Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
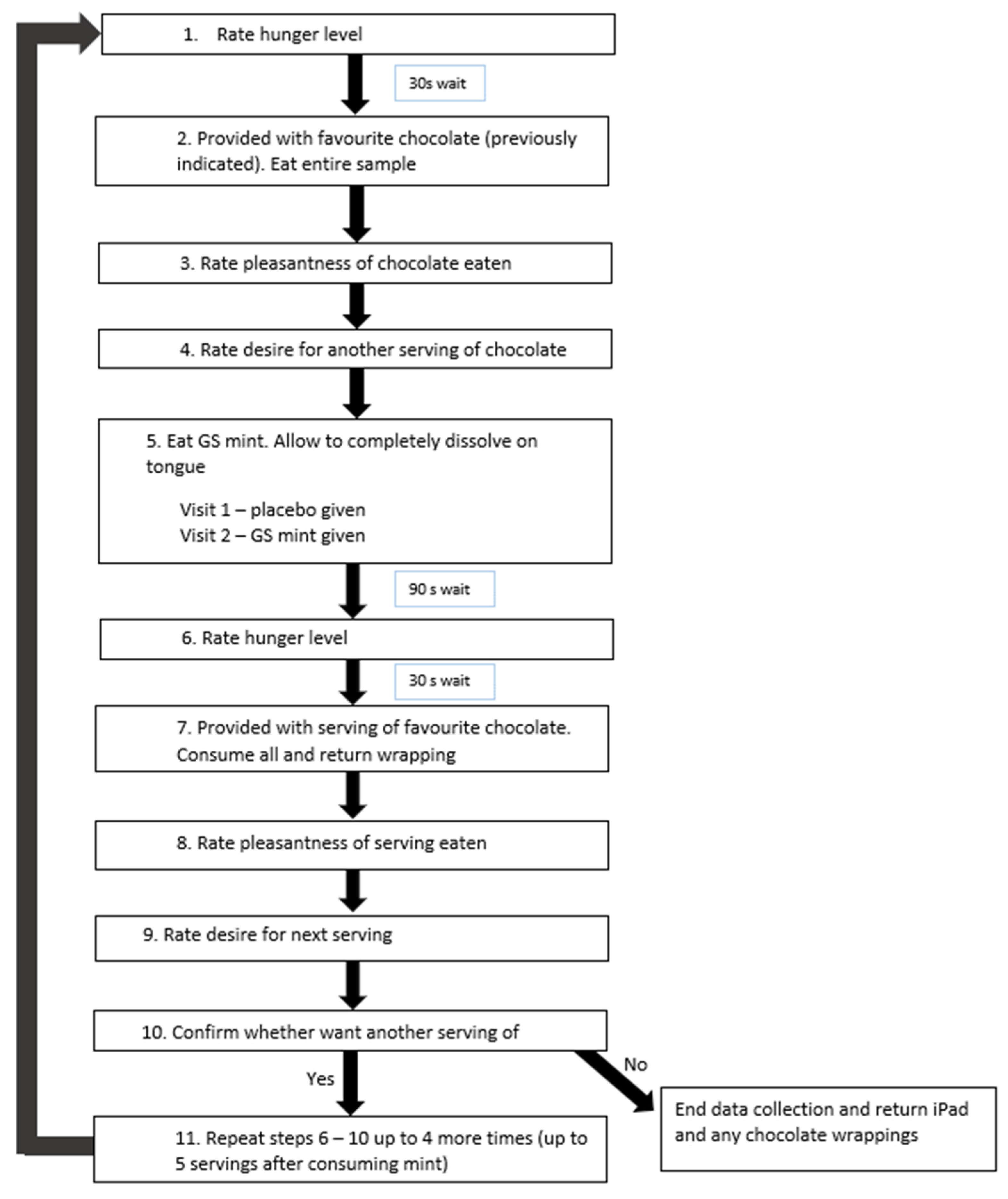
2.2. Research Design
2.3. Measures
2.3.1. Anthropomorphic Measures
2.3.2. PROP Testing
2.3.3. Questionnaire
2.3.4. Sensory Testing
2.3.5. Statistical Methods
3. Results
3.1. Sweet Tooth and PROP Taster Status
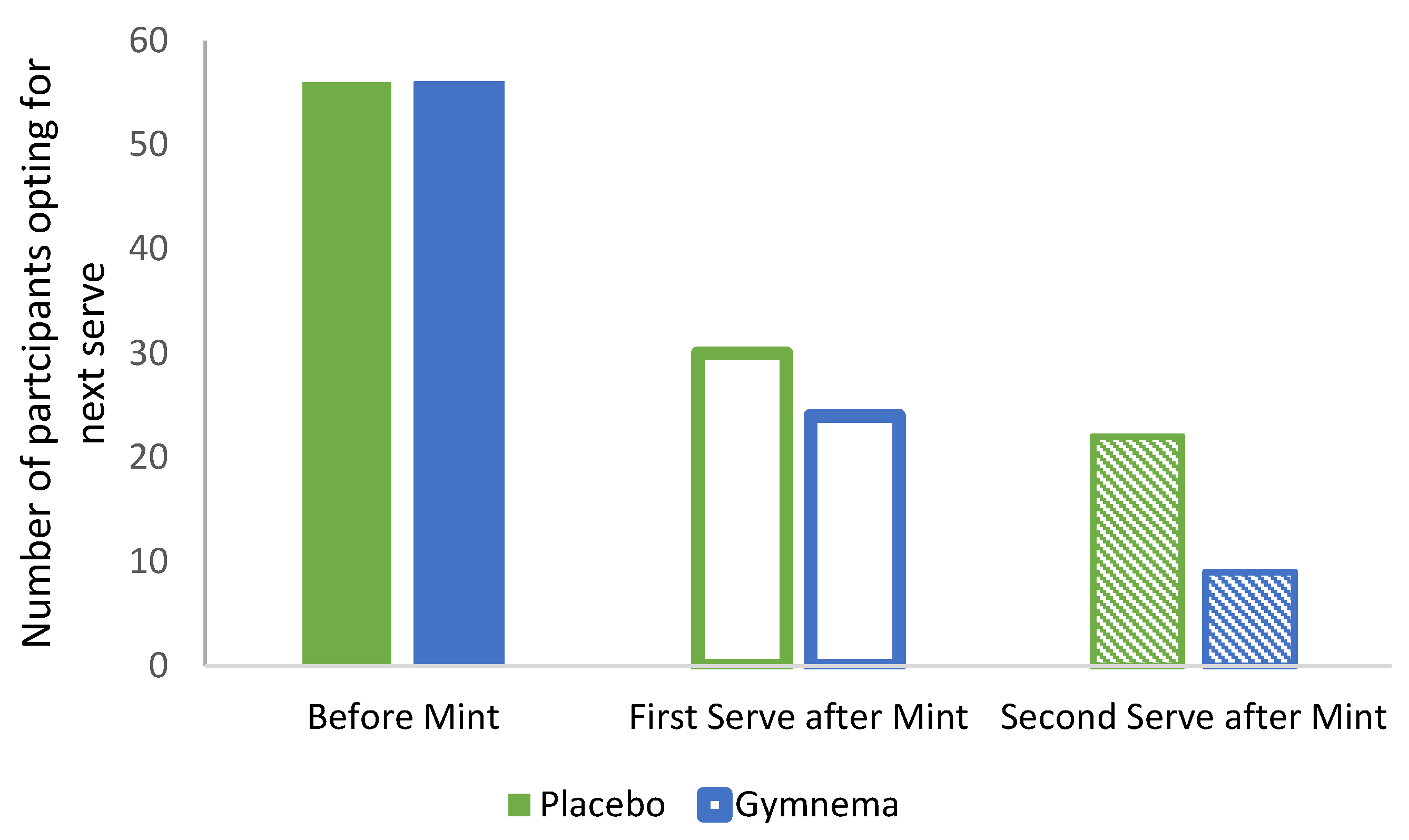
3.2. Effect of GS Mint on High-Sugar Sweet Food Consumption.
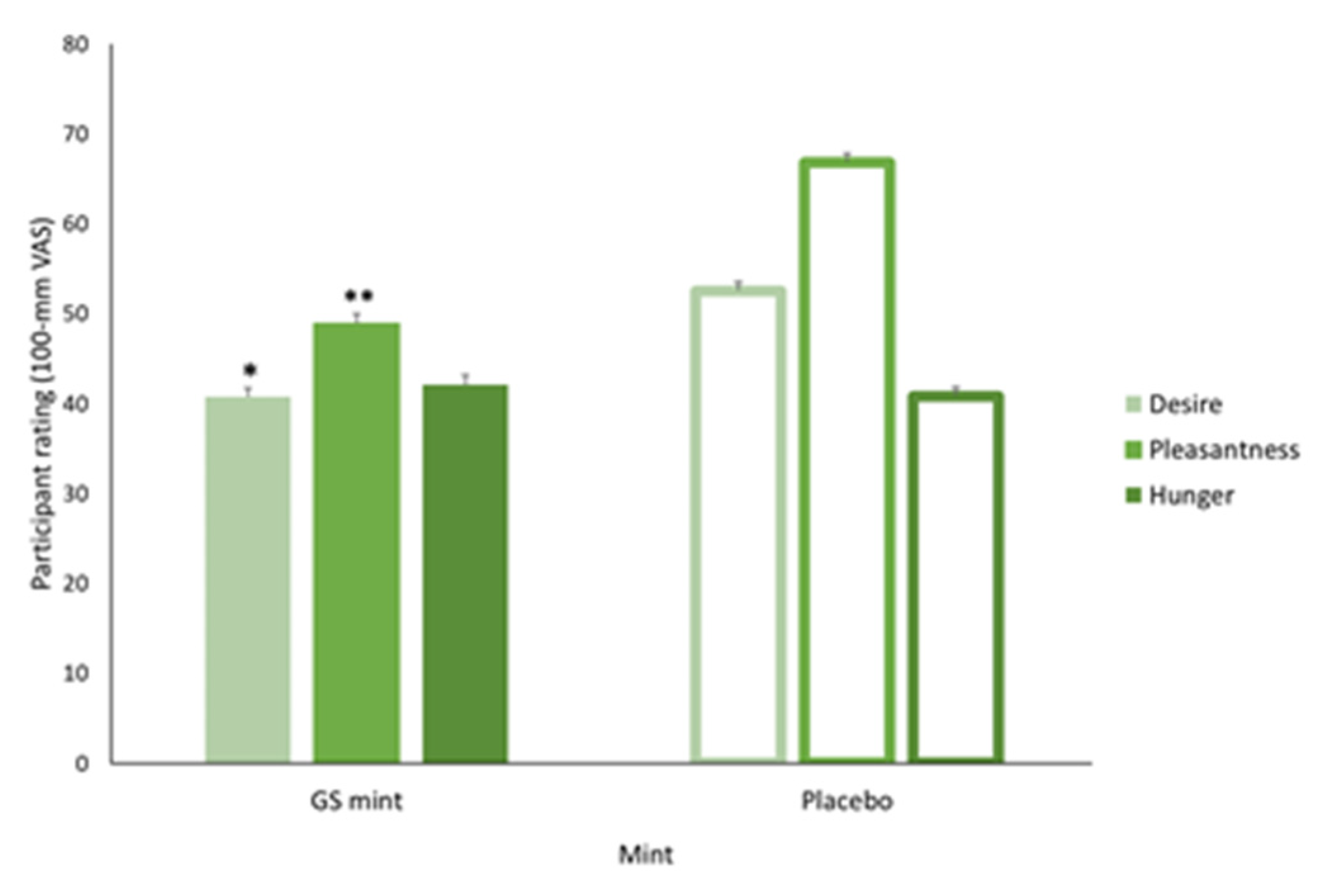
3.3. Desire for Next Serving, Pleasantness of High-Sugar Sweet Foods and Hunger Ratings
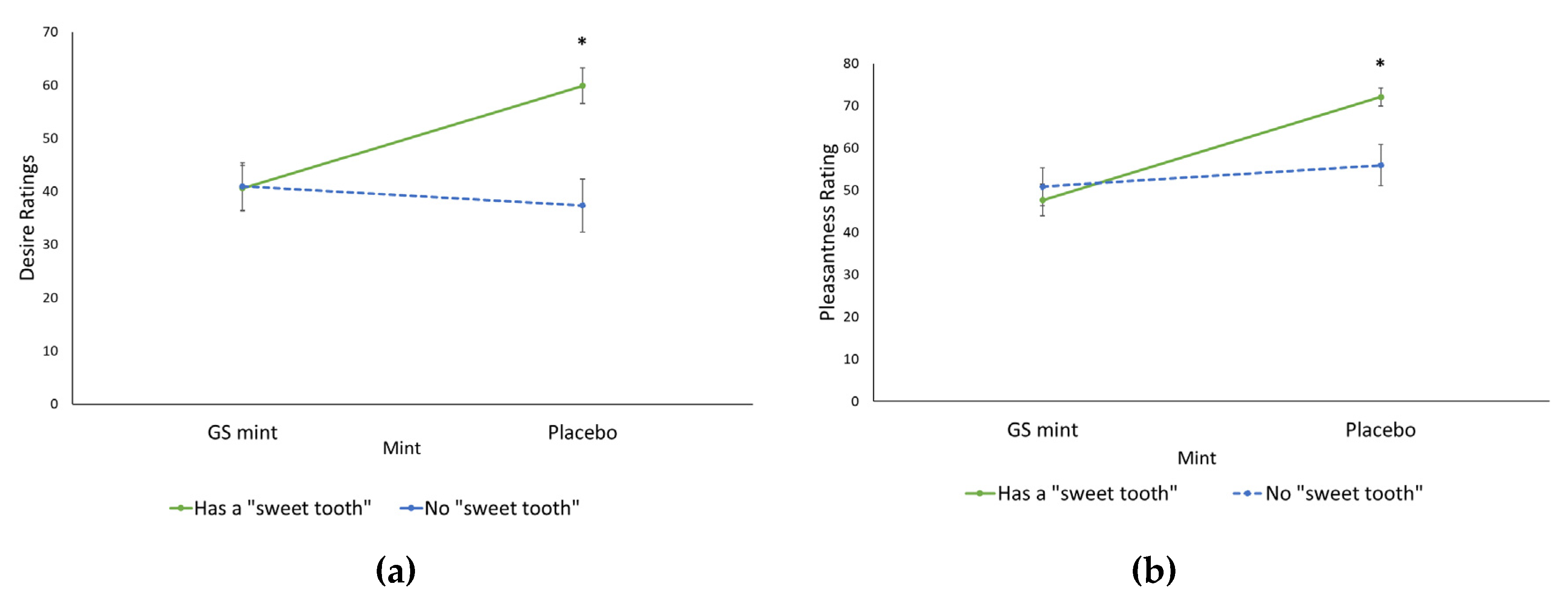
3.4. Effect of Demographic Variables on Desire and Pleasantness
4. Discussion
4.1. Effect on Taste Receptors
4.2. GS Mint Reduces Acute Consumption of High-Sugar Sweet Foods (Acute vs Chronic Consumption)
4.3. Behavioural Aspects of Consuming GS Mints
4.4. Effect of the GS Mint on Those with a “Sweet Tooth”
4.5. Relationship between GS Mint and Gender
4.6. Relationship between GS Mint Consumption and BMI
4.7. Relationship between GS Mint Consumption and PROP Taster Status
4.8. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popkin, B.M.; Hawkes, C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Newens, K.J.; Walton, J. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet. 2016, 29, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. Br. Med J. 2013, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Bartoshuk, L.M. Comparing Sensory Experiences Across Individuals: Recent Psychophysical Advances Illuminate Genetic Variation in Taste Perception. Chem. Senses 2000, 25, 447–460. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. BioMed Res. Int. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Pothuraju, R.; Sharma, R.K.; Chagalamarri, J.; Jangra, S.; Kumar Kavadi, P. A systematic review of Gymnema sylvestre in obesity and diabetes management. J. Sci. Food Agric. 2014, 94, 834–840. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Flannery-Schroeder, E. Use of the herb Gymnema sylvestre to illustrate the principles of gustatory sensation: An undergraduate neuroscience laboratory exercise. J. Undergrad. Neurosci. Educ. 2005, 3, 59–62. [Google Scholar]
- Noel, C.A.; Sugrue, M.; Dando, R. Participants with pharmacologically impaired taste function seek out more intense, higher calorie stimuli. Appetite 2017, 117, 74–81. [Google Scholar] [CrossRef]
- Thakur, G.S.; Sharma, R.; Sanodiya, B.S.; Pandey, M.; Prasad, G.B.K.S.; Bisen, P.S. Gymnema sylvestre: An Alternative Therapeutic Agent for Management of Diabetes. J. Appl. Pharm. Sci. 2012, 2, 001–006. [Google Scholar] [CrossRef]
- Kurihara, Y. Characteristics of antisweet substances, sweet proteins, and sweetness—Inducing proteins. Crit. Rev. Food Sci. Nutr. 1992, 32, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic Effects of Gymnemic Acid IV, a Compound Derived from Gymnema sylvestre Leaves in Streptozotocin-Diabetic Mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.M.; Pfaffmann, C. Suppression of sweet sensitivity by potassium gymenmate. J. Appl. Physiol. 1959, 14, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Treesukosol, Y.; Smith, K.R.; Spector, A.C. The functional role of the T1R family of receptors in sweet taste and feeding. Physiol. Behav. 2011, 105, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Sanematsu, K.; Kusakabe, Y.; Shigemura, N.; Hirokawa, T.; Nakamura, S.; Imoto, T.; Ninomiya, Y. Molecular Mechanisms for Sweet-suppressing Effect of Gymnemic Acids. J. Biol. Chem. 2014, 289, 25711–25720. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.D.; Sims, C.A.; Odabasi, A.Z.; Colquhoun, T.A.; Snyder, D.J.; Stamps, J.J.; Dotson, S.C.; Puentes, L.; Bartoshuk, L.M. Flavor Alterations Associated with Miracle Fruit and Gymnema sylvestre. Chem. Senses 2018, 43, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Perera, O.D.A.N.; Pavitha, P. Development of a sauce using Gymnema sylvestre leaves. Sri Lanka J. Food Agric. 2017, 3, 29–36. [Google Scholar] [CrossRef]
- Devi, K.; Jain, N. Clinical evaluation of the anti-sweet effects of Gymnema sylvestre extract developed into a dispersable oral tablet. J. Herb. Med. 2015, 5, 184–189. [Google Scholar] [CrossRef]
- Nobel, S.; Baker, C.; Loullis, C. Crave Crush lozenge containing gymnemic acids reduce consumption of high sugar foods. Adv. Med. Plant Res. 2017, 5, 63–67. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Gau, J.M. Gymnemic acids lozenge reduces short-term consumption of high-sugar food: A placebo controlled experiment. J. Psychopharmacol. 2017, 31, 1496–1502. [Google Scholar] [CrossRef]
- Hayes, J.E.; Duffy, V.B. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol. Behav. 2008, 95, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B. Associations between oral sensation, dietary behaviors and risk of cardiovascular disease (CVD). Appetite 2004, 43, 5–9. [Google Scholar] [CrossRef]
- Duffy, V.B.; Peterson, J.M.; Dinehart, M.E.; Bartoshuk, L.M. Genetic and Environmental Variation in Taste: Associations with Sweet Intensity, Preference, and Intake. Top. Clin. Nutr. 2003, 18, 209–220. [Google Scholar] [CrossRef]
- Holt, S.H.A.; Cobiac, L.; Beaumont-Smith, N.E.; Easton, K.; Best, D.J. Dietary habits and the perception and liking of sweetness among Australian and Malaysian students: A cross-cultural study. Food Qual. Prefer. 2000, 11, 299–312. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Sartor, F.; Donaldson, L.F.; Markland, D.A.; Loveday, H.; Jackson, M.J.; Kubis, H.-P. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite 2011, 57, 237–246. [Google Scholar] [CrossRef]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef]
- Monneuse, M.-O.; Bellisle, F.; Louis-Sylvestre, J. Impact of sex and age on sensory evaluation of sugar and fat in dairy products. Physiol. Behav. 1991, 50, 1111–1117. [Google Scholar] [CrossRef]
- Wansink, B.; Cheney, M.M.; Chan, N. Exploring comfort food preferences across age and gender. Physiol. Behav. 2003, 79, 739–747. [Google Scholar] [CrossRef]
- Green, B.G.; Shaffer, G.S.; Gilmore, M.M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses 1993, 18, 683–702. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Sanematsu, K.; Shigemura, N.; Ninomiya, Y. Binding properties between human sweet receptor and sweet-inhibitor, gymnemic acids. J. Oral Biosci. 2017, 59, 127–130. [Google Scholar] [CrossRef]
- Ng, M.; Chaya, C.; Hort, J. The influence of sensory and packaging cues on both liking and emotional, abstract and functional conceptualisations. Food Qual. Prefer. 2013, 29, 146–156. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S. Effects of gymnemic acids lozenge on reward region response to receipt and anticipated receipt of high-sugar food. Physiol. Behav. 2018, 194, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behaviour change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, D.; Chaloupka, F.J. Smoking status and subjective well-being. Tob. Control 2017, 26, 195–201. [Google Scholar] [CrossRef]
- Reed, D.R.; McDaniel, A.H. The human sweet tooth. BMC Oral Health 2006, 6 (Suppl. S1). [Google Scholar] [CrossRef]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [CrossRef]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and food preference. J. Nutr. 2012, 142, 1142S–1148S. [Google Scholar] [CrossRef]
- Vignini, A.; Borroni, F.; Sabbatinelli, J.; Pugnaloni, S.; Alia, S.; Taus, M.; Ferrante, L.; Mazzanti, L.; Fabri, M. General Decrease of Taste Sensitivity Is Related to Increase of BMI: A Simple Method to Monitor Eating Behavior. Dis. Markers 2019, 2019, 8. [Google Scholar] [CrossRef]
- Gent, J.F.; Bartoshuk, L.M. Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6- n -propylthiouracil. Chem. Senses 1983, 7, 265–272. [Google Scholar] [CrossRef]
- Deshaware, S.; Singhal, R. Genetic variation in bitter taste receptor gene TAS2R38, PROP taster status and their association with body mass index and food preferences in Indian population. Gene 2017, 627, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Henderson, S.A.; Shore, A.B.; Barratt-Fornell, A. Nontasters, tasters, and supertaster of 6-n-Propylthiouracil (PROP) and hedonic response to sweet. Physiol. Behav. 1997, 62, 649–655. [Google Scholar] [CrossRef]
- Tepper, J.B.; White, E.A.; Koelliker, Y.; Lanzara, C.; d’Adamo, P.; Gasparini, P. International Symposium on Olfaction and Taste. Ann. N. Y. Acad. Sci. 2009, 1170, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP Tasting: Anatomy, Psychophysics, and Sex Effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- McAnally, H.M.; Poulton, R.; Hancox, R.J.; Prescott, J.; Welch, D. Psychosocial correlates of 6-n-propylthiouracil (PROP) ratings in a birth cohort. Appetite 2007, 49, 700–703. [Google Scholar] [CrossRef]
- Guo, S.W.; Reed, D.R. The genetics of phenylthiocarbamide perception. Ann. Hum. Biol. 2001, 28, 111–142. [Google Scholar]
| Characteristic Presented as (mean ± SD) or n (%) | Total Group | Men | Women |
|---|---|---|---|
| Gender | 56 (100) | 20 (35.7) | 36 (64.3) |
| Age (years) | 23.2 ± 5.7 | 24.5 ± 7.6 | 22.4 ± 4.3 |
| Median | 21 | 23 | 21 |
| Range | 18–47 | 18–47 | 18–39 |
| BMI (kg·m−2) mean ± SD | 23.2 ± 3.1 | 24.4 ± 3.4 | 22.4 ± 2.7 |
| BMI groupn (%) | |||
| Underweight | 3 (5.4) | 0 (0) | 3 (8.3) |
| Normal | 39 (69.6) | 12 (60.0) | 27 (75.0) |
| Above normal (overweight + obese) 1 | 14 (25.0) | 8 (40.0) | 6 (16.7) |
| Weight (kg) mean ± SD | 64.7 ± 12.1 | 74.9 ± 11.7 | 59.1 ± 8.0 |
| Ethnicity 2n (%) | |||
| European | 12 (20.7) | 6 (26.1) | 6 (17.1) |
| Asian | 44 (75.9) | 15 (65.2) | 29 (82.8) |
| MELAA 3 | 2 (3.4) | 2 (8.7) | 0 (0) |
| Chocolate | Participant Selection (n) 1 | (%) | Weight (g) | Energy (kJ) | Sugar per Serve (g) | Sugar per 100 g (g) | Cocoa Content (%) |
|---|---|---|---|---|---|---|---|
| Nestle KitKat | 18 | 32 | 17 | 370 | 8.6 | 50.5 | 22 |
| Whittaker’s Almond Gold | 12 | 21 | 15 | 360 | 5.2 | 34.6 | 33 |
| Whittaker’s Creamy Milk | 8 | 14 | 15 | 352.5 | 6.7 | 44.7 | 33 |
| Whittaker’s Peanut Slab | 6 | 11 | 15 | 333 | 7.2 | 48.0 | 33 |
| Twix | 3 | 5 | 14.5 | 308 | 7.0 | 48.0 | 25 |
| Cadbury Moro Gold | 2 | 4 | 17.5 | 327 | 7.3 | 48.6 | 26 |
| Whittaker’s Dark Peppermint | 2 | 4 | 15 | 342 | 7.8 | 52.1 | 50 |
| Nestle Milky Bar | 2 | 4 | 14.5 | 340 | 8.0 | 54.9 | 0 |
| Cadbury Crunchie | 1 | 2 | 15 | 292 | 10.3 | 68.7 | 26 |
| Cadbury Flake | 1 | 2 | 14 | 313 | 7.9 | 56.5 | 26 |
| Snickers | 1 | 2 | 18 | 370 | 9.3 | 50.6 | 25 |
| Characteristic Presented as Mean ± SD or n (%) | Total Group | Men | Women |
|---|---|---|---|
| Sweet tooth status n (%) | |||
| Has a “sweet tooth” | 34 (60.7) | 8 (23.5) | 26 (76.5) |
| No “sweet tooth” | 22 (39.3) | 12 (54.5) | 10 (45.5) |
| PROP taster group 1 | |||
| Non-taster/medium taster 2 | 17 (33.3) | 9 (52.9) | 8 (23.5) |
| Super taster | 34 (66.7) | 8 (47.1) | 26 (76.5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, S.; Diako, C.; Kruger, R.; Wong, M.; Wood, W.; Rutherfurd-Markwick, K.; Ali, A. Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods. Nutrients 2020, 12, 1046. https://doi.org/10.3390/nu12041046
Turner S, Diako C, Kruger R, Wong M, Wood W, Rutherfurd-Markwick K, Ali A. Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods. Nutrients. 2020; 12(4):1046. https://doi.org/10.3390/nu12041046
Chicago/Turabian StyleTurner, Sophie, Charles Diako, Rozanne Kruger, Marie Wong, Warrick Wood, Kay Rutherfurd-Markwick, and Ajmol Ali. 2020. "Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods" Nutrients 12, no. 4: 1046. https://doi.org/10.3390/nu12041046
APA StyleTurner, S., Diako, C., Kruger, R., Wong, M., Wood, W., Rutherfurd-Markwick, K., & Ali, A. (2020). Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods. Nutrients, 12(4), 1046. https://doi.org/10.3390/nu12041046







