Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader–Willi Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Physiologic and Metabolic Variables
2.3. Dietary Analysis
2.4. Gut Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Dietary Analysis of Children and Adolescents with Prader–Willi Syndrome
3.3. Associations between Dietary Variables and the Degree of Obesity
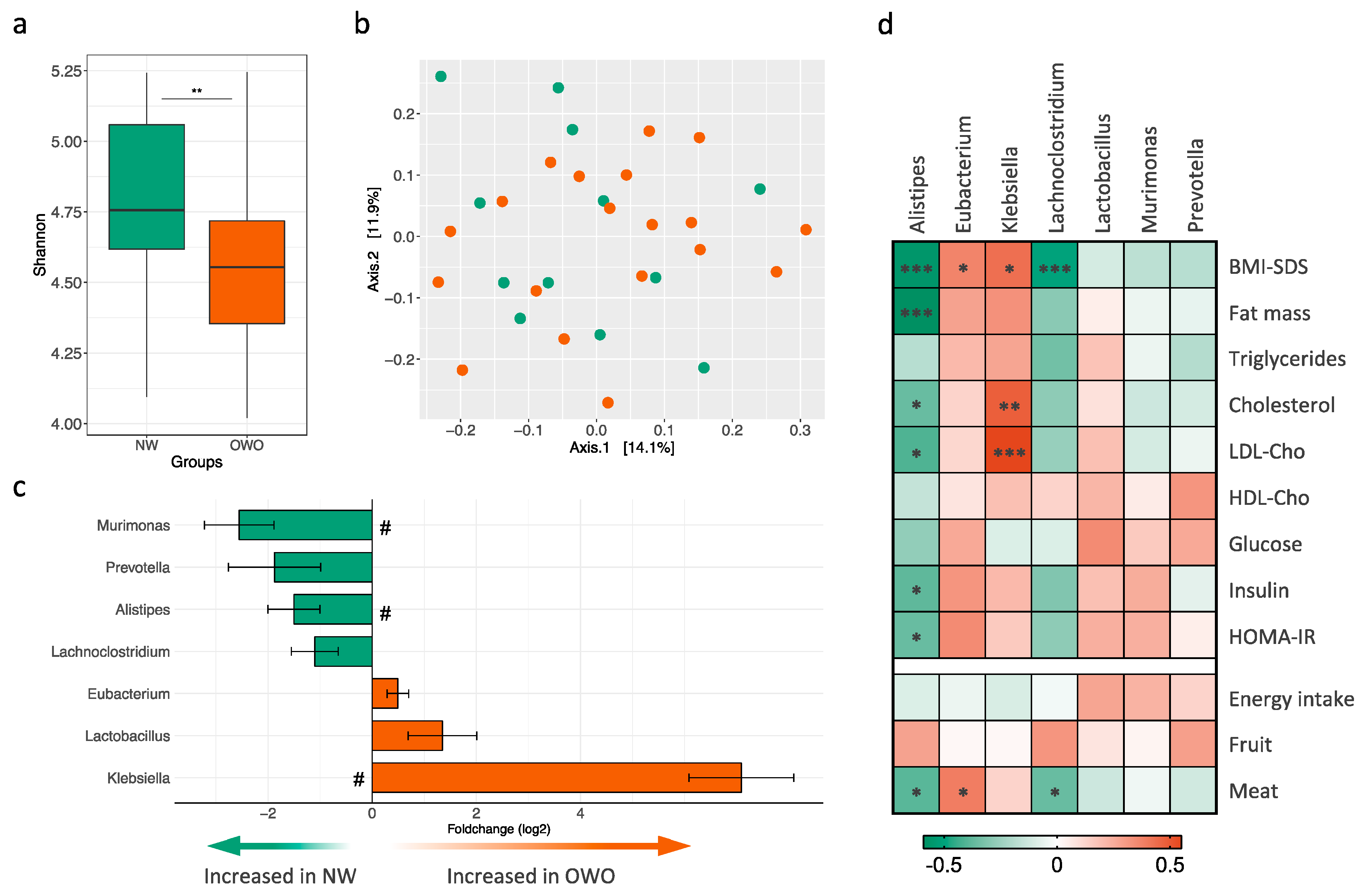
3.4. Gut Microbiota Composition in Children and Adolescents with Prader–Willi Syndrome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Lynn, C.H.; Driscoll, D.C.; Goldstone, A.P.; Gold, J.-A.; Kimonis, V.; Dykens, E.; Butler, M.G.; Shuster, J.J.; Driscoll, D.J. Nutritional phases in Prader–Willi syndrome. Am. J. Med. Genet. Part A 2011, 155, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Skokauskas, N.; Sweeny, E.; Meehan, J.; Gallagher, L. Mental health problems in children with prader-willi syndrome. J. Can. Acad. Child Adolesc. Psychiatry 2012, 21, 194–203. [Google Scholar] [CrossRef]
- Alsaif, M.; Elliot, S.A.; MacKenzie, M.L.; Prado, C.M.; Field, C.J.; Haqq, A.M. Energy Metabolism Profile in Individuals with Prader-Willi Syndrome and Implications for Clinical Management: A Systematic Review. Adv. Nutr. 2017, 8, 905–915. [Google Scholar] [CrossRef]
- Hedgeman, E.; Ulrichsen, S.P.; Carter, S.; Kreher, N.C.; Malobisky, K.P.; Braun, M.M.; Fryzek, J.; Olsen, M.S. Long-term health outcomes in patients with Prader-Willi syndrome. A nationwide cohort study in Denmark. Int. J. Obes. 2017, 41, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Bonfig, W.; Dokoupil, K.; Schmidt, H. A special, strict, fat-reduced, and carbohydrate-modified diet leads to marked weight reduction even in overweight adolescents with Prader-Willi syndrome (PWS). Sci. World J. 2009, 9, 934–939. [Google Scholar] [CrossRef]
- Miller, J.L.; Lynn, C.H.; Shuster, J.; Driscoll, D.J. A reduced-energy intake, well-balanced diet improves weight control in children with Prader-Willi syndrome. J. Hum. Nutr. Diet. 2013, 26, 2–9. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1131. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.M.; Poitou, C.; Tremaroli, V.; Coupaye, M.; Aron-Wisnewsky, J.; Backhed, F.; Clement, K.; Caesar, R. Gut microbiota of obese subjects with Prader-Willi syndrome is linked to metabolic health. Gut 2019. [Google Scholar] [CrossRef] [PubMed]
- Blössner, M.; Siyam, A.; Borghi, E.; Onyango, A.; de Onis, M. WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents; WHO: Geneva, Switzerland, 2009; Available online: http://www.who.int/growthref/tools/en/ (accessed on 10 April 2020).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Andrés, P.; Requejo, A.M.; Aparicio, A.; Molinero, L.M. DIAL Software for Assessing Diets and Food Calculations (for Windows, version 3.5.0.3). Department of Nutrition (UCM) & Alce Ingeniería, S.L.; Madrid, Spain. Available online: http://www.alceingenieria.net/nutricion/descarga.htm (accessed on 19 February 2020).
- Yuan, S.; Cohen, D.B.; Ravel, J.; Abdo, Z.; Forney, L.J. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 2012, 7, e33865. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef]
- EFSA. Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef]
- Schmidt, H.; Pozza, S.B.; Bonfig, W.; Schwarz, H.P.; Dokoupil, K. Successful early dietary intervention avoids obesity in patients with Prader-Willi syndrome: A ten-year follow-up. J. Pediatr. Endocrinol. Metab. 2008, 21, 651–655. [Google Scholar] [CrossRef]
- Mackenzie, M.L.; Triador, L.; Gill, J.K.; Pakseresht, M.; Mager, D.; Field, C.J.; Haqq, A.M. Dietary intake in youth with prader-willi syndrome. Am. J. Med. Genet. A 2018, 176, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.A.; Nowak, J.; McLaren, E.; Patino, M.; Castner, D.M.; Dumont-Driscoll, M.C. Nutritional intakes in children with Prader-Willi syndrome and non-congenital obesity. Food Nutr. Res. 2015, 59, 29427. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; Jenkins, A.; Smircic-Duvnjak, L.; Sievenpiper, J.; et al. Can dietary viscous fiber affect body weight independently of an energy-restrictive diet? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Chu, A.Y.; Kang, J.H.; Jensen, M.K.; Curhan, G.C.; Pasquale, L.R.; Ridker, P.M.; Hunter, D.J.; Willett, W.C.; Rimm, E.B.; et al. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 2012, 367, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Dietary fat plays a major role in obesity: No. Obes. Rev. 2002, 3, 59–68. [Google Scholar] [CrossRef]
- Willett, W.C. Is dietary fat a major determinant of body fat? Am. J. Clin. Nutr. 1998, 67, 556s–562s. [Google Scholar] [CrossRef]
- Field, A.E.; Willett, W.C.; Lissner, L.; Colditz, G.A. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity 2007, 15, 967–976. [Google Scholar] [CrossRef]
- Rouhani, M.H.; Salehi-Abargouei, A.; Surkan, P.J.; Azadbakht, L. Is there a relationship between red or processed meat intake and obesity? A systematic review and meta-analysis of observational studies. Obes. Rev. 2014, 15, 740–748. [Google Scholar] [CrossRef]
- Wang, Y.; Beydoun, M.A. Meat consumption is associated with obesity and central obesity among US adults. Int. J. Obes. 2009, 33, 621–628. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, L.B.; Ruhlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hubenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Bussolo de Souza, C.; Venema, K. The Gut Microbiota from Lean and Obese Subjects Contribute Differently to the Fermentation of Arabinogalactan and Inulin. PLoS ONE 2016, 11, e0159236. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Qin, X.; Jia, H.; Chen, S.; Sun, W.; Wang, X. The Association between Gut Microbiota Composition and Body Mass Index in Chinese Male College Students, as Analyzed by Next-generation Sequencing. Br. J. Nutr. 2019, 122, 986–995. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
| Variables | All Subjects (N = 31) | NW (n = 12) | OWO (n = 19) | p-Value a |
|---|---|---|---|---|
| Gender (Females) | 19 (61%) | 5 (42%) | 14 (74%) | 0.075 |
| Age (years) | 12.0 (4.0) | 11.4 (3.9) | 12.4 (4.2) | 0.498 |
| Pre-pubertal status | 12 (39%) | 6 (50%) | 6 (32%) | 0.306 |
| Genotype (Deletions) | 18 (58%) | 5 (42%) | 13 (68%) | 0.243 |
| Hyperphagia (HQ-CT Score) | 6.8 (6.0) | 7.3 (7.4) | 6.6 (5.1) | 0.787 |
| Physical activity (>2 h/week) | 17 (55%) | 8 (67%) | 9 (47%) | 0.290 |
| Growth hormone therapy | 30 (97%) | 12 (100%) | 18 (95%) | 0.317 |
| Metformin therapy | 8 (26%) | 1 (8%) | 7 (37%) | 0.061 |
| BMI-SDS | 1.51 (1.38) | 0.22 (0.55) | 2.32 (1.09) | <0.001 |
| Body fat mass (%) | 43.2 (8.4) | 35.9 (3.9) | 47.9 (7.0) | <0.001 |
| Lipid profile | ||||
| Triglycerides (mg/dL) | 70 (25) | 64 (26) | 74 (25) | 0.294 |
| Cholesterol (mg/dL) | 170 (36) | 155 (34) | 179 (35) | 0.069 |
| LDL-cholesterol (mg/dL) | 102 (31) | 88 (30) | 112 (28) | 0.036 |
| HDL-cholesterol (mg/dL) | 56 (13) | 55 (13) | 58 (14) | 0.529 |
| Glucose metabolism | ||||
| Glucose (mg/dL) | 87 (9) | 85 (10) | 88 (9) | 0.352 |
| HbA1c (%) | 5.3 (0.2) | 5.2 (0.3) | 5.3 (0.2) | 0.692 |
| Insulin (mU/L) | 12.6 (9.2) | 8.0 (7.2) | 15.6 (9.3) | 0.017 |
| HOMA-IR | 2.82 (2.18) | 1.76 (1.62) | 3.49 (2.26) | 0.020 |
| Dietary Intake | All Subjects N = 31 | NW n = 12 | OWO n = 19 | p-Value |
|---|---|---|---|---|
| Energy Intake (kcal/day) | 1571 (349) | 1525 (308) | 1600 (378) | 0.552 |
| (% RCI) | 80 (22) | 84 (26) | 78 (20) | 0.493 |
| Protein (% kcal) | 17.7 (2.7) | 17.3 (2.7) | 18.0 (2.9) | 0.530 |
| Fat (% kcal) | 32.7 (6.0) | 31.1 (6.1) | 33.7 (5.9) | 0.256 |
| SFA (% kcal) | 9.4 (2.6) | 8.6 (2.7) | 9.9 (2.4) | 0.170 |
| MUFA (% kcal) | 14.9 (2.7) | 14.1 (2.8) | 15.4 (2.6) | 0.198 |
| PUFA (% kcal) | 5.1 (1.3) | 5.2 (1.2) | 5.0 (1.3) | 0.727 |
| Carbohydrate (% kcal) | 46.6 (5.6) | 48.2 (4.6) | 45.5 (6.0) | 0.176 |
| Fiber (g/day) | 23.8 (12.0) | 24.9 (10.0) | 23.1 (13.4) | 0.681 |
| Food Groups | All Subjects N = 31 | NW n = 12 | OWO n = 19 | p-Value |
|---|---|---|---|---|
| Grains | 27.6 (7.0) | 25.6 (8.2) | 29.0 (6.1) | 0.235 |
| Legumes | 4.4 (3.6) | 4.4 (4.4) | 4.3 (3.2) | 0.960 |
| Vegetables | 5.4 (2.1) | 6.0 (2.0) | 5.1 (2.1) | 0.245 |
| Fruit | 11.8 (6.5) | 14.6 (5.9) | 10.0 (6.3) | 0.050 |
| Dairy | 16.9 (6.6) | 18.4 (8.3) | 16.0 (5.2) | 0.394 |
| Meat | 9.9 (5.8) | 7.0 (3.8) | 11.7 (6.3) | 0.015 |
| Fish | 3.6 (1.7) | 3.9 (1.7) | 3.5 (1.8) | 0.499 |
| Eggs | 1.7 (1.2) | 1.8 (1.1) | 1.7 (1.2) | 0.946 |
| Oils and fats | 11.6 (2.6) | 11.9 (3.0) | 11.4 (2.4) | 0.589 |
| Beverages | 1.6 (2.0) | 1.6 (1.9) | 1.7 (1.6) | 0.899 |
| Dietary Variables | BMI-SDS | Body Fat Mass (%) | ||
|---|---|---|---|---|
| B (CI 95%) | p-Value | B (CI 95%) | p-Value | |
| Caloric intake (kcal/day) a | 0.00 (0.00 to 0.00) | 0.293 | −0.01 (−0.02 to 0.00) | 0.133 |
| Protein (% kcal) | 0.11 (−0.11 to 0.33) | 0.302 | 0.11 (−1.18 to 1.41) | 0.856 |
| Fat (% kcal) | 0.08 (0.00 to 0.17) | 0.061 | 0.44 (−0.06 to 0.94) | 0.084 |
| SFA (% kcal) | 0.26 (0.06 to 0.47) | 0.014 | 1.49 (0.31 to 2.67) | 0.016 |
| MUFA (% kcal) | 0.16 (−0.02 to 0.35) | 0.081 | 0.88 (−0.20 to 1.96) | 0.105 |
| PUFA (% kcal) | −0.12 (−0.56 to 0.32) | 0.571 | −1.09 (−3.61 to 1.43) | 0.379 |
| Carbohydrates (% kcal) | −0.09 (−0.18 to −0.01) | 0.038 | −0.39 (−0.92 to 0.14) | 0.145 |
| Fiber (g/day) | −0.03 (−0.08 to 0.02) | 0.168 | −0.21 (−0.50 to 0.08) | 0.144 |
| Food Groups | BMI-SDS | Body Fat Mass (%) | ||
|---|---|---|---|---|
| B (CI 95%) | p-Value | B (CI 95%) | p-Value | |
| Grains | 0.03 (−0.04 to 0.11) | 0.360 | 0.19 (−0.25 to 0.63) | 0.375 |
| Legumes | −0.05 (−0.23 to 0.12) | 0.535 | −0.19 (−1.20 to 0.83) | 0.707 |
| Vegetables | −0.22 (−0.48 to 0.04) | 0.098 | −0.64 (−2.21 to 0.93) | 0.406 |
| Fruit | −0.12 (−0.2 to −0.04) | 0.008 | −0.64 (−1.14 to −0.14) | 0.015 |
| Dairy | −0.01 (−0.11 to 0.08) | 0.751 | −0.08 (−0.62 to 0.47) | 0.776 |
| Meat | 0.12 (0.05 to 0.20) | 0.002 | 0.52 (0.05 to 0.99) | 0.033 |
| Fish | −0.12 (−0.46 to 0.21) | 0.459 | −0.95 (−2.86 to 0.97) | 0.318 |
| Eggs | 0.23 (−0.24 to 0.69) | 0.329 | 1.45 (−1.24 to 4.15) | 0.276 |
| Oils and fat | −0.05 (−0.27 to 0.17) | 0.632 | −0.03 (−1.31 to 1.26) | 0.966 |
| Beverages | −0.17 (−0.44 to 0.11) | 0.216 | −0.38 (−2.01 to 1.25) | 0.632 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Ribera, S.; Amat-Bou, M.; Climent, E.; Llobet, M.; Chenoll, E.; Corripio, R.; Ibáñez, L.; Ramon-Krauel, M.; Lerin, C. Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader–Willi Syndrome. Nutrients 2020, 12, 1063. https://doi.org/10.3390/nu12041063
Garcia-Ribera S, Amat-Bou M, Climent E, Llobet M, Chenoll E, Corripio R, Ibáñez L, Ramon-Krauel M, Lerin C. Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader–Willi Syndrome. Nutrients. 2020; 12(4):1063. https://doi.org/10.3390/nu12041063
Chicago/Turabian StyleGarcia-Ribera, Sonika, Montse Amat-Bou, Eric Climent, Marina Llobet, Empar Chenoll, Raquel Corripio, Lourdes Ibáñez, Marta Ramon-Krauel, and Carles Lerin. 2020. "Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader–Willi Syndrome" Nutrients 12, no. 4: 1063. https://doi.org/10.3390/nu12041063
APA StyleGarcia-Ribera, S., Amat-Bou, M., Climent, E., Llobet, M., Chenoll, E., Corripio, R., Ibáñez, L., Ramon-Krauel, M., & Lerin, C. (2020). Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader–Willi Syndrome. Nutrients, 12(4), 1063. https://doi.org/10.3390/nu12041063





