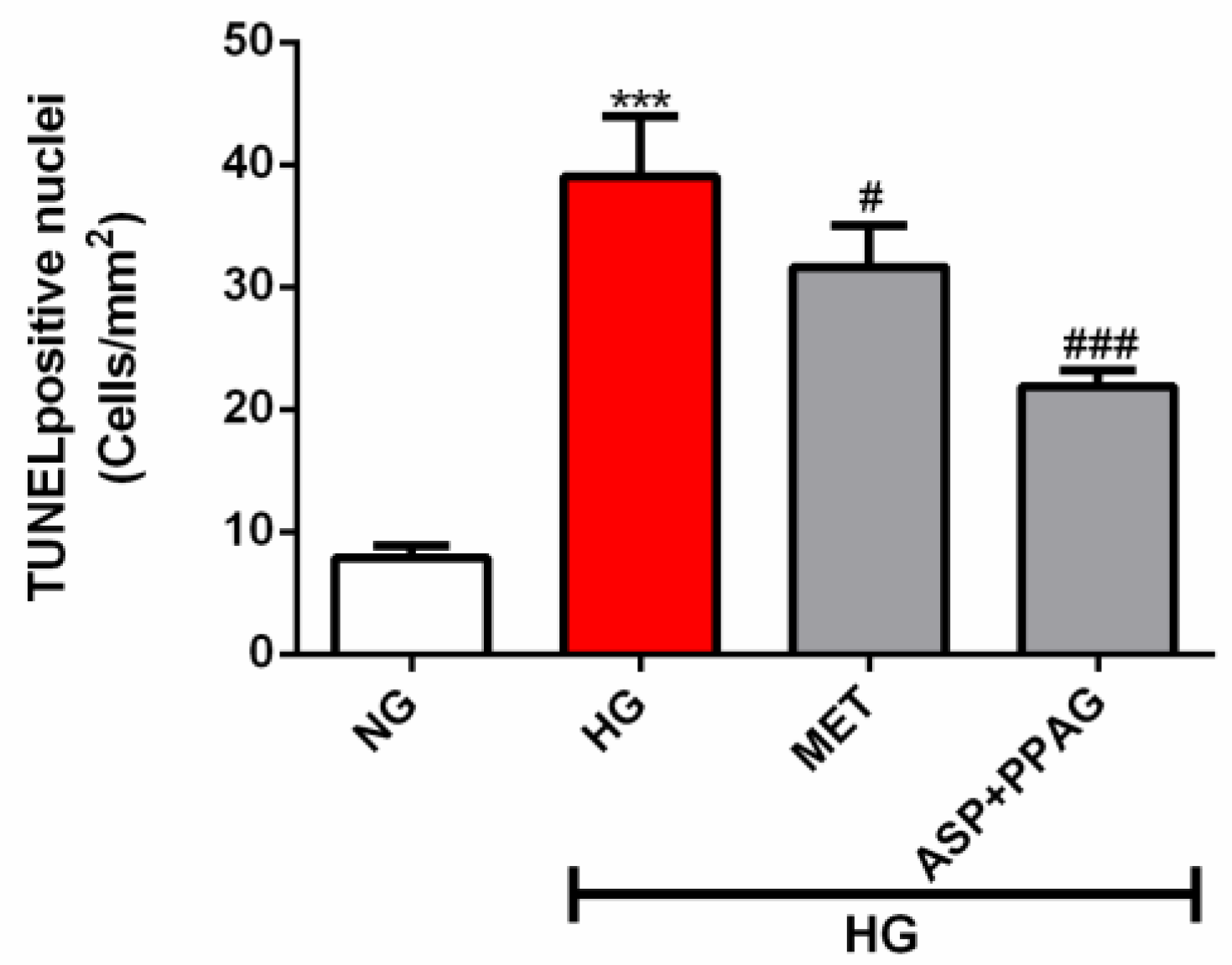
2.7. DNA Damage Assessment
2.8. Statistical Analysis
3. Results
3.1. The Combination Effect of Aspalathin and PPAG on Myocardial Substrate Metabolism
3.2. The Combination Effect of Aspalathin and PPAG on Altered Mitochondrial Membrane Potential
3.3. The Combination Effect of Aspalathin and PPAG on the Modulation of Genes Involved in Energy Metabolism
3.4. The Combination Effect of Aspalathin and PPAG on Oxidative Stress Markers
3.5. The Combination Effect of Aspalathin and PPAG on Ameliorating DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 February 2020).
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. Type 2 diabetes mellitus and heart failure: A scientific statement from the American Heart Association and the Heart Failure Society of America. Circulation 2019, 140, e294–e324. [Google Scholar] [CrossRef] [PubMed]
- Belke, D.D.; Larsen, T.S.; Gibbs, E.M.; Severson, D.L. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1104–E1113. [Google Scholar] [CrossRef]
- Lionetti, V.; Stanley, W.C.; Recchia, F.A. Modulating fatty acid oxidation in heart failure. Cardiovasc. Res. 2011, 90, 202–209. [Google Scholar] [CrossRef]
- Boudina, S.; Sena, S.; Theobald, H.; Sheng, X.; Wright, J.J.; Hu, X.X.; Aziz, S.; Johnson, J.I.; Bugger, H.; Zaha, V.G.; et al. Mitochondrial energetics in the heart in obesity-related diabetes: Direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 2007, 56, 2457–2466. [Google Scholar] [CrossRef]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef]
- Lawal, A.O.; Davids, L.M.; Marnewick, J.L. Rooibos (Aspalathus linearis) and honeybush (Cyclopia species) modulate the oxidative stress associated injury of diesel exhaust particles in human umbilical vein endothelial cells. Phytomedicine 2019, 59, 152898. [Google Scholar] [CrossRef]
- Ulicna, O.; Vancova, O.; Bozek, P.; Carsky, J.; Sebekova, K.; Boor, P.; Nakano, M.; Greksák, M. Rooibos tea (Aspalathus linearis) partially prevents oxidative stress in streptozotocin-induced diabetic rats. Physiol. Res. 2006, 55, 157–164. [Google Scholar]
- Muller, C.J.F.; Malherbe, C.J.; Chellan, N.; Yagasaki, K.; Miura, Y.; Joubert, E. Potential of rooibos, its major C-glucosyl flavonoids, and Z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2018, 58, 227–246. [Google Scholar] [CrossRef]
- Sasaki, M.; Nishida, N.; Shimada, M. A beneficial role of rooibos in diabetes mellitus: A systematic review and meta-analysis. Molecules 2018, 23, 839. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, O.R.; Ayeleso, A.O.; Adefolaju, G.A. The potential of South African herbal tisanes, rooibos and honeybush in the management of type 2 diabetes mellitus. Molecules 2018, 23, 3207. [Google Scholar] [CrossRef]
- Smith, C.; Swart, A. Aspalathus linearis (Rooibos)—A functional food targeting cardiovascular disease. Food Funct. 2018, 9, 5041–5058. [Google Scholar] [CrossRef] [PubMed]
- Webster, I.; Imperial, E.G.; Westcott, C.; Strijdom, H. The cardiovascular effects of Aspalathus linearis supplementation in male Wistar rats receiving fixed-dose combination first-line antiretroviral therapy. Cardiovasc. J. Afr. 2019, 30, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-d-glucoside. Nutr. Metab. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Joubert, E.; Louw, J.; Essop, M.F.; Gabuza, K.B.; Ghoor, S.; Huisamen, B.; Johnson, R. Aspalathin protects the heart against hyperglycemia-induced oxidative damage by up-regulating Nrf2 expression. Molecules 2017, 22, 129. [Google Scholar] [CrossRef]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016, 60, 922–934. [Google Scholar] [CrossRef]
- Dludla, P.V.; Muller, C.J.; Joubert, E.; Louw, J.; Gabuza, K.B.; Huisamen, B.; Essop, M.F.; Johnson, R. Phenylpyruvic Acid-2-O-β-d-glucoside attenuates high glucose-induced apoptosis in H9c2 cardiomyocytes. Planta Med. 2016, 82, 1468–1474. [Google Scholar] [CrossRef]
- Himpe, E.; Cunha, D.A.; Song, I.; Bugliani, M.; Marchetti, P.; Cnop, M.; Bouwens, L. Phenylpropenoic acid glucoside from rooibos protects pancreatic beta cells against cell death induced by acute injury. PLoS ONE 2016, 11, e0157604. [Google Scholar] [CrossRef]
- Mathijs, I.; Da Cunha, D.A.; Himpe, E.; Ladriere, L.; Chellan, N.; Roux, C.R.; Joubert, E.; Muller, C.; Cnop, M.; Louw, J.; et al. Phenylpropenoic acid glucoside augments pancreatic beta cell mass in high-fat diet-fed mice and protects beta cells from ER stress-induced apoptosis. Mol. Nutr. Food Res. 2014, 58, 1980–1990. [Google Scholar] [CrossRef]
- Dludla, P.V.; Muller, C.J.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomedicine 2014, 21, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Johnson, R.; Mazibuko-Mbeje, S.E.; Muller, C.J.; Louw, J.; Joubert, E.; Orlando, P.; Silvestri, S.; Chellan, C.; Nkambule, B.B.; et al. Fermented rooibos extract attenuates hyperglycemia-induced myocardial oxidative damage by improving mitochondrial energetics and intracellular antioxidant capacity. S. Afr. J. Bot. 2020, 131, 143–150. [Google Scholar] [CrossRef]
- Nugent, C.; Prins, J.B.; Whitehead, J.P.; Wentworth, J.M.; Chatterjee, V.K.; O’Rahilly, S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2001, 276, 9149–9157. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, R.C.; Yang, Z.H.; Sun, G.B.; Wang, M.; Ma, X.J.; Yang, L.J.; Sun, X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [CrossRef]
- Abid, M.R.; Spokes, K.C.; Shih, S.C.; Aird, W.C. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 2007, 282, 35373–35385. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Kimar, C.; Symington, B.; Milne, R.; Essop, M.F. The detrimental effects of acute hyperglycemia on myocardial glucose uptake. Life Sci. 2014, 105, 31–42. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Muller, C.J.F.; Nxele, X.; Kappo, A.P.; Louw, J.; Johnson, R. Aspalathin ameliorates doxorubicin-induced oxidative stress in H9c2 cardiomyoblasts. Toxicol. In Vitro. 2019, 55, 134–139. [Google Scholar] [CrossRef]
- Ng, K.W.; Allen, M.L.; Desai, A.; Macrae, D.; Pathan, N. Cardioprotective effects of insulin: How intensive insulin therapy may benefit cardiac surgery patients. Circulation 2012, 125, 721–728. [Google Scholar] [CrossRef]
- El Messaoudi, S.; Rongen, G.A.; de Boer, R.A.; Riksen, N.P. The cardioprotective effects of metformin. Curr. Opin. Lipidol. 2011, 22, 445–453. [Google Scholar] [CrossRef]
- Mizuno, Y.; Harada, E.; Nakagawa, H.; Morikawa, Y.; Shono, M.; Kugimiya, F.; Yoshimura, M.; Yasue, H. The diabetic heart utilizes ketone bodies as an energy source. Metabolism 2017, 77, 65–72. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Davargaon, R.S.; Sambe, A.D.; Muthangi, V.V.S. Toxic effect of high glucose on cardiomyocytes, H9c2 cells: Induction of oxidative stress and ameliorative effect of trolox. J. Biochem. Mol. Toxicol. 2019, 33, e22272. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic cardiomyopathy: Current and future therapies. Beyond glycemic control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef]
- Hong, I.S.; Lee, H.Y.; Kim, H.P. Anti-oxidative effects of Rooibos tea (Aspalathus linearis) on immobilization-induced oxidative stress in rat brain. PLoS ONE 2014, 9, e87061. [Google Scholar] [CrossRef]
- Orlando, P.; Chellan, N.; Louw, J.; Tiano, L.; Cirilli, I.; Dludla, P.; Joubert, E.; Muller, C.J.F. Aspalathin-rich green rooibos extract lowers LDL-cholesterol and oxidative status in high-fat diet-induced diabetic Vervet monkeys. Molecules 2019, 24, 1713. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef]
- Lee, T.W.; Bai, K.J.; Lee, T.I.; Chao, T.F.; Kao, Y.H.; Chen, Y.J. PPARs modulate cardiac metabolism and mitochondrial function in diabetes. J. Biomed. Sci. 2017, 24, 5. [Google Scholar] [CrossRef]
- Pantsi, W.G.; Marnewick, J.L.; Esterhuyse, A.J.; Rautenbach, F.; van Rooyen, J. Rooibos (5) offers cardiac protection against ischaemia/reperfusion in the isolated perfused rat heart. Phytomedicine 2011, 18, 1220–1228. [Google Scholar] [CrossRef]
- Johnson, R.; Dludla, P.V.; Muller, C.J.; Huisamen, B.; Essop, M.F.; Louw, J. The transcription profile unveils the cardioprotective effect of aspalathin against lipid toxicity in an in vitro H9c2 model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef]
- Dludla, P.V.; Gabuza, K.B.; Muller, C.J.F.; Joubert, E.; Louw, J.; Johnson, R. Aspalathin, a C-glucosyl dihydrochalcone from rooibos improves the hypoglycemic potential of metformin in type 2 diabetic (db/db) mice. Physiol. Res. 2018, 14, 813–818. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dludla, P.V.; Muller, C.J.F.; Louw, J.; Mazibuko-Mbeje, S.E.; Tiano, L.; Silvestri, S.; Orlando, P.; Marcheggiani, F.; Cirilli, I.; Chellan, N.; et al. The Combination Effect of Aspalathin and Phenylpyruvic Acid-2-O-β-d-glucoside from Rooibos against Hyperglycemia-Induced Cardiac Damage: An In Vitro Study. Nutrients 2020, 12, 1151. https://doi.org/10.3390/nu12041151
Dludla PV, Muller CJF, Louw J, Mazibuko-Mbeje SE, Tiano L, Silvestri S, Orlando P, Marcheggiani F, Cirilli I, Chellan N, et al. The Combination Effect of Aspalathin and Phenylpyruvic Acid-2-O-β-d-glucoside from Rooibos against Hyperglycemia-Induced Cardiac Damage: An In Vitro Study. Nutrients. 2020; 12(4):1151. https://doi.org/10.3390/nu12041151
Chicago/Turabian StyleDludla, Phiwayinkosi V., Christo J. F. Muller, Johan Louw, Sithandiwe E. Mazibuko-Mbeje, Luca Tiano, Sonia Silvestri, Patrick Orlando, Fabio Marcheggiani, Ilenia Cirilli, Nireshni Chellan, and et al. 2020. "The Combination Effect of Aspalathin and Phenylpyruvic Acid-2-O-β-d-glucoside from Rooibos against Hyperglycemia-Induced Cardiac Damage: An In Vitro Study" Nutrients 12, no. 4: 1151. https://doi.org/10.3390/nu12041151
APA StyleDludla, P. V., Muller, C. J. F., Louw, J., Mazibuko-Mbeje, S. E., Tiano, L., Silvestri, S., Orlando, P., Marcheggiani, F., Cirilli, I., Chellan, N., Ghoor, S., Nkambule, B. B., Essop, M. F., Huisamen, B., & Johnson, R. (2020). The Combination Effect of Aspalathin and Phenylpyruvic Acid-2-O-β-d-glucoside from Rooibos against Hyperglycemia-Induced Cardiac Damage: An In Vitro Study. Nutrients, 12(4), 1151. https://doi.org/10.3390/nu12041151







