Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet
Abstract
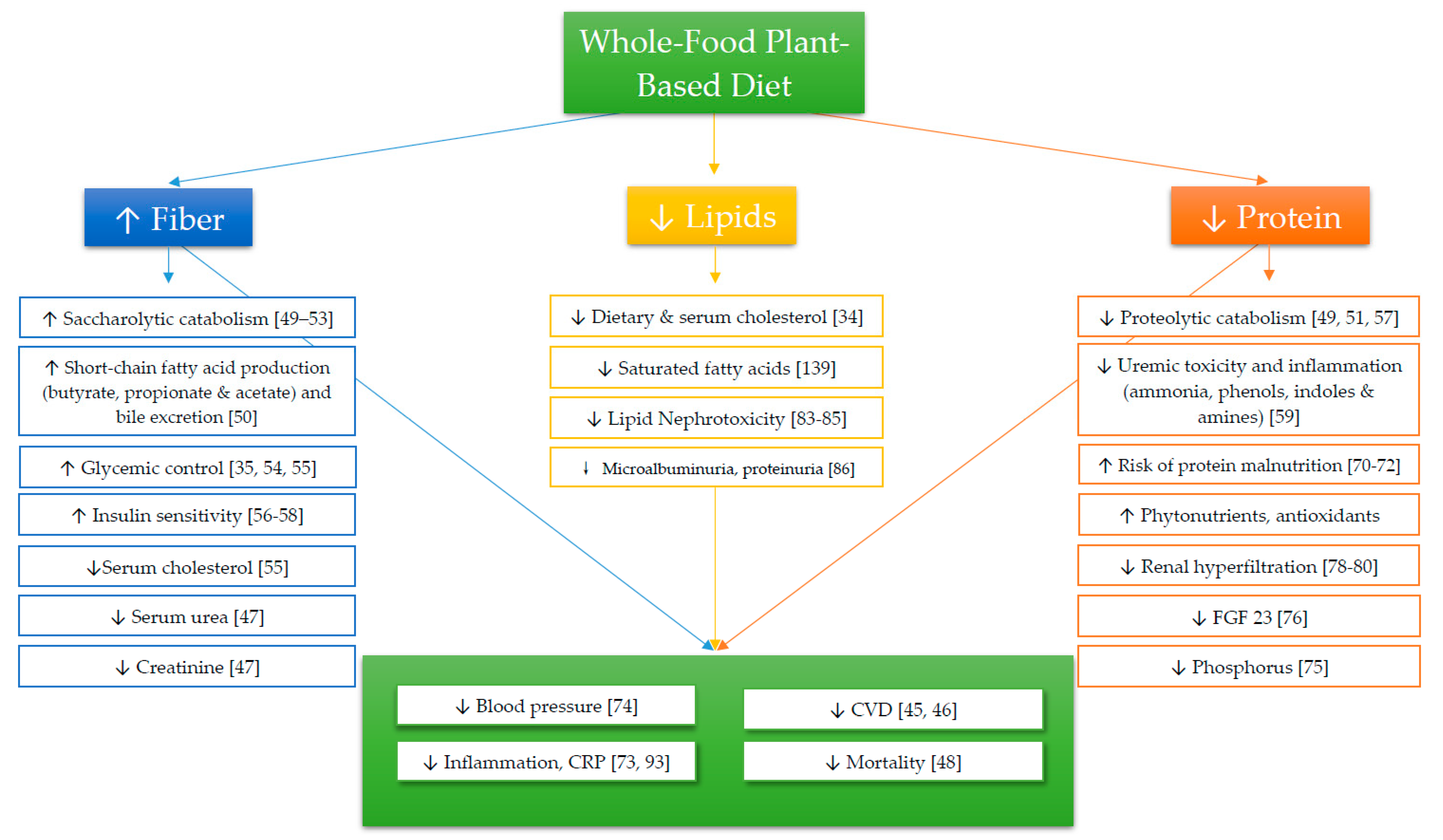
1. Introduction
2. Carbohydrates
Fiber
3. Protein
4. Lipids
4.1. Cholesterol
4.2. Omega-3 Fatty Acids
5. Inflammation
6. Acidity and Alkalinity of Foods
× dietary phosphorus (mg) − 0.0205 × dietary potassium (mg)
− 0.0125 × calcium (mg) − 0.0263 × magnesium (mg)
7. Electrolyte Balance
7.1. Hyperkalemia
7.2. Hyperphosphatemia
7.3. Hypocalcemia
7.4. Hypernatremia
8. Potential Drawbacks to a Plant-Based Diet
8.1. Vitamins and Minerals
8.2. Calorie Consumption
8.3. Accessibility
9. Conclusions
10. Literature Search
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018, 71 (Suppl. S1), A7. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G.; et al. The State of US Health, 1990-2010: Burden of Diseases, Injuries, and Risk Factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact. Sheet, 2017; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017.
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095. [Google Scholar] [CrossRef]
- Honeycutt, A.A.; Segel, J.E.; Zhuo, X.; Hoerger, T.J.; Imai, K.; Williams, D. Medical Costs of CKD in the Medicare Population. J. Am. Soc. Nephrol. 2013, 24, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimburger, O.; Paultre, F.; Diczfalusy, U. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M. Souto Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- Bailey, J.L. Metabolic acidosis: An unrecognized cause of morbidity in the patient with chronic kidney disease. Kidney Int. 2005, 68, S15–S23. [Google Scholar] [CrossRef]
- Chen, W.; Abramowitz, M.K. Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol. 2014, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Goraya_CKD Metabolic Acidosis_ACKD_2016.pdf. Powered By Box. Available online: https://baylor.app.box.com/file/315804092410 (accessed on 10 September 2018).
- Kraut, J.A.; Madias, N.E. Metabolic Acidosis of CKD: An Update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L. Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef]
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014. JAMA 2016, 316, 602. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 24. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA J. Am. Med. Assoc. 2003, 289, 2560. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J. Temporal Trends in the Prevalence of Diabetic Kidney Disease in the United States. JAMA J. Am. Med. Assoc. 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A.L.; Kopple, J.D. Vitamin Status and Needs for People with Stages 3-5 Chronic Kidney Disease. J. Ren. Nutr. 2011, 21, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W. The Effects of Dietary Protein Restriction and Blood-Pressure Control on the Progression of Chronic Renal Disease. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Chan, M.; Kelly, J.; Tapsell, L. Dietary Modeling of Foods for Advanced CKD Based on General Healthy Eating Guidelines: What Should Be on the Plate? Am. J. Kidney Dis. 2017, 69, 436–450. [Google Scholar] [CrossRef]
- Campbell, T.C.; Campbell II, T.M. The China Study; Revised and Expanded Edition; BenBella Books, Inc.: Dallas, TX, USA, 2016. [Google Scholar]
- McDougall, J.; Thomas, L.E.; McDougall, C.; Moloney, G. Effects of 7 days on an ad libitum low-fat vegan diet: The McDougall Program cohort. Nutr. J. 2014, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Jr, C.B.E. Prevent and Reverse Heart Disease: The Revolutionary, Scientifically Proven, Nutrition-Based Cure, 1st ed.; Avery: New York, NY, USA, 2008; ISBN 978-1-58333-300-6. [Google Scholar]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional Update for Physicians: Plant-Based Diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- The BROAD study: A Randomised Controlled Trial Using a Whole Food Plant-Based diet in the Community for Obesity, Ischaemic Heart Disease or Diabetes. Nutrition & Diabetes. Available online: https://www.nature.com/articles/nutd20173 (accessed on 1 October 2018).
- Greger, M.; Stone, G. How Not To Die: Discover the Foods Scientifically Proven to Prevent and Reverse Disease; Flatiron Books: New York, NY USA, 2015; ISBN 978-1-250-06611-4. [Google Scholar]
- Parish, R.C.; Miller, L.J. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf. 1992, 7, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Wojcicka, G.; Jamroz-Wisniewska, A. Adverse effects of statins-mechanisms and consequences. Curr. Drug Saf. 2009, 4, 209–228. [Google Scholar] [CrossRef]
- Chiu, Y.-W.; Teitelbaum, I.; Misra, M.; de Leon, E.M.; Adzize, T.; Mehrotra, R. Pill Burden, Adherence, Hyperphosphatemia, and Quality of Life in Maintenance Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1089–1096. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Anderson, J.W.; Ward, K. High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. Am. J. Clin. Nutr. 1979, 32, 2312–2321. [Google Scholar] [CrossRef]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses’ Health Study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [CrossRef]
- Steffen, L.M.; Jacobs, D.R.; Stevens, J.; Shahar, E. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) study. Am. J. Clin. Nutr. 2003, 78, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Hyre, A.D.; Pedersen, B.; Yi, Y. Effect of dietary fiber intake on blood pressure: A meta-analysis of randomized, controlled clinical trials. J. Hypertens. 2005, 23, 475. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Aromaa, A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D.; Arnault, N.; Bertrais, S.; Planells, R.; Clero, E.; Hercberg, S.; Boutron-Ruault, M.-C. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am. J. Clin. Nutr. 2005, 82, 1185–1194. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Hoy, M.; Goldman, J. Fiber Intake of the U.S. Population: What We Eat in America; NHANES 2009–2010 Food Surveys Research Group Dietary Data; United States Department of Agriculture: Washington, DC, USA, 2014.
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th, ed.; United States Department of Agriculture: Washington, DC, USA, December 2015; 144p.
- Hajishafiee, M.; Saneei, P.; Benisi-Kohansal, S.; Esmaillzadeh, A. Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. Camb. 2016, 116, 343–352. [Google Scholar] [CrossRef]
- North, C.J.; Venter, C.S.; Jerling, J.C. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur. J. Clin. Nutr. 2009, 63, 921–933. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. Lond. 2015, 69, 761–768. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G. What Would You Like to Eat, Mr CKD Microbiota? A Mediterranean Diet, please. Kidney Blood Press. Res. 2014, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, E.; Kolida, S.; Yaqoob, P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients 2017, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.K.I.; Meijers, B.K.I.; Bammens, B.R.M. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009, 76, S12–S19. [Google Scholar] [CrossRef]
- Cummings, J.H. Fermentation in the human large intestine: Evidence and implications for health. Lancet 1983, 1, 1206. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef]
- Biörklund, M.; van Rees, A.; Mensink, R.P.; Önning, G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with β -glucans from oats or barley: A randomised dose-controlled trial. Eur. J. Clin. Nutr. 2005, 59, 1272–1281. [Google Scholar] [CrossRef]
- Vuksan, V.; Sievenpiper, J.L.; Owen, R.; Swilley, J.A.; Spadafora, P.; Jenkins, D.J.; Vidgen, E.; Brighenti, F.; Josse, R.G.; Leiter, L.A.; et al. Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: Results of a controlled metabolic trial. Diabetes Care 2000, 23, 9–14. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, J.; Ferreri, S. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188. [Google Scholar] [CrossRef]
- Garcia, A.L.; Otto, B.; Reich, S.-C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Möhlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claus, D.; Geypens, B.; Hiele, M.; Geboes, K.; Rutgeerts, P.; Ghoos, Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G935–G943. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; O’Neil, C.E.; Greenway, F.L. Dietary fiber and satiety: The effects of oats on satiety. Nutr. Rev. 2016, 74, 131–147. [Google Scholar] [CrossRef]
- Wu, M.-J.; Chang, C.-S.; Cheng, C.-H.; Chen, C.-H. Colonic transit time in long-term dialysis patients. Am. J. Kidney Dis. 2004, 44, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial Effects of High Dietary Fiber Intake in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar] [CrossRef]
- Frank, H.; Graf, J.; Amann-Gassner, U.; Bratke, R.; Daniel, H.; Heemann, U.; Hauner, H. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. Am. J. Clin. Nutr. 2009, 90, 1509–1516. [Google Scholar] [CrossRef]
- Rosenvinge Skov, A.; Toubro, S.; Bülow, J.; Krabbe, K.; Parving, H.-H.; Astrup, A. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int. J. Obes. 1999, 23, 1170–1177. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Lakatua, J.D.; Ma, J.Z.; Louis, T.A. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1998, 31, 954. [Google Scholar] [CrossRef]
- Beto, J.A.; Bansal, V.K. Medical nutrition therapy in chronic kidney failure: Integrating clinical practice guidelines. J. Am. Diet. Assoc. 2004, 104, 404–409. [Google Scholar] [CrossRef]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 37, S66–S70. [Google Scholar] [CrossRef]
- Mak, R.H.; Cheung, W. Energy homeostasis and cachexia in chronic kidney disease. Pediatr. Nephrol. 2006, 21, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E. Malnutrition: A frequent misdiagnosis for hemodialysis patients. J. Clin. Invest. 2002, 110, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, E.G.; Lew, N.L. Death Risk in Hemodialysis Patients: The Predictive Value of Commonly Measured Variables and an Evaluation of Death Rate Differences Between Facilities. Am. J. Kidney Dis. 1990, 15, 458–482. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef]
- Elliott, P.; Stamler, J.; Dyer, A.R.; Appel, L. Association between protein intake and blood pressure—The INTERMAP study. Arch. Intern. Med. 2006, 166, 79–87. [Google Scholar] [CrossRef]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 257–264. [Google Scholar] [CrossRef]
- Seiler, S.; Heine, G.H.; Fliser, D. Clinical relevance of FGF-23 in chronic kidney disease. Kidney Int. 2009, 76, S34–S42. [Google Scholar] [CrossRef]
- Evenepoel, P.; Meijers, B.K. Dietary fiber and protein: Nutritional therapy in chronic kidney disease and beyond. Kidney Int. 2012, 81, 227–229. [Google Scholar] [CrossRef]
- Simon, A.H.; Lima, P.R.; Almerinda, M.; Alves, V.F.; Bottini, P.V.; de Faria, J.B. Renal haemodynamic responses to a chicken or beef meal in normal individuals. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 1998, 13, 2261–2264. [Google Scholar] [CrossRef]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takasawa, M.; Kasahara, S.; Tsuda, A.; Momotsu, T.; Ito, S.; Shibata, A. Effects of acute protein loads of different sources on renal function of patients with diabetic nephropathy. Tohoku J. Exp. Med. 1989, 159, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kimmelstiel, P.; Wilson, C. Intercapillary Lesions in the Glomeruli of the Kidney. Am. J. Pathol. 1936, 12, 83–98.7. [Google Scholar] [PubMed]
- Virchow, R. Cellular Pathology as Based Upon Physiological and Pathological Histology...; J. B. Lippincott & Co.: Philadelphia, PA, USA, 1863. [Google Scholar]
- Gyebi, L.; Soltani, Z.; Reisin, E. Lipid nephrotoxicity: New concept for an old disease. Curr. Hypertens. Rep. 2012, 14, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, J.F.; El-Nahas, M.; Chan, M.K.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982, 320, 1309–1311. [Google Scholar] [CrossRef]
- Hartroft, W.S. Fat emboli in glomerular capillaries of choline-deficient rats and of patients with diabetic glomerulosclerosis. Am. J. Pathol. 1955, 31, 381. [Google Scholar]
- Lin, J.; Hu, F.B.; Curhan, G.C. Associations of diet with albuminuria and kidney function decline. Clin. J. Am. Soc. Nephrol. 2010, 5, 836–843. [Google Scholar] [CrossRef]
- Bowden, R.G.; Wilson, R.L.; Deike, E.; Gentile, M. Fish Oil Supplementation Lowers C-Reactive Protein Levels Independent of Triglyceride Reduction in Patients With End-Stage Renal Disease. Nutr. Clin. Pract. 2009, 24, 508–512. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Bucher, H.C.; Hengstler, P.; Schindler, C.; Meier, G. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am. J. Med. 2002, 112, 298–304. [Google Scholar] [CrossRef]
- Mirfatahi, M.; Imani, H.; Tabibi, H.; Nasrollahi, A. Effects of Flaxseed Oil on Serum Bone Turnover Markers in Hemodialysis Patients A Randomized Controlled Trial. Iran. J. Kidney Dis. 2018, 12, 215–222. [Google Scholar] [PubMed]
- Nettleton, J.A. Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet. Assoc. 1991, 91, 331–337. [Google Scholar] [PubMed]
- Boekholdt, S.M.; Hack, C.E.; Sandhu, M.S.; Luben, R.; Bingham, S.A.; Wareham, N.J.; Peters, R.J.G.; Jukema, J.W.; Day, N.E.; Kastelein, J.J.P.; et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: The EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis 2006, 187, 415–422. [Google Scholar] [CrossRef]
- King, D.E.; Egan, B.M.; Geesey, M.E. Relation of dietary fat and fiber to elevation of C-reactive protein. Am. J. Cardiol. 2003, 92, 1335–1339. [Google Scholar] [CrossRef]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J. The Associations of Plant Protein Intake With All-Cause Mortality in CKD. Am. J. Kidney Dis. 2016, 67, 423–430. [Google Scholar] [CrossRef]
- Kelley, D.S.; Rasooly, R.; Jacob, R.A.; Kader, A.A.; Mackey, B.E. Consumption of Bing Sweet Cherries Lowers Circulating Concentrations of Inflammation Markers in Healthy Men and Women. J. Nutr. 2006, 136, 981–986. [Google Scholar] [CrossRef]
- Ajani, U.A.; Ford, E.S.; Mokdad, A.H. Dietary Fiber and C-Reactive Protein: Findings from National Health and Nutrition Examination Survey Data. J. Nutr. 2004, 134, 1181–1185. [Google Scholar] [CrossRef]
- Lefevre, M.; Jonnalagadda, S. Effect of whole grains on markers of subclinical inflammation. Nutr. Rev. 2012, 70, 387–396. [Google Scholar] [CrossRef]
- McGraw, N.J.; Krul, E.S.; Grunz-Borgmann, E.; Parrish, A.R. Soy-based renoprotection. World J. Nephrol. 2016, 5, 233–257. [Google Scholar] [CrossRef]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardio-renal indices and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Siefker, K.; DiSilvestro, R.A. Safety and antioxidant effects of a modest soy protein intervention in hemodialysis patients. J. Med. Food 2006, 9, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Miraghajani, M.S.; Esmaillzadeh, A.; Najafabadi, M.M.; Mirlohi, M.; Azadbakht, L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care 2012, 35, 1981–1985. [Google Scholar] [CrossRef] [PubMed]
- Fanti, P.; Asmis, R.; Stephenson, T.J.; Sawaya, B.P.; Franke, A.A. Positive effect of dietary soy in ESRD patients with systemic inflammation—Correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol. Dial. Transplant. 2006, 21, 2239–2246. [Google Scholar] [CrossRef]
- Maxwell, M.H.; Kleeman, C.R. (Eds.) Clinical Disorders of Fluid and Electrolyte Metabolism; McGraw-Hill Publishing Co. Ltd.: London, UK, 1962. [Google Scholar]
- Lennon, E.J.; Lemann, J.; Litzow, J.R. The effects of diet and stool composition on the net external acid balance of normal subjects. J. Clin. Invest. 1966, 45, 1601–1607. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Morris Jr, R.C.; Sebastian, A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am. J. Physiol. Ren. Physiol. 2007, 293, F521–F525. [Google Scholar] [CrossRef]
- Remer, T. Influence of nutrition on acid-base balance—Metabolic aspects. Eur. J. Nutr. 2001, 40, 214–220. [Google Scholar] [CrossRef]
- Barsotti, G.; Morelli, E.; Cupisti, A.; Meola, M. A Low-Nitrogen Low-Phosphorus Vegan Diet for Patients with Chronic Renal Failure. Nephron 1996, 74, 390–394. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential Renal Acid Load of Foods and its Influence on Urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Khosravi, H.; Azizi, F. Associations between dietary acid-base load and cardiometabolic risk factors in adults: The tehran lipid and glucose study. Endocrinol. Metab. 2015, 30, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kanda, E.; Ai, M.; Kuriyama, R.; Yoshida, M. Dietary Acid Intake and Kidney Disease Progression in the Elderly. Am. J. Nephrol. 2014, 39, 145–152. [Google Scholar] [CrossRef]
- Cosgrove, K.; Johnston, C.S. Examining the Impact of Adherence to a Vegan Diet on Acid-Base Balance in Healthy Adults. Plant. Foods Hum. Nutr. 2017, 72, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.M.; Zhan, M.; Hsu, V.D.; Walker, L.D.; Moen, M.F.; Seliger, S.L.; Weir, M.R.; Fink, J.C. The Frequency of Hyperkalemia and Its Significance in Chronic Kidney Disease. Arch. Intern. Med. 2009, 169, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.J.; Donnelly, S.M.; Ethier, J.H.; Quaggin, S.E.; Kaiser, U.B.; Vasuvattakul, S.; Kamel, K.S.; Halperin, M.L. Modulation of the secretion of potassium by accompanying anions in humans. Kidney Int. 1991, 39, 1206–1212. [Google Scholar] [CrossRef]
- FGF23—Clinical: Fibroblast Growth Factor 23 (FGF23), Plasma. Available online: https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/88662 (accessed on 1 October 2018).
- Larsson, T.; Nisbeth, U.L.F.; Ljunggren, Ö.; Jüppner, H.; Jonsson, K.B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003, 64, 2272–2279. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Bioavailable dietary phosphate, a mediator of cardiovascular disease, may be decreased with plant-based diets, phosphate binders, niacin, and avoidance of phosphate additives. Nutr. Burbank Los Angel. Cty. Calif 2014, 30, 739–747. [Google Scholar] [CrossRef]
- McCann, L. KDIGO Guidelines for Nutritional Management of CKD; Kidney Disease Improving Global Outcomes: Brussels, Belgium, 2019. [Google Scholar]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Shantouf, R.; Budoff, M.J.; Ahmadi, N.; Tiano, J.; Flores, F.; Kalantar-Zadeh, K. Effects of Sevelamer and Calcium-Based Phosphate Binders on Lipid and Inflammatory Markers in Hemodialysis Patients. Am. J. Nephrol. 2008, 28, 275–279. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Armstrong, C.L.H.; Janda, K.; Ponsler-Sipes, K. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2015, 40, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Porres, J.M. Phytase enzymology, applications, and biotechnology. Biotechnol. Lett. 2003, 25, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Malatesta, K.; Norris, K. Vitamin D and Chronic Kidney Disease. Ethn. Dis. 2009, 19 (Suppl. S5), S5. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Scheingraber, S.; Rehm, M.; Sehmisch, C.; Finsterer, U. Rapid Saline Infusion Produces Hyperchloremic Acidosis in Patients Undergoing Gynecologic Surgery. Anesthesiol. J. Am. Soc. Anesthesiol. 1999, 90, 1265–1270. [Google Scholar] [CrossRef]
- He, J.; Shen, X.; Fang, A.; Song, J.; Li, H.; Guo, M.; Li, K. Association between predominantly plant-based diets and iron status in Chinese adults: A cross-sectional analysis. Br. J. Nutr. Camb. 2016, 116, 1621–1632. [Google Scholar] [CrossRef]
- Savva, S.C.; Kafatos, A. Is Red Meat Required for the Prevention of Iron Deficiency Among Children and Adolescents? Curr. Pediatr. Rev. 2014, 10, 177–183. [Google Scholar] [CrossRef]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- Larsson, C.L.; Johansson, G.K. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am. J. Clin. Nutr. 2002, 76, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, S1627–S1633. [Google Scholar] [CrossRef] [PubMed]
- Inc, G. Snapshot: Few Americans Vegetarian or Vegan. Available online: https://news.gallup.com/poll/238328/snapshot-few-americans-vegetarian-vegan.aspx (accessed on 25 October 2018).
- Banerjee, T.; Liu, Y.; Crews, D.C. Dietary Patterns and CKD Progression. Blood Purif. 2016, 41, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Dharmarajan, S. Food Insecurity, CKD, and Subsequent ESRD in US Adults. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 70, 38. [Google Scholar] [CrossRef]
- Attini, R.; Leone, F.; Parisi, S.; Fassio, F. Vegan-vegetarian low-protein supplemented diets in pregnant CKD patients: Fifteen years of experience. BMC Nephrol. 2016, 17, 132. [Google Scholar] [CrossRef]
| GFR Category | Description | Estimated GFR (mL/min/1.73 m2) |
|---|---|---|
| Stage 1 | Normal or high | > 90 |
| Stage 2 | Mildly decreased | 60–89 |
| Stage 3a | Mildly to moderately decreased | 45–59 |
| Stage 3b | Moderately to severely decreased | 30–44 |
| Stage 4 | Severely decreased | 15–29 |
| Stage 5 | Kidney Failure | <15 |
| Category | Food | PRAL (per 100 g) |
|---|---|---|
| Fruits | Apples | −1.9 |
| Bananas | −6.9 | |
| Lemons | −2.3 | |
| Medjool Dates | −13.7 | |
| Oranges | −3.6 | |
| Vegetables | Broccoli | −4.0 |
| Carrots | −5.7 | |
| Kale | −8.3 | |
| Spinach | −11.8 | |
| Sweet Potato | −5.6 | |
| Grains | Brown Rice | 7.5 |
| Oats | 13.3 | |
| Spaghetti (white) | 7.3 | |
| White Bread | 6.0 | |
| Legumes | Garbanzo Beans (Chickpeas) | 0.3 |
| Kidney Beans | −8.4 | |
| Lentils | 5.4 | |
| Peanuts | 6.2 | |
| Soybeans | −4.7 | |
| Tofu (raw) | −0.3 | |
| White Beans | −23.2 | |
| Nuts and Seeds | Almonds | 2.3 |
| Flaxseeds | 2.1 | |
| Pecans | 2.1 | |
| Sunflower Seeds | 12.1 | |
| Fats | Butter | 0.6 |
| Corn Oil | 0 | |
| Margarine | −0.5 | |
| Olive Oil | 0 | |
| Meats | Beef (Lean) | 8.7 |
| Chicken | 13.2 | |
| Fish (average) | 7.9 | |
| Turkey | 9.0 | |
| Dairy and Eggs | Cottage Cheese | 23.4 |
| Egg (Whole) | 0.6 | |
| Hard cheese | 34.2 | |
| Ice Cream | 28.7 | |
| Whole Milk | 1.2 | |
| Whole Milk Yogurt | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adair, K.E.; Bowden, R.G. Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients 2020, 12, 1007. https://doi.org/10.3390/nu12041007
Adair KE, Bowden RG. Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients. 2020; 12(4):1007. https://doi.org/10.3390/nu12041007
Chicago/Turabian StyleAdair, Kathleen E., and Rodney G. Bowden. 2020. "Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet" Nutrients 12, no. 4: 1007. https://doi.org/10.3390/nu12041007
APA StyleAdair, K. E., & Bowden, R. G. (2020). Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients, 12(4), 1007. https://doi.org/10.3390/nu12041007






