A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought
Abstract
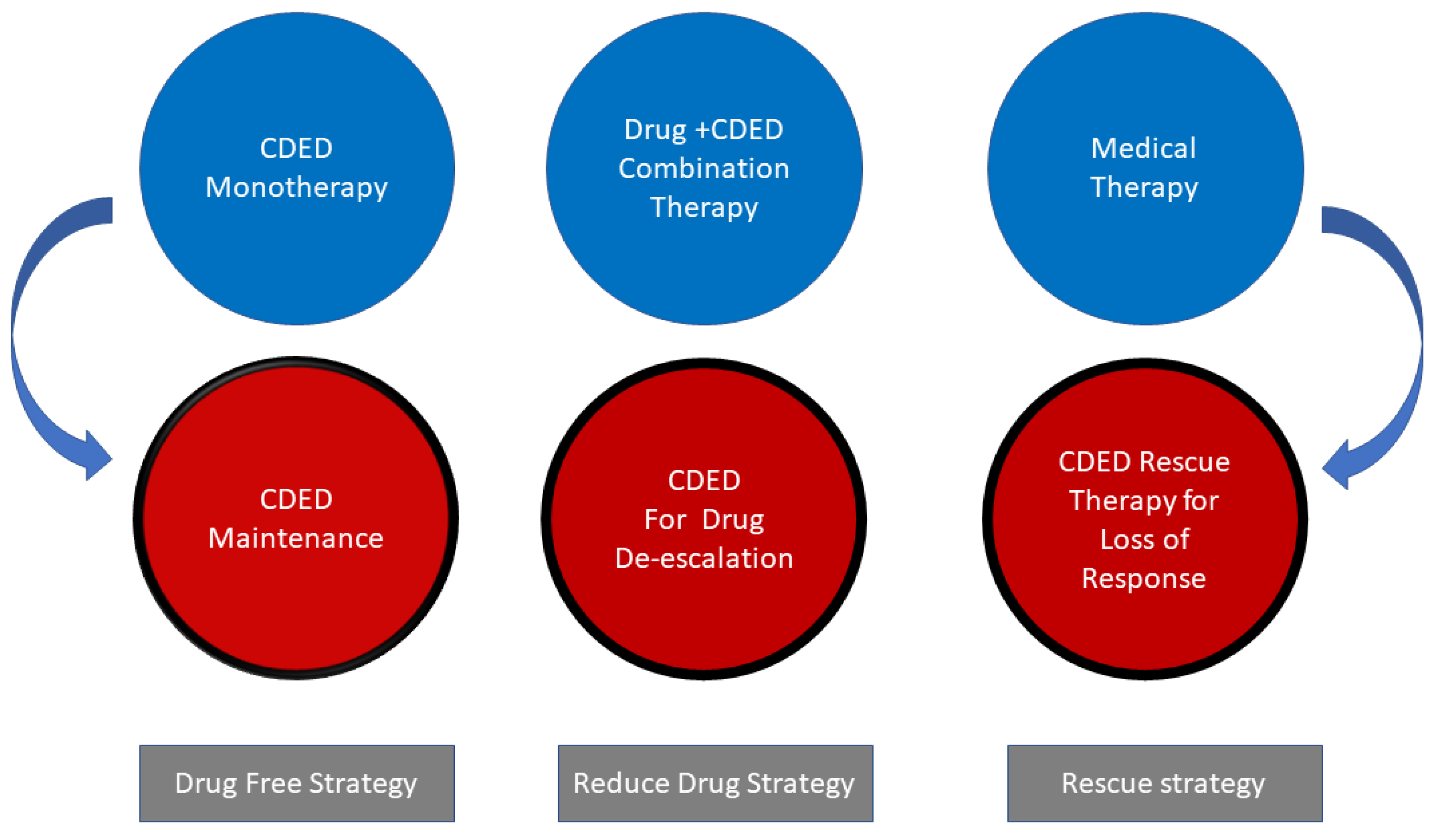
1. Introduction
2. Principles of the Crohn’s Disease Exclusion Diet
Dr. Wael El-Matary: Dr Levine, how would you Describe the Crohn’s Disease Exclusion Diet in a Nutshell?
3. How Effective is the Diet?
3.1. Dr. Wael El-Matary, Editor
3.1.1. Case 1
3.1.2. Case 2
3.2. Dr. Wael El-Matary, Editor
Case 3
3.3. Dr Wael El-Matary, Editor
Case 4
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Hakansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, W. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Dolev, N.; Sladek, M.; Hussey, S.; Turner, D.; Veres, G.; Koletzko, S.; de Carpi, J.M.; Staiano, A.; Shaoul, R.; Lionetti, P.; et al. Differences in Outcomes Over Time With Exclusive Enteral Nutrition Compared With Steroids in Children With Mild to Moderate Crohn’s Disease: Results From the GROWTH CD Study. J. Crohns Colitis 2018, 12, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Dunn, K.A.; Allott, J.; Bandsma, R.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; Van Limbergen, J. The relationship between fecal bile acids and microbiome community structure in pediatric Crohn’s disease. ISME J. 2020, 14, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Basseri, S.; Grant, A.; Giffin, N.; Mahdi, G.; Noble, A.; Rashid, M.; Otley, A.; Van Limbergen, J. Exclusive Enteral Nutrition Therapy in Paediatric Crohn’s Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis. J. Crohns Colitis 2017, 11, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy With the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohns Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Hansen, A.K.; Heitmann, B.L. Potential impact of diet on treatment effect from anti-TNF drugs in inflammatory bowel disease. Nutrients 2017, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.L.; Palmer, L.B.; Nguyen, E.T.; McClave, S.A.; Martindale, R.G.; Bechtold, M.L. Specialized enteral nutrition therapy in Crohn’s disease patients on maintenance infliximab therapy: A meta-analysis. Therap. Adv. Gastroenterol. 2015, 8, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Ishihara, H.; Yada, S.; Esaki, M.; Ohwan, T.; Nozaki, R.; Ashizuka, S.; Inatsu, H.; Ohi, H.; Aoyagi, K.; et al. Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn’s disease in adults. Dig. Dis. Sci. 2013, 58, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Sazuka, S.; Katsuno, T.; Nakagawa, T.; Saito, M.; Matsumura, T.; Arai, M.; Sato, T.; Yokosuka, O. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn’s disease. Eur. J. Clin. Nutr. 2012, 66, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.N.; Falzon, L.; Li Ferry, S. Systematic review with meta-analysis: Enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 46, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Brulisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking cessation alters intestinal microbiota: Insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Takeda, T.; Takada, Y.; Kishi, M.; Beppu, T.; Takastu, N.; Miyaoka, M.; Hisabe, T.; Yao, K.; Ueki, T. Efficacy of enteral nutrition in patients with Crohn’s disease on maintenance anti-TNF-alpha antibody therapy: A meta-analysis. J. Gastroenterol. 2020, 55, 133–141. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levine, A.; El-Matary, W.; Van Limbergen, J. A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients 2020, 12, 880. https://doi.org/10.3390/nu12030880
Levine A, El-Matary W, Van Limbergen J. A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients. 2020; 12(3):880. https://doi.org/10.3390/nu12030880
Chicago/Turabian StyleLevine, Arie, Wael El-Matary, and Johan Van Limbergen. 2020. "A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought" Nutrients 12, no. 3: 880. https://doi.org/10.3390/nu12030880
APA StyleLevine, A., El-Matary, W., & Van Limbergen, J. (2020). A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients, 12(3), 880. https://doi.org/10.3390/nu12030880





