Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention
Abstract
:1. Introduction
2. Flavonoids
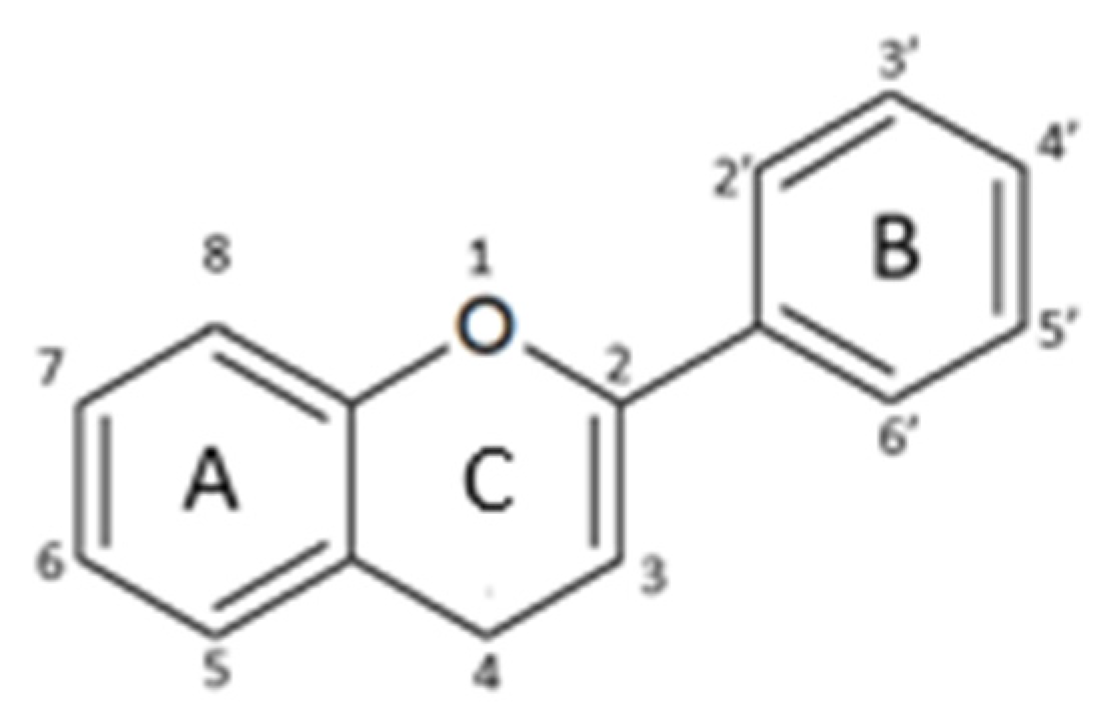
2.1. Structure and Metabolism
- -
- Flavones: have a double bond between C2C3 and a C4-oxo function;
- -
- Flavonols: are flavone analogues with a 3-hydroxylic group;
- -
- Flavanones: are flavone analogues but with a C2-C3 single bond;
- -
- Isoflavonoids: have the B ring attached at C3, rather than C2 position of the C ring;
- -
- Flavanols or catechins: are the 3-hydroxy derivatives of flavanones, they have the hydroxyl group always bound to position 3 of the C ring;
- -
- Anthocyanins: have a basic chemical structure with a flavylium cation, which binds the hydroxyl and/or methoxyl group(s) in R₁, R₂, and R₃ position.
2.2. Biological Activity of Flavonoids
2.2.1. Anti-Oxidant and Pro-Oxidant Activity
2.2.2. Anti-Proliferative and Cytotoxic Effect
2.2.3. Cell Cycle Arrest and Apoptosis Induction
3. Epigenetic Modifications Induced by Flavonoids
3.1. Epigenetics in Prostate Cancer
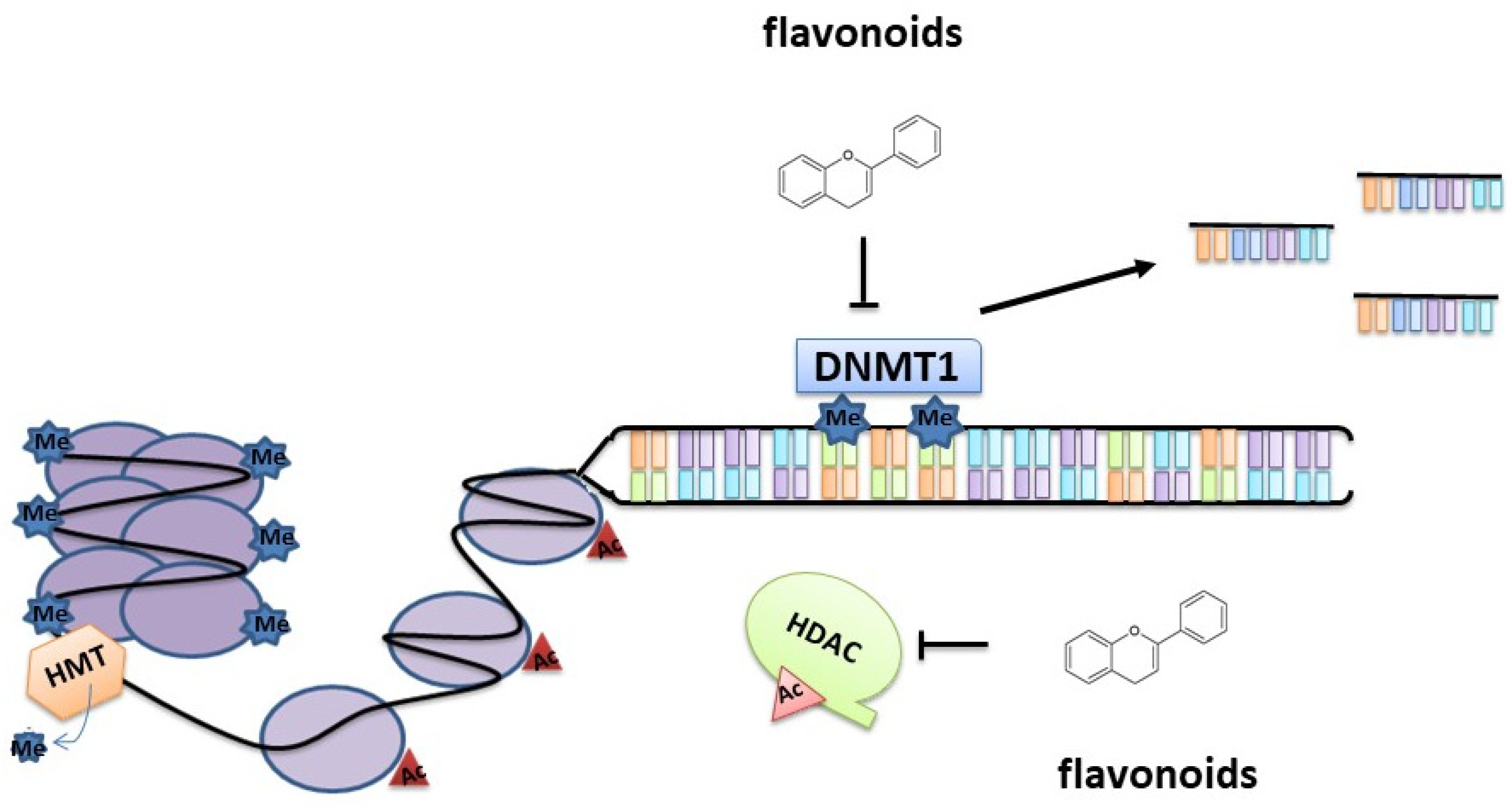
3.1.1. DNA Methylation
3.1.2. Histone Modifications
3.1.3. miRNA
3.1.4. Long Noncoding RNA (lncRNA)
4. Flavonoids as Epigenetic Modulators in Prostate Cancer
4.1. Flavones
4.2. Flavonols
4.3. Flavanones
4.4. Isoflavonoids
4.5. Catechins
4.6. Anthocyanidins
5. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ziglioli, F.; Granelli, G.; Cavalieri, D.M.; Bocchialini, T.; Maestroni, U. What chance do we have to decrease prostate cancer overdiagnosis and overtreatment? A narrative review. Acta Biomed. 2019, 90, 423–426. [Google Scholar] [PubMed]
- Chen, F.Z.; Zhao, X.K. Prostate cancer: Current treatment and prevention strategies. Iran. Red Crescent Med. J. 2013, 15, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Damber, J.E.; Aus, G. Prostate cancer. Lancet 2008, 371, 1710–1721. [Google Scholar] [CrossRef]
- Fromont, G.; Yacoub, M.; Valeri, A.; Mangin, P.; Vallancien, G.; Cancel-Tassin, G.; Cussenot, O. Differential expression of genes related to androgen and estrogen metabolism in hereditary versus sporadic prostate cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1505–1509. [Google Scholar] [CrossRef] [Green Version]
- Alberti, C. Hereditary/familial versus sporadic prostate cancer: Few indisputable genetic differences and many similar clinicopathological features. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 31–41. [Google Scholar]
- Baade, P.D.; Youlden, D.R.; Krnjacki, L.J. International epidemiology of prostate cancer: Geographical distribution and secular trends. Mol. Nutr. Food Res. 2009, 53, 171–184. [Google Scholar] [CrossRef]
- Kheirandish, P.; Chinegwundoh, F. Ethnic differences in prostate cancer. Br. J. Cancer 2011, 105, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Henderson, B.E.; Mack, T.M.; Yatani, R. Cancers of the prostate and breast among japanese and white immigrants in los angeles county. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Demissie, K.; Lu, S.E.; Rhoads, G.G. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control 2007, 11, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Vaccarella, L.; Bientinesi, R.; Gandi, C. Lifestyle in urology: Cancer. Urol. J. 2019, 86, 105–114. [Google Scholar]
- Klein, E.A.; Thompson, I.M. Chemoprevention of prostate cancer: An updated view. World J. Urol. 2012, 30, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tombal, B. Chemoprevention of prostate cancer. In Management of Prostate Cancer: A Multidisciplinary Approach; Van Poppel, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 13–24. ISBN 9783642275975. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr. Relat. Cancer 2006, 13, 751–778. [Google Scholar] [CrossRef] [Green Version]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 213, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; Van Gameren, Y.; Cnossen, E.P.J.; De Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New frontier for immuno-regulation and breast cancer control. Antioxidants 2019, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Cutler, G.J.; Nettleton, J.A.; Ross, J.A.; Harnack, L.J.; Jacobs, D.R.; Scrafford, C.G.; Barraj, L.M.; Mink, P.J.; Robien, K. Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. Int. J. Cancer 2008, 123, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Sak, K. Current epidemiological knowledge about the role of flavonoids in prostate carcinogenesis. Exp. Oncol. 2017, 39, 98–105. [Google Scholar] [CrossRef]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-cancer effects of green tea polyphenols against prostate cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reale, G.; Russo, G.I.; Di Mauro, M.; Regis, F.; Campisi, D.; Giudice, A.L.; Marranzano, M.; Ragusa, R.; Castelli, T.; Cimino, S.; et al. Association between dietary flavonoids intake and prostate cancer risk: A case-control study in Sicily. Complement. Ther. Med. 2018, 39, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Brausi, M.; Rizzi, F.; Bettuzzi, S. Chemoprevention of Human Prostate Cancer by Green Tea Catechins: Two Years Later. A Follow-up Update. Eur. Urol. 2008, 54, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.M.Y.; Ng, C.F.; Liu, Z.M.; Ho, W.M.; Lee, M.K.; Wang, F.; Kan, H.D.; He, Y.H.; Ng, S.S.M.; Wong, S.Y.S.; et al. Reduced prostate cancer risk with green tea and epigallocatechin 3-gallate intake among Hong Kong Chinese men. Prostate Cancer Prostatic Dis. 2017, 20, 318–322. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Annali-Istituto Superiore Sanita 2007, 43, 348–361. [Google Scholar]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; Van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4531–4538. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of Flavonoids in Human: A Comprehensive Review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef]
- Caporali, A.; Davalli, P.; Astancolle, S.; D’Arca, D.; Brausi, M.; Bettuzzi, S.; Corti, A. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis 2004, 25, 2217–2224. [Google Scholar] [CrossRef] [Green Version]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 Years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2006, 27, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.T.; Jung, J.I.; Song, H.R.; Woo, E.Y.; Jun, J.G.; Kim, J.K.; Her, S.; Park, J.H.Y. Piceatannol inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased interleukin-6 signaling. J. Nutr. Biochem. 2012, 27, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.Y.; Schoene, N.W.; Kim, Y.S.; Mizuno, C.S.; Rimando, A.M. Differential effects of resveratrol and its naturally occurring methylether analogs on cell cycle and apoptosis in human androgen-responsive LNCaP cancer cells. Mol. Nutr. Food Res. 2010, 54, 335–344. [Google Scholar] [CrossRef]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer—I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000, 3, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Dorai, T.; Cao, Y.C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47, 293. [Google Scholar] [CrossRef]
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012, 51, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Hagen, R.M.; Chedea, V.S.; Mintoff, C.P.; Bowler, E.; Morse, H.R.; Ladomery, M.R. Epigallocatechin-3-gallate promotes apoptosis and expression of the caspase 9a splice variant in PC3 prostate cancer cells. Int. J. Oncol. 2013, 43, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Siddique, N.A.; Mujeeb, M.; Najmi, A.K.; Khan, H.N.; Farooqi, H. WITHDRAWN: Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. J. Saudi Chem. Soc. 2010, 4, 1–5. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Dikici, E.; Tozoglu, F.; Gulcin, I. Antioxidant activity of Melissa officinalis leaves. J. Med. Plants Res. 2011, 5, 217–222. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.M. Molecular mechanisms and bioavailability of polyphenols in prostate cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, A.; Pechan, T.; Willett, K.L. Differential protein expression of peroxiredoxin I and II by benzo(a)pyrene and quercetin treatment in 22Rv1 and PrEC prostate cell lines. Toxicol. Appl. Pharmacol. 2007, 210, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofrè, S.; Di Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phyther. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Bode, A.M.; Dong, Z. Signal transduction and molecular targets of selected flavonoids. Antioxid. Redox Signal. 2013, 19, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.J.; Lee, K.W.; Kwon, J.Y.; Hwang, M.K.; Rogozin, E.A.; Heo, Y.S.; Bode, A.M.; Lee, H.J.; Dong, Z. Delphinidin attenuates neoplastic transformation in JB6 Cl41 mouse epidermal cells by blockingraf/mitog en-activated protein kinase kinase/extracellular signal-regulated kinase signaling. Cancer Prev. Res. 2008, 20, 522–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Kang, N.J.; Rogozin, E.A.; Kim, H.G.; Cho, Y.Y.; Bode, A.M.; Lee, H.J.; Surh, Y.J.; Bowden, G.T.; Dong, Z. Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1. Carcinogenesis 2007, 28, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Ki, W.L.; Nam, J.K.; Heo, Y.S.; Rogozin, E.A.; Pugliese, A.; Mun, K.H.; Bowden, G.T.; Bode, A.M.; Hyong, J.L.; Dong, Z. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008, 68, 946–955. [Google Scholar]
- Byun, S.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, M.K.; Lim, S.H.; Bode, A.M.; Lee, H.J.; Dong, Z. Luteolin inhibits protein kinase Cε and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010, 70, 2415–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, K.J.; Ki, W.L.; Byun, S.; Nam, J.K.; Sung, H.L.; Heo, Y.S.; Bode, A.M.; Bowden, G.T.; Hyong, J.L.; Dong, Z. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008, 68, 6021–6029. [Google Scholar]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.Y.; Baldwin, A.S. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Yang, H.J.; Youn, H.; Yun, Y.J.; Seong, K.M.; Youn, B. Myricetin Inhibits Akt Survival Signaling and Induces Bad-mediated Apoptosis in a Low Dose Ultraviolet (UV)-B-irradiated HaCaT Human Immortalized Keratinocytes. J. Radiat. Res. 2010, 51, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.K.; Lee, K.W.; Byun, S.; Lee, E.J.; Kim, J.E.; Bode, A.M.; Dong, Z.; Lee, H.J. Myricetin inhibits UVB-induced angiogenesis by regulating PI-3 kinase in vivo. Carcinogenesis 2009, 31, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.Y.; Lee, K.W.; Kim, J.E.; Jung, S.K.; Kang, N.J.; Hwang, M.K.; Heo, Y.S.; Bode, A.M.; Dong, Z.; Lee, H.J. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis 2009, 30, 1932–1940. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Hu, X.; Xing, Z.; Xing, R.; Lv, R.; Cheng, X.; Su, J.; Zhou, Z.; Xu, Z.; Nilsson, S.; et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol. Cell. Biochem. 2015, 406, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Serttas, R.; Turkekul, K.; Dibirdik, I.; Bilir, A. The flavonoid apigenin reduces prostate cancer CD44+ stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016, 162, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Wu, J.; Li, S.; Wang, X.; Liang, Z.; Xu, X.; Xu, X.; Hu, Z.; Lin, Y.; Chen, H.; et al. Apigenin inhibits migration and invasion via modulation of epithelial mesenchymal transition in prostate cancer. Mol. Med. Rep. 2015, 11, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzi, F.; Naponelli, V.; Silva, A.; Modernelli, A.; Ramazzina, I.; Bonacini, M.; Tardito, S.; Gatti, R.; Uggeri, J.; Bettuzzi, S. Polyphenon E®, a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis 2014, 35, 828–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.C.; Yen, C.Y.; Wu, R.S.C.; Yang, J.S.; Lu, H.F.; Lu, K.W.; Lo, C.; Chen, H.Y.; Tang, N.Y.; Wu, C.C.; et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014, 29, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008, 44, 1833–1845. [Google Scholar] [CrossRef] [Green Version]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr. Cancer 2012, 64, 580–587. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2009, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bredfeldt, T.G.; Walker, C.L. Epigenetics. In Comprehensive Toxicology, 2nd ed.; Elsevier Science: Oxford, UK, 2010; ISBN 9780080468846. [Google Scholar]
- Albany, C.; Alva, A.S.; Aparicio, A.M.; Singal, R.; Yellapragada, S.; Sonpavde, G.; Hahn, N.M. Epigenetics in Prostate Cancer. Prostate Cancer 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, N.; Kumar, A.P.; Ghosh, R. DNA Methylation and Flavonoids in Genitourinary Cancers. Curr. Pharmacol. Rep. 2015, 1, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, M. Methylation and Inactivation of Estrogen, Progesterone, and Androgen Receptors in Prostate Cancer. J. Natl. Cancer Inst. 2002, 94, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Lodygin, D.; Epanchintsev, A.; Menssen, A.; Diebold, J.; Hermeking, H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005, 65, 4218–4227. [Google Scholar] [CrossRef] [Green Version]
- Li, L.C.; Carroll, P.R.; Dahiya, R. Epigenetic changes in prostate cancer: Implication for diagnosis and treatment. J. Natl. Cancer Inst. 2005, 97, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Sathyanarayana, U.G.; Padar, A.; Suzuki, M.; Maruyama, R.; Shigematsu, H.; Hsieh, J.T.; Frenkel, E.P.; Gazdar, A.F. Aberrant Promoter Methylation of Laminin-5-Encoding Genes in Prostate Cancers and Its Relationship to Clinicopathological Features. Clin. Cancer Res. 2003, 9, 6395–6400. [Google Scholar]
- Li, L.C.; Zhao, H.; Nakajima, K.; Oh, B.R.; Alves Ribeiro Filho, L.; Carroll, P.; Dahiya, R. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J. Urol. 2001, 166, 705–709. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Zhang, Z.; Humphreys, E.B.; Bennett, C.; Mangold, L.A.; Carducci, M.A.; Partin, A.W.; Garrett-Mayer, E.; DeMarzo, A.M.; Herman, J.G. Effect of DNA Methylation on Identification of Aggressive Prostate Cancer. Urology 2008, 72, 1234–1239. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.R.; Filipe, L.; Costa, V.L.; Ribeiro, F.R.; Martins, A.T.; Teixeira, M.R.; Jerónimo, C.; Henrique, R. Detailed analysis of expression and promoter methylation status of apoptosis-related genes in prostate cancer. Apoptosis 2010, 15, 956–965. [Google Scholar] [CrossRef]
- Jurkowska, R.Z.; Jurkowski, T.P.; Jeltsch, A. Structure and Function of Mammalian DNA Methyltransferases. ChemBioChem 2011, 12, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Yegnasubramanian, S.; Haffner, M.C.; Zhang, Y.; Gurel, B.; Cornish, T.C.; Wu, Z.; Irizarry, R.A.; Morgan, J.; Hicks, J.; DeWeese, T.L.; et al. DNA Hypomethylation Arises Later in Prostate Cancer Progression than CpG Island Hypermethylation and Contributes to Metastatic Tumor Heterogeneity. Cancer Res. 2008, 68, 8954–8967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowacka-Zawisza, M.; Wiśnik, E. DNA methylation and histone modifications as epigenetic regulation in prostate cancer (review). Oncol. Rep. 2017, 38, 2587–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chervona, Y.; Costa, M. Histone modifications and cancer: Biomarkers of prognosis? Am. J. Cancer Res. 2012, 2, 589–597. [Google Scholar]
- Kurdistani, S.K. Histone modifications in cancer biology and prognosis. Prog. Drug Res. 2011, 67, 91–106. [Google Scholar]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. γH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Nanni, S.; Priolo, C.; Grasselli, A.; D’Eletto, M.; Merola, R.; Moretti, F.; Gallucci, M.; De Carli, P.; Sentinelli, S.; Cianciulli, A.M.; et al. Epithelial-restricted gene profile of primary cultures from human prostate tumors: A molecular approach to predict clinical behavior of prostate cancer. Mol. Cancer Res. 2006, 4, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, R.; Plaga, A.R.; Liu, X.; Shukla, G.C.; Gupta, S. MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer Lett. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Walter, B.A.; Valera, V.A.; Pinto, P.A.; Merino, M.J. Comprehensive microRNA profiling of prostate cancer. J. Cancer 2013, 99, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Josson, S.; Chung, L.W.K.; Gururajan, M. MicroRNAs and prostate cancer. In Advances in Experimental Medicine and Biology; SpringerNature: Basel, Switzerland, 2015; pp. 105–118. [Google Scholar]
- Sekhon, K.; Bucay, N.; Majid, S.; Dahiya, R.; Saini, S. MicroRNAs and epithelial-mesenchymal transition in prostate cancer. Oncotarget 2016, 7, 67597–67611. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, L. Members of the microRNA-200 family are promising therapeutic targets in cancer (Review). Exp. Ther. Med. 2017, 14, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekhail, S.M.; Yousef, P.G.; Jackinsky, S.W.; Pasic, M.; Yousef, G.M. miRNA in Prostate Cancer: New Prospects for Old Challenges. EJIFCC 2014, 25, 79–98. [Google Scholar] [PubMed]
- Ma, X.; Zou, L.; Li, X.; Chen, Z.; Lin, Q.; Wu, X. MicroRNA-195 regulates docetaxel resistance by targeting clusterin in prostate cancer. Biomed. Pharmacother. 2018, 99, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Bettuzzi, S. The clusterin paradigm in prostate and breast carcinogenesis. Endocr. Relat. Cancer 2010, 17, R1–R17. [Google Scholar] [CrossRef]
- Scaltriti, M.; Brausi, M.; Amorosi, A.; Caporali, A.; D’Arca, D.; Astancolle, S.; Corti, A.; Bettuzzi, S. Clusterin (SGP-2, ApoJ) expression is downregulated in low- and high-grade human prostate cancer. Int. J. Cancer 2004, 108, 23–30. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Davalli, P.; Davoli, S.; Chayka, O.; Rizzi, F.; Belloni, L.; Pellacani, D.; Fregni, G.; Astancolle, S.; Fassan, M.; et al. Genetic inactivation of ApoJ/clusterin: Effects on prostate tumourigenesis and metastatic spread. Oncogene 2009, 28, 4344–4352. [Google Scholar] [CrossRef] [Green Version]
- Bonacini, M.; Negri, A.; Davalli, P.; Naponelli, V.; Ramazzina, I.; Lenzi, C.; Bettuzzi, S.; Rizzi, F. Clusterin Silencing in Prostate Cancer Induces Matrix Metalloproteinases by an NF-B-Dependent Mechanism. J. Oncol. 2019, 266, 16425–16430. [Google Scholar] [CrossRef]
- Ramnarine, V.R.; Kobelev, M.; Gibb, E.A.; Nouri, M.; Lin, D.; Wang, Y.; Buttyan, R.; Davicioni, E.; Zoubeidi, A.; Collins, C.C. The evolution of long noncoding RNA acceptance in prostate cancer initiation, progression, and its clinical utility in disease management. Eur. Urol. 2019, 31, 11050–11058. [Google Scholar] [CrossRef]
- Malik, B.; Feng, F. Long noncoding RNAs in prostate cancer: Overview and clinical implications. Asian J. Androl. 2016, 18, 568–574. [Google Scholar]
- Saghafi, T.; Ali Taheri, R.; Parkkila, S.; Emameh, R.Z. Phytochemicals as modulators of long non-coding RNAs and inhibitors of cancer-related carbonic anhydrases. Int. J. Mol. Sci. 2019, 20, 2939. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganai, S.A. Plant-derived flavone Apigenin: The small-molecule with promising activity against therapeutically resistant prostate cancer. Biomed. Pharmacother. 2017, 85, 47–56. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; He, K.; Jiang, W.; Xu, C.; Li, Z.; Wang, H.; Wang, W.; Wang, H.; Teng, X.; et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J. Transl. Med. 2015, 13, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Peng, H.; Li, K.; Zhao, R.; Li, L.; Yu, Y.; Wang, X.; Han, Z. Luteolin exerts an anticancer effect on NCI-H460 human non-small cell lung cancer cells through the induction of Sirt1-mediated apoptosis. Mol. Med. Rep. 2015, 12, 4196–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Meng, W.; Zhang, J.J.; Zhou, Y.; Wang, Y.L.; Su, Y.; Lin, S.C.; Gan, Z.H.; Sun, Y.N.; Min, D.L. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. Onco Targets Ther. 2016, 9, 3085–3094. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kim, S.H.; Shin, J.; Harikishore, A.; Lim, J.K.; Jung, Y.; Lyu, H.N.; Baek, N.I.; Choi, K.Y.; Yoon, H.S.; et al. Luteolin suppresses cancer cell proliferation by targeting vaccinia-related kinase 1. PLoS ONE 2014, 9, e109655. [Google Scholar] [CrossRef] [Green Version]
- Hong, Z.; Cao, X.; Li, N.; Zhang, Y.; Lan, L.; Zhou, Y.; Pan, X.; Shen, L.; Yin, Z.; Luo, L. Luteolin is effective in the non-small cell lung cancer model with L858R/T790M EGF receptor mutation and erlotinib resistance. Br. J. Pharmacol. 2014, 171, 2842–2853. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, M.A.; Ozaki, Y.; Okuzaki, D.; Naito, Y.; Sasakura, T.; Okamoto, A.; Tabara, H.; Inoue, T.; Hagiyama, M.; Ito, A.; et al. Gefitinib and luteolin cause growth arrest of human prostate cancer PC-3 cells via inhibition of cyclin G-associated kinase and induction of miR-630. PLoS ONE 2014, 9, e100124. [Google Scholar] [CrossRef] [Green Version]
- Markaverich, B.M.; Vijjeswarapu, M. Multiple sites of type II site ligand (Luteolin and BMHPC) regulation of gene expression in PC-3 cells. Int. J. Biomed. Sci. 2012, 8, 219–232. [Google Scholar] [PubMed]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Phromnoi, K.; Aggarwal, B.B. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem. Pharmacol. 2013, 85, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, W.S.; Go, S., II; Nagappan, A.; Han, M.H.; Hong, S.H.; Kim, G.S.; Kim, G.Y.; Kwon, T.K.; Ryu, C.H.; et al. Morin, a flavonoid from moraceae, induces apoptosis by induction of bad protein in human leukemic cells. Int. J. Mol. Sci. 2015, 16, 645–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, I.; Páez, A.; Ferruelo, A.; Luján, M.; Berenguer, A. Polyphenols in red wine inhibit the proliferation and induce apoptosis of LNCaP cells. BJU Int. 2002, 89, 950–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferruelo, A.; Romero, I.; Cabrera, P.M.; Arance, I.; Andrés, G.; Angulo, J.C. Effects of resveratrol and other wine polyphenols on the proliferation, apoptosis and androgen receptor expression in LNCaP cells. Actas Urológicas Españolas 2014, 38, 397–404. [Google Scholar] [CrossRef]
- Li, B.; Jin, X.; Meng, H.; Hu, B.; Zhang, T.; Yu, J.; Chen, S.; Guo, X.; Wang, W.; Jiang, W.; et al. Morin promotes prostate cancer cells chemosensitivity to paclitaxel through miR-155/GATA3 axis. Oncotarget 2017, 8, 47849–47860. [Google Scholar] [CrossRef] [Green Version]
- Moheb, A.; Grondin, M.; Ibrahim, R.K.; Roy, R.; Sarhan, F. Winter wheat hull (husk) is a valuable source for tricin, a potential selective cytotoxic agent. Food Chem. 2013, 138, 931–937. [Google Scholar] [CrossRef]
- Ghasemi, S.; Lorigooini, Z.; Wibowo, J.P.; Amini-khoei, H. Tricin isolated from Allium atroviolaceum potentiated the effect of docetaxel on PC3 cell proliferation: Role of miR-21. Nat. Prod. Res. 2019, 33, 1828–1831. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Stepani, V.; Novak, R.; Gall, K. Epigenome, Cancer Prevention and Flavonoids and Curcumin. In Epigenetics and Epigenomics; InTech: Rijeka, Croatia, 2014; pp. 173–210. [Google Scholar]
- ElAttar, T.M.A.; Virji, A.S. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs 1999, 10, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Ebermann, R.; Marian, B. Quercetin-induced apoptosis in colorectal tumor cells: Possible role of EGF receptor signaling. Nutr. Cancer 1999, 34, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Kedhari Sundaram, M.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Liu, M.; Li, W.; Che, J.P.; Wang, G.C.; Zheng, J.H. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol. Med. Rep. 2015, 11, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mydlarz, W.; Uemura, M.; Ahn, S.; Hennessey, P.; Chang, S.; Demokan, S.; Sun, W.; Shao, C.; Bishop, J.; Krosting, J.; et al. Clusterin is a gene-specific target of microrna-21 in head and neck squamous cell carcinoma. Clin. Cancer Res. 2014, 20, 868–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitomsky, N.; Böhm, M.; Klempnauer, K.H. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene 2004, 23, 7484–7493. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-S.; Jansen, A.P.; Komar, A.A.; Zheng, X.; Merrick, W.C.; Costes, S.; Lockett, S.J.; Sonenberg, N.; Colburn, N.H. The Transformation Suppressor Pdcd4 Is a Novel Eukaryotic Translation Initiation Factor 4A Binding Protein That Inhibits Translation. Mol. Cell. Biol. 2003, 23, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Ting, H.; Deep, G.; Agarwal, R. Molecular mechanisms of silibinin-mediated cancer chemoprevention with major emphasis on prostate cancer. AAPS J. 2013, 15, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Anestopoulos, I.; Sfakianos, A.P.; Franco, R.; Chlichlia, K.; Panayiotidis, M.I.; Kroll, D.J.; Pappa, A. A novel role of silibinin as a putative epigenetic modulator in human prostate carcinoma. Molecules 2017, 22, 62. [Google Scholar] [CrossRef]
- Ellinger, J.; Kahl, P.; Von Der Gathen, J.; Heukamp, L.C.; Gütgemann, I.; Walter, B.; Hofstädter, F.; Bastian, P.J.; Von Ruecker, A.; Müller, S.C.; et al. Global histone H3K27 methylation levels are different in localized and metastatic prostate cancer. Cancer Investig. 2012, 30, 92–97. [Google Scholar] [CrossRef]
- De Souza, P.L.; Russell, P.J.; Kearsley, J.H.; Howes, L.G. Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr. Rev. 2010, 68, 542–555. [Google Scholar] [CrossRef]
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr. Cancer 2009, 61, 598–606. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Yan, G.R.; Yin, X.F.; Xiao, C.L.; Tan, Z.L.; Xu, S.H.; He, Q.Y. Identification of novel signaling components in genistein-regulated signaling pathways by quantitative phosphoproteomics. J. Proteom. 2011, 75, 695–707. [Google Scholar] [CrossRef]
- Li, Y.; Sarkar, F.H. Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar]
- Liss, M.A.; Schlicht, M.; Kahler, A.; Fitzgerald, R.; Thomassi, T.; Degueme, A.; Hessner, M.; Datta, M.W. Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genom. Proteom. 2010, 7, 245–252. [Google Scholar]
- Mahmoud, A.M.; Al-Alem, U.; Ali, M.M.; Bosland, M.C. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J. Steroid Biochem. Mol. Biol. 2015, 152, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein Inhibits Prostate Cancer Cell Growth by Targeting miR-34a and Oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef]
- Hansen, T.B.; Wiklund, E.D.; Bramsen, J.B.; Villadsen, S.B.; Statham, A.L.; Clark, S.J.; Kjems, J. MiRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011, 30, 4414–4422. [Google Scholar] [CrossRef] [Green Version]
- Braconi, C.; Kogure, T.; Valeri, N.; Huang, N.; Nuovo, G.; Costinean, S.; Negrini, M.; Miotto, E.; Croce, C.M.; Patel, T. MicroRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene 2011, 30, 4750–4756. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, G.; Zhang, X.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.H. A new class of flavonol-based anti-prostate cancer agents: Design, synthesis, and evaluation in cell models. Bioorg. Med. Chem. Lett. 2016, 26, 4241–4245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Li, S.L.; Wang, S.Y. Construction and analysis of mRNA, miRNA, lncRNA, and TF regulatory networks reveal the key genes associated with prostate cancer. PLoS ONE 2018, 13, e0198055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Zhao, J.C.; Kim, J.; Fong, K.W.; Yang, Y.A.; Chakravarti, D.; Mo, Y.Y.; Yu, J. LncRNA HOTAIR Enhances the Androgen-Receptor-Mediated Transcriptional Program and Drives Castration-Resistant Prostate Cancer. Cell Rep. 2015, 13, 209–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 23, 965. [Google Scholar] [CrossRef]
- Chan, M.M.; Chen, R.; Fong, D. Targeting cancer stem cells with dietary phytochemical—Repositioned drug combinations. Cancer Lett. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Jin, G.; Yang, Y.; Liu, K.; Zhao, J.; Chen, X.; Liu, H.; Bai, R.; Li, X.; Jiang, Y.; Zhang, X.; et al. Combination curcumin and (-)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis 2017, 433, 53–64. [Google Scholar] [CrossRef]
- Schramm, L. Going Green: The Role of the Green Tea Component EGCG in Chemoprevention. J. Carcinog. Mutagen. 2013, 6, e384. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea Polyphenol (-)-Epigallocatechin-3-Gallate Inhibits DNA Methyltransferase and Reactivates Methylation-Silenced Genes in Cancer Cell Lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Oya, Y.; Mondal, A.; Rawangkan, A.; Umsumarng, S.; Iida, K.; Watanabe, T.; Kanno, M.; Suzuki, K.; Li, Z.; Kagechika, H.; et al. Down-regulation of histone deacetylase 4, -5 and -6 as a mechanism of synergistic enhancement of apoptosis in human lung cancer cells treated with the combination of a synthetic retinoid, Am80 and green tea catechin. J. Nutr. Biochem. 2017, 42, 7–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Han, L.; Zhou, Y.; Sun, S. Green tea polyphenol EGCG reverse cisplatin resistance of A549/DDP cell line through candidate genes demethylation. Biomed. Pharmacother. 2015, 69, 285–290. [Google Scholar] [CrossRef]
- Jin, H.; Chen, J.X.; Wang, H.; Lu, G.; Liu, A.; Li, G.; Tu, S.; Lin, Y.; Yang, C.S. NNK-induced DNA methyltransferase 1 in lung tumorigenesis in A/J mice and inhibitory effects of (-)-epigallocatechin-3-gallate. Nutr. Cancer 2015, 67, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Bosutti, A.; Zanconati, F.; Grassi, G.; Dapas, B.; Passamonti, S.; Scaggiante, B. Epigenetic and miRNAs Dysregulation in Prostate Cancer: The role of Nutraceuticals. Anticancer Agents Med. Chem. 2016, 16, 1385–1402. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Rao, M.; Wang, C.; Sakamaki, T.; Wang, J.; Di Vizio, D.; Zhang, X.; Albanese, C.; Balk, S.; Chang, C.; et al. Acetylation of Androgen Receptor Enhances Coactivator Binding and Promotes Prostate Cancer Cell Growth. Mol. Cell. Biol. 2003, 23, 8563–8575. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kwak, J.; Choi, H.K.; Choi, K.C.; Kim, S.; Lee, J.; Jun, W.; Park, H.J.; Yoon, H.G. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.S.; Gupta, K.; Gupta, S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis 2012, 33, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Shukla, S.; Gupta, S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int. J. Cancer 2010, 126, 2520–2533. [Google Scholar] [CrossRef] [Green Version]
- Shinojima, T.; Yu, Q.; Huang, S.K.; Li, M.; Mizuno, R.; Liu, E.T.; Hoon, D.S.B.; Lessard, L. Heterogeneous epigenetic regulation of TIMP3 in prostate cancer. Epigenetics 2012, 7, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Pulukuri, S.M.; Patibandla, S.; Patel, J.; Estes, N.; Rao, J.S. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene 2007, 26, 5229–5237. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Cao, X.; Zhang, H.; Sun, G.; Fan, G.; Chen, L.; Wang, S. Imbalance between MMP-2, 9 and TIMP-1 promote the invasion and metastasis of renal cell carcinoma via SKP2 signaling pathways. Tumor Biol. 2014, 35, 9807–9813. [Google Scholar] [CrossRef]
- Deb, G.; Shankar, E.; Thakur, V.S.; Ponsky, L.E.; Bodner, D.R.; Fu, P.; Gupta, S. Green tea–induced epigenetic reactivation of tissue inhibitor of matrix metalloproteinase-3 suppresses prostate cancer progression through histone-modifying enzymes. Mol. Carcinog. 2019, 58, 1194–1207. [Google Scholar] [CrossRef]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-κB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef] [Green Version]
- Syed, D.N.; Suh, Y.; Afaq, F.; Mukhtar, H. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008, 265, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.H.; Ko, H.; Jeon, H.; Sung, G.J.; Park, S.Y.; Jun, W.J.; Lee, Y.H.; Lee, J.; Lee, S.W.; Yoon, H.G.; et al. Delphinidin induces apoptosis via cleaved HDAC3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget 2016, 7, 56767–56780. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |
| Chemical Structure | Bonds | Compounds | |
|---|---|---|---|
| FLAVONES | 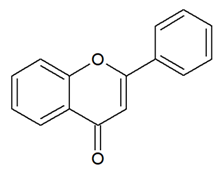 | Double bond between C2-C3 and a ketone in C4 of the C ring. | Apigenin Luteolin Morin Tricin |
| FLAVONOLS | 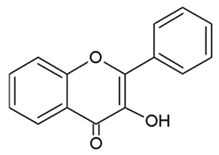 | Hydroxylic group, a double bond between C2-C3 and a ketone in C ring. | Quercetin Myrecitin Fisetin Kaempferol |
| FLAVANONES | 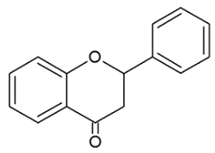 | Lack the double bond between C2-C3 in C ring; only hydroxyl and methoxy groups as substituents. | Silibinin Naringenin Hesperdin |
| ISOFLAVONOIDS | 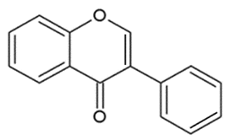 | Large diversity of structure in the C ring. The B ring is attached at C3 rather C2 of the C ring. | Genistein DaidzeinGlycitein |
| FLAVANOLS OR CATECHINS | 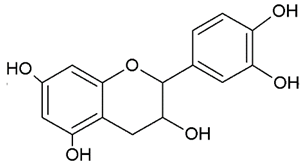 | No double bond of C2-C3 in the hydroxyl group in position 3. | EGCG Epicatechin Epicatechin-3-gallate |
| ANTHOCYANINS |  | Flavylium cation binding hydroxyl groups and/or methoxy group(s) in R₁, R₂, and R₃ position. | Delphinidin Cyanidin PeonidinN |
| Cell Lines/Animal Models/Clinical Trials | Molecular Target | Cellular Mechanism | Ref | ||
|---|---|---|---|---|---|
| Flavones | Apigenin | PC3-22Rv1 cells | ↓ HDAC1, HDAC3 ↑ acetylation H3-H4 | Cell cycle arrest apoptosis | [50] |
| PC3 xenograft mice | ↓ HDAC1, HDAC3 | Tumor growth reduction | [50] | ||
| Luteolin | PC3-LNCaP cells | ↓ miR-301 | Inhibition proliferation apoptosis | [121] | |
| +/- gefitinib | PC3 cells | ↑ miR-603 | Growth arrest Apoptosis | [124] | |
| PC3 cells | Binding to H4 | Cell cycle arrest | [125] | ||
| Morin + paclitaxel | DU145, PC3 cells DU145 xenograft mice | ↓ miR-155 ↓ miR-155 | Apoptosis Suppression of PCa progression | [131] | |
| Tricin + docetaxel | PC3 cells | ↓ miR-21 | Inhibition proliferation | [133] | |
| Flavonols | Quercetin + hyperoside | PC3 cells | ↓ miR-21 | Apoptosis, cell cycle arrest and reduced invasive capacity | [139] |
| Flavanones | Silibinin | DU145, PC3 cells | ↓ EZH2, ↑ DNMT ↓ HDAC1, HDAC2 | Cell cycle arrest Apoptosis | [144] |
| Isoflavonoids | Genistein | LNCaP, LAPC-4 cells | ↓ DNMT1, DNMT3b | Inhibition proliferation | [152] |
| PC3, DU145 cells | ↑ miR-34a, ↓ HOTAIR | Cell cycle arrest Apoptosis Inibition proliferation | [153] | ||
| Catechins | EGCG | PC3 cells | ↓ DNMT | Cell growth inhibition | [165] |
| EGCG | LNCaP cells | ↓ HAT | Cell growth inhibition | [171] | |
| EGCG | LNCaP 22Rv1 cells 22Rv1 xenograft mice | ↓ AR ↓ AR, miR-21, ↑ miR-330 | Cell growth inhibition Suppression of PCa progression | [172] | |
| Polyphenon E | LNCaP, PC3 cells | ↓ HDAC1, HDAC2, HDAC3, HDAC8 ↑ acetylation H3 | Cell cycle arrest Apoptosis | [173] | |
| Polyphenon E | LNCaP cells | ↓ DNMT1 | Growth arrest | [174] | |
| Polyphenon E/EGCG | DUPRO, LNCaP cells Patients undergoing prostatectomy | ↓ HDAC1, EZH2 ↓ HDAC1 | Migration, invasion abrogation Suppression of PCa progression | [178] | |
| Anthocyanidins | Delphinidin | LNCaP cell lines | ↓ HDAC3 | apoptosis | [181] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, S.; Naponelli, V.; Bettuzzi, S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12, 1010. https://doi.org/10.3390/nu12041010
Izzo S, Naponelli V, Bettuzzi S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients. 2020; 12(4):1010. https://doi.org/10.3390/nu12041010
Chicago/Turabian StyleIzzo, Simona, Valeria Naponelli, and Saverio Bettuzzi. 2020. "Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention" Nutrients 12, no. 4: 1010. https://doi.org/10.3390/nu12041010
APA StyleIzzo, S., Naponelli, V., & Bettuzzi, S. (2020). Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients, 12(4), 1010. https://doi.org/10.3390/nu12041010







