Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study
Abstract
1. Introduction
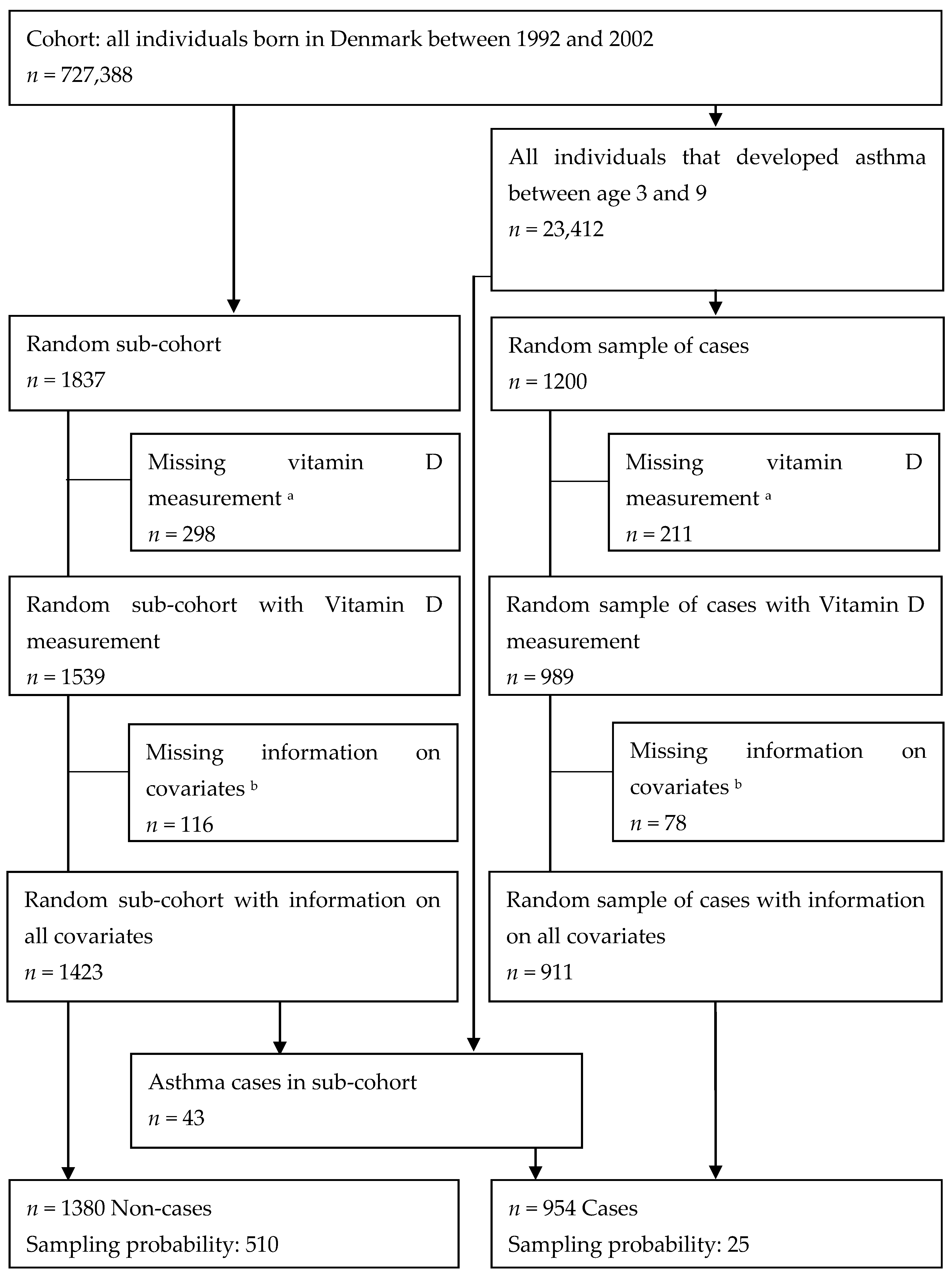
2. Methods
2.1. Data Sources
2.2. Study Population
2.3. Assessment of Vitamin D Status
2.4. Covariates
2.5. Statistical Analyses
2.6. Ethical Considerations
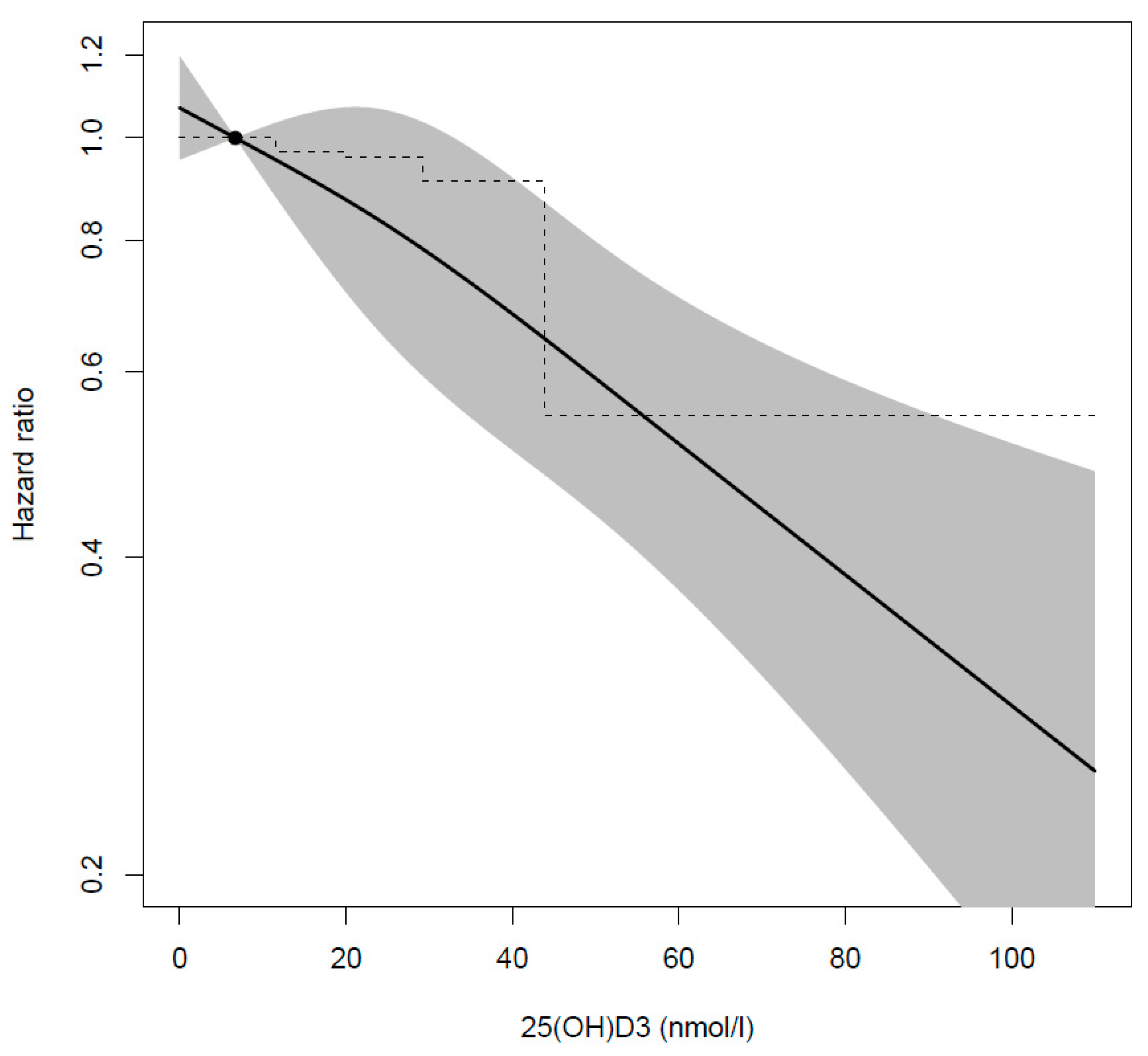
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lai, C.K.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S. Global variation in the prevalence and severity of asthma symptoms: Phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Drever, N.; Saade, G.R.; Bytautiene, E. Fetal programming: Early-life modulations that affect adult outcomes. Curr. Allergy Asthma Rep. 2010, 10, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Just, J.; Saint Pierre, P.; Amat, F.; Gouvis-Echraghi, R.; Lambert-Guillemot, N.; Guiddir, T.; Annesi Maesano, I. What lessons can be learned about asthma phenotypes in children from cohort studies? Pediatr. Allergy Immunol. 2015, 26, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Salle, B.L.; Delvin, E.E.; Lapillonne, A.; Bishop, N.J.; Glorieux, F.H. Perinatal metabolism of vitamin D. Am. J. Clin. Nutr. 2000, 71, 1317S–1324S. [Google Scholar] [CrossRef] [PubMed]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Baca, K.M.; Simhan, H.N.; Platt, R.W.; Bodnar, L.M. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann. Epidemiol. 2016, 26, 853–857.e851. [Google Scholar] [CrossRef]
- Zhang, M.X.; Pan, G.T.; Guo, J.F.; Li, B.Y.; Qin, L.Q.; Zhang, Z.L. Vitamin D Deficiency Increases the Risk of Gestational Diabetes Mellitus: A Meta-Analysis of Observational Studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef]
- Keller, A.; Stougard, M.; Frederiksen, P.; Thorsteinsdottir, F.; Vaag, A.; Damm, P.; Jacobsen, R.; Heitmann, B.L. In utero exposure to extra vitamin D from food fortification and the risk of subsequent development of gestational diabetes: The D-tect study. Nutr. J. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Mann, E.H.; Hornsby, E.; Chambers, E.S.; Chen, Y.H.; Rice, L.; Hawrylowicz, C.M. Vitamin D influences asthmatic pathology through its action on diverse immunological pathways. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 5), S314–S321. [Google Scholar] [CrossRef] [PubMed]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.M.; Arianto, L.; Wolsk, H.M.; Vinding, R.K.; Hallas, H.W.; et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: A randomized clinical trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Bacharier, L.B.; Sandel, M.; Iverson, R.E.; Macones, G.A.; et al. Effect of prenatal supplementation with vitamin d on asthma or recurrent wheezing in offspring by age 3 years: The vdaart randomized clinical trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef] [PubMed]
- Chawes, B.L.; Bonnelykke, K.; Jensen, P.F.; Schoos, A.M.; Heickendorff, L.; Bisgaard, H. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: The COPSAC2000 birth cohort study. PLoS ONE 2014, 9, e99856. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, A.; Hourihane, J.O.; Malvisi, L.; Irvine, A.D.; Kenny, L.C.; Murray, D.M.; Kiely, M.E. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork Baseline Birth Cohort Study. Allergy 2018. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 2008, 62, 68–77. [Google Scholar] [CrossRef]
- Morales, E.; Romieu, I.; Guerra, S.; Ballester, F.; Rebagliato, M.; Vioque, J.; Tardon, A.; Rodriguez Delhi, C.; Arranz, L.; Torrent, M.; et al. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology 2012, 23, 64–71. [Google Scholar] [CrossRef]
- Pedersen, C.B. The Danish Civil Registration System. Scand. J. Public Health 2011, 39, 22–25. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sorensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef]
- Norgaard-Pedersen, B.; Hougaard, D.M. Storage policies and use of the Danish Newborn Screening Biobank. J. Inherit. Metab. Dis. 2007, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Morley, R.; Anderson, C.; Ko, P.; Burne, T.; Permezel, M.; Mortensen, P.B.; Norgaard-Pedersen, B.; Hougaard, D.M.; McGrath, J.J. The utility of neonatal dried blood spots for the assessment of neonatal vitamin D status. Paediatr. Perinat. Epidemiol. 2010, 24, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Wisner, K.L.; Klebanoff, M.A. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br. J. Nutr. 2009, 101, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Anderson, C.; Ko, P.; Jones, A.; Thomas, A.; Burne, T.; Mortensen, P.B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; McGrath, J. A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin. Chim. Acta 2009, 403, 145–151. [Google Scholar] [CrossRef]
- Bliddal, M.; Broe, A.; Pottegard, A.; Olsen, J.; Langhoff-Roos, J. The Danish Medical Birth Register. Eur. J. Epidemiol. 2018, 33, 27–36. [Google Scholar] [CrossRef]
- Martinez, F.D.; Wright, A.L.; Taussig, L.M.; Holberg, C.J.; Halonen, M.; Morgan, W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N. Engl. J. Med. 1995, 332, 133–138. [Google Scholar] [CrossRef]
- Carter, G.D.; Berry, J.; Durazo-Arvizu, R.; Gunter, E.; Jones, G.; Jones, J.; Makin, H.L.J.; Pattni, P.; Sempos, C.T.; Twomey, P.; et al. Hydroxyvitamin D assays: An historical perspective from DEQAS. J. Steroid Biochem. Mol. Biol. 2018, 177, 30–35. [Google Scholar] [CrossRef]
- Prentice, R.L. A Case-Cohort Design for Epidemiologic Cohort Studies and Disease Prevention Trials. Biometrika 1986, 73, 1–11. [Google Scholar] [CrossRef]
- Gray, R.J. Weighted analyses for cohort sampling designs. Lifetime Data Anal. 2009, 15, 24–40. [Google Scholar] [CrossRef]
- Vasiliou, J.E.; Lui, S.; Walker, S.A.; Chohan, V.; Xystrakis, E.; Bush, A.; Hawrylowicz, C.M.; Saglani, S.; Lloyd, C.M. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy 2014, 69, 1380–1389. [Google Scholar] [CrossRef]
- Chen, L.; Wilson, R.; Bennett, E.; Zosky, G.R. Identification of vitamin D sensitive pathways during lung development. Respir. Res. 2016, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, E.; Pfeffer, P.E.; Laranjo, N.; Cruikshank, W.; Tuzova, M.; Litonjua, A.A.; Weiss, S.T.; Carey, V.J.; O’Connor, G.; Hawrylowicz, C. Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial. J. Allergy Clin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Hart, P.H.; Whitehouse, A.J.; Kusel, M.M.; Ang, W.; Foong, R.E.; Chen, L.; Holt, P.G.; Sly, P.D.; Hall, G.L. Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann. Am. Thorac. Soc. 2014, 11, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Huang, S.Y.; Peng, Y.C.; Tsai, M.H.; Hua, M.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr. Allergy Immunol. 2015, 26, 337–343. [Google Scholar] [CrossRef]
- Baiz, N.; Dargent-Molina, P.; Wark, J.D.; Souberbielle, J.C.; Annesi-Maesano, I. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J. Allergy Clin. Immunol. 2014, 133, 147–153. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr.; Ingham, T.; Wickens, K.; Thadhani, R.; Silvers, K.M.; Epton, M.J.; Town, G.I.; Pattemore, P.K.; Espinola, J.A.; Crane, J. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011, 127, e180–e187. [Google Scholar] [CrossRef]
- Rothers, J.; Wright, A.L.; Stern, D.A.; Halonen, M.; Camargo, C.A., Jr. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J. Allergy Clin. Immunol. 2011, 128, 1093–1099.e5. [Google Scholar] [CrossRef]
- Gazibara, T.; den Dekker, H.T.; de Jongste, J.C.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Reiss, I.K.; Franco, O.H.; Tiemeier, H.; Jaddoe, V.W.; et al. Associations of maternal and fetal 25-hydroxyvitamin D levels with childhood lung function and asthma: The Generation R Study. Clin. Exp. Allergy 2016, 46, 337–346. [Google Scholar] [CrossRef]
- Wills, A.K.; Shaheen, S.O.; Granell, R.; Henderson, A.J.; Fraser, W.D.; Lawlor, D.A. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clin. Exp. Allergy 2013, 43, 1180–1188. [Google Scholar] [CrossRef]
- Pike, K.C.; Inskip, H.M.; Robinson, S.; Lucas, J.S.; Cooper, C.; Harvey, N.C.; Godfrey, K.M.; Roberts, G.; Southampton Women’s Survey Study Group. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax 2012, 67, 950–956. [Google Scholar] [CrossRef]
- Gordon, C.M.; Feldman, H.A.; Sinclair, L.; Williams, A.L.; Kleinman, P.K.; Perez-Rossello, J.; Cox, J.E. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch. Pediatr. Adolesc. Med. 2008, 162, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fenger-Gron, J.; Fenger-Gron, M.; Blunck, C.H.; Schonemann-Rigel, H.; Wielandt, H.B. Low breastfeeding rates and body mass index in Danish children of women with gestational diabetes mellitus. Int. Breastfeed. J. 2015, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Crozier, S.R.; Dennison, E.M.; Davies, J.H.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Harvey, N.C. Tracking of 25-hydroxyvitamin D status during pregnancy: The importance of vitamin D supplementation. Am. J. Clin. Nutr. 2015, 102, 1081–1087. [Google Scholar] [CrossRef]
- Klahn, I.R. Fra Ro, Renlighed og Regelmæssighed til Barnets Naturlige Rytme; Københavns Universitet, Det Humanistiske Fakultet: København, Denmark, 2013. [Google Scholar]
- Brustad, N.; Eliasen, A.U.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H.; Chawes, B.L. High-Dose Vitamin D Supplementation during Pregnancy and Asthma in Offspring at the Age of 6 Years. Jama 2019, 321, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Williamson, E.J.; Ebeling, P.R.; Kvaskoff, D.; Eyles, D.W.; English, D.R. Measurements of 25-hydroxyvitamin D concentrations in archived dried blood spots are reliable and accurately reflect those in plasma. J. Clin. Endocrinol. Metab. 2014, 99, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmidt, S.A.J.; Adelborg, K.; Sundbøll, J.; Laugesen, K.; Ehrenstein, V.; Sørensen, H.T. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019, 11, 563–591. [Google Scholar] [CrossRef]
- Moth, G.; Vedsted, P.; Schiøtz, P.O. National registry diagnoses agree with medical records on hospitalized asthmatic children. Acta Pædiatr. 2007, 96, 1470–1473. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Polinski, K.J.; Liu, J.; Boghossian, N.S.; McLain, A.C. Maternal Obesity, Gestational Weight Gain, and Asthma in Offspring. Prev. Chronic Dis. 2017, 14. [Google Scholar] [CrossRef]
- Bendixen, H.; Holst, C.; Sorensen, T.I.; Raben, A.; Bartels, E.M.; Astrup, A. Major increase in prevalence of overweight and obesity between 1987 and 2001 among Danish adults. Obes. Res. 2004, 12, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.J.; Eyles, D.W.; Pedersen, C.B.; Anderson, C.; Ko, P.; Burne, T.H.; Norgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, P.B. Neonatal vitamin D status and risk of schizophrenia: A population-based case-control study. Arch. Gen. Psychiatry 2010, 67, 889–894. [Google Scholar] [CrossRef] [PubMed]
| Asthma Cases | Random Sub-Cohort † | P-Value | |
|---|---|---|---|
| N | 911 | 1423 | |
| 25(OH)D3 nmol/L median (Q1–Q3) | 23 (14–35) | 25 (14–40) | 0.16 |
| Sex n (%) | <0.000 | ||
| Girls | 328 (36) | 682 (48) | |
| Boys | 583 (64) | 741 (52) | |
| Season of birth n (%) | 0.15 | ||
| August–January | 459 (50) | 673 (47) | |
| February–July | 452 (50) | 750 (53) | |
| Preterm n (%) | 0.02 | ||
| Yes | 73 (8) | 84 (6) | |
| Caesarean section n (%) | 0.01 | ||
| Yes | 120 (13) | 139 (10) | |
| Birthweight in grams mean (SD) | 3490 (640) | 3517 (577) | 0.56 |
| Size for gestational age n (%) | 0.56 | ||
| Small for gestational age | 117 (13) | 167 (12) | |
| Normal for gestational age | 688 (76) | 1074 (76) | |
| Large for gestational age | 106 (12) | 182 (13) | |
| Parity n (%) | 0.86 | ||
| Primiparous | 390 (43) | 604 (43) | |
| Multiparous | 521 (57) | 819 (58) | |
| Maternal age in years mean (SD) | 28.8 (5) | 29.1 (5) | |
| Maternal ethnicity n (%) | 0.95 | ||
| European | 836 (92) | 1307 (92) | |
| Non-European | 75 (8) | 116 (8) | |
| Maternal education n (%) | 0.002 | ||
| School | 274 (30) | 342 (24) | |
| High school | 421 (46) | 748 (53) | |
| University | 216 (24) | 333 (23) | |
| Maternal smoking n (%) | 0.04 | ||
| Yes | 223 (25) | 297 (21) | |
| Missing | 136 (15) | 214 (15) | |
| Maternal asthma n (%) | <0.000 | ||
| Yes | 86 (9) | 53 (4) | |
| Paternal asthma n (%) | <0.000 | ||
| Yes | 53 (6) | 39 (3) |
| Unadjusted (n = 2334) | Adjusted ‡ (n = 2334) | Adjusted § (n = 1984) | |
|---|---|---|---|
| Quintiles limit, nmol/L | |||
| Q1 (0.0–11.6) | 1 (ref) | 1 (ref) | 1 (ref) |
| Q2 (11.6–20.0) | 0.93 (0.72, 1.20) | 0.97 (0.74, 1.28) | 0.90 (0.66, 1.23) |
| Q3 (20.0–29.3) | 1.00 (0.78, 1.28) | 0.96 (0.72, 1.28) | 0.94 (0.68, 1.30) |
| Q4 (29.3–43.9) | 0.97 (0.75, 1.25) | 0.91 (0.67, 1.23) | 0.82 (0.58, 1.14) |
| Q5 (43.9–110.8) | 0.61 (0.46, 0.80) | 0.55 (0.39, 0.77) | 0.51 (0.35, 0.75) |
| Wald test | 0.002 | 0.001 | 0.003 |
| Unadjusted (n = 2334) | Adjusted ‡ (n = 2334) | Adjusted § (n = 1984) | |
|---|---|---|---|
| Quintiles limit, nmol/L | |||
| Girls (n = 1010) | |||
| Q1 (0.0–11.6) | 1 (ref) | 1 (ref) | 1 (ref) |
| Q2 (11.6–20.0) | 0.88 (0.59, 1.31) | 0.88 (0.57, 1.38) | 0.84 (0.51, 1.39) |
| Q3 (20.0–29.3) | 1.07 (0.73, 1.56) | 1.11 (0.72, 1.71) | 0.96 (0.58, 1.59) |
| Q4 (29.3–43.9) | 0.95 (0.63, 1.42) | 0.94 (0.58, 1.54) | 0.86 (0.49, 1.50) |
| Q5 (43.9–110.8) | 0.58 (0.37, 0.91) | 0.62 (0.36, 1.07) | 0.52 (0.28, 0.96) |
| Boys (n = 1324) | |||
| Q1 (0.0–11.6) | 1 (ref) | 1 (ref) | 1 (ref) |
| Q2 (11.6–20.0) | 0.96 (0.69, 1.33) | 1.05 (0.73, 1.51) | 0.97 (0.65, 1.45) |
| Q3 (20.0–29.3) | 0.97 (0.69, 1.36) | 0.93 (0.63, 1.38) | 1.02 (0.66, 1.58) |
| Q4 (29.3–43.9) | 0.93 (0.67, 1.29) | 0.93 (0.63, 1.37) | 0.84 (0.55, 1.29) |
| Q5 (43.9–110.8) | 0.60 (0.42, 0.85) | 0.53 (0.34, 0.83) | 0.54 (0.33, 0.88) |
| February–July (n = 1302) | |||
| Q1 (0.0–11.6) | 1 (ref) | 1 (ref) | 1 (ref) |
| Q2 (11.6–20.0) | 0.94 (0.66, 1.34) | 0.92 (0.63, 1.35) | 0.83 (0.53, 1.28) |
| Q3 (20.0–29.3) | 1.06 (0.75, 1.51) | 0.98 (0.66, 1.44) | 0.98 (0.63, 1.53) |
| Q4 (29.3–43.9) | 1.21 (0.85, 1.72) | 1.17 (0.80, 1.72) | 1.06 (0.69, 1.63) |
| Q5 (43.9–110.8) | 0.72 (0.49, 1.04) | 0.66 (0.43, 0.99) | 0.63 (0.40, 1.00) |
| August–January (n = 1226) | |||
| Q1 (0.0–11.6) | 1 (ref) | 1 (ref) | 1 (ref) |
| Q2 (11.6–20.0) | 0.90 (0.63, 1.30) | 1.00 (0.67, 1.50) | 0.97 (0.63, 1.51) |
| Q3 (20.0–29.3) | 0.92 (0.64, 1.32) | 0.97 (0.64, 1.47) | 0.91 (0.58, 1.43) |
| Q4 (29.3–43.9) | 0.75 (0.52, 1.09) | 0.81 (0.54, 1.21) | 0.71 (0.46, 1.11) |
| Q5 (43.9–110.8) | 0.50 (0.34, 0.74) | 0.51 (0.33, 0.79) | 0.48 (0.30, 0.78) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsteinsdottir, F.; Cardoso, I.; Keller, A.; Stougaard, M.; Frederiksen, P.; Cohen, A.S.; Maslova, E.; Jacobsen, R.; Backer, V.; Heitmann, B.L. Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study. Nutrients 2020, 12, 842. https://doi.org/10.3390/nu12030842
Thorsteinsdottir F, Cardoso I, Keller A, Stougaard M, Frederiksen P, Cohen AS, Maslova E, Jacobsen R, Backer V, Heitmann BL. Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study. Nutrients. 2020; 12(3):842. https://doi.org/10.3390/nu12030842
Chicago/Turabian StyleThorsteinsdottir, Fanney, Isabel Cardoso, Amélie Keller, Maria Stougaard, Peder Frederiksen, Arieh Sierra Cohen, Ekaterina Maslova, Ramune Jacobsen, Vibeke Backer, and Berit Lilienthal Heitmann. 2020. "Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study" Nutrients 12, no. 3: 842. https://doi.org/10.3390/nu12030842
APA StyleThorsteinsdottir, F., Cardoso, I., Keller, A., Stougaard, M., Frederiksen, P., Cohen, A. S., Maslova, E., Jacobsen, R., Backer, V., & Heitmann, B. L. (2020). Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study. Nutrients, 12(3), 842. https://doi.org/10.3390/nu12030842






