Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis
Abstract
1. Introduction
1.1. Human Microbiota and Reproductive Impact
1.2. Dietary Nutritional Supplements: Probiotics and Nutraceuticals
1.3. Probiotics Administered in Fertility Dysbiosis
1.4. Type of Administration and Site of Colonisation of Probiotics
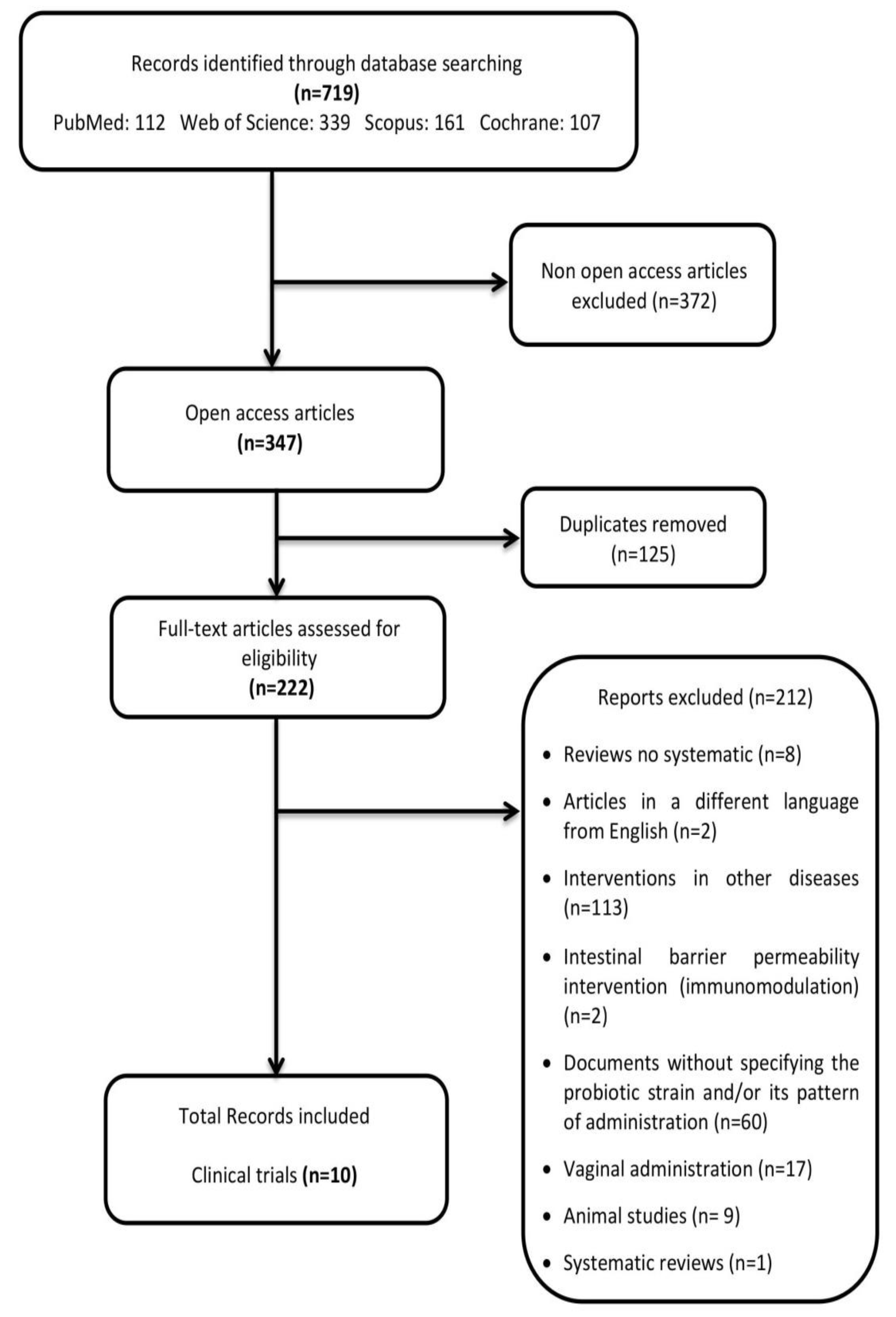
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
Data Analysis and Extraction
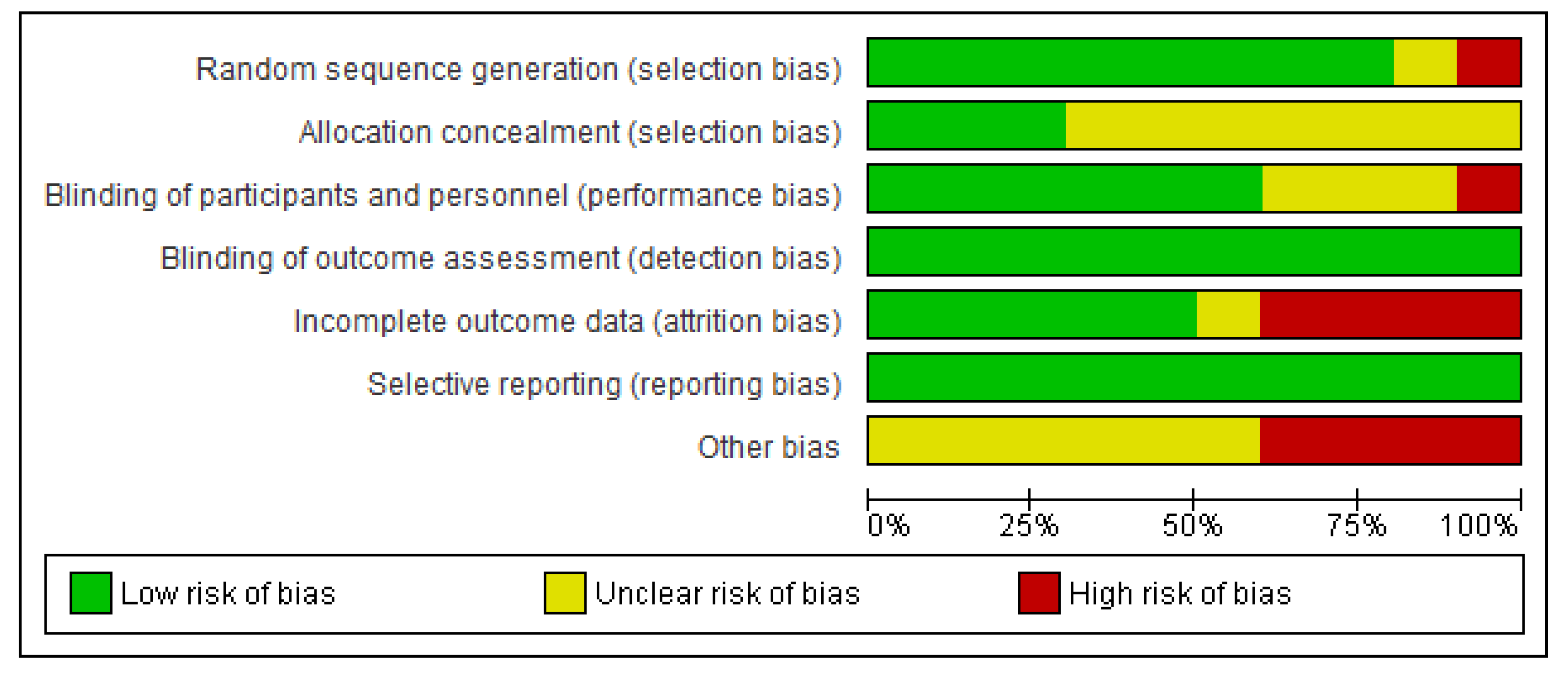
2.3. Risk of Bias (Quality) Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Velasco, J.; Menabrito, M.; Catalán, I. What fertility specialists should know about the vaginal microbiome: A review. Reprod. BioMed. Online 2017, 35, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.; Fernández-Blázquez, A.; García, B. Vaginosis. Microbiota vaginal. Enferm. Infecc. Microbiol. Clin. 2019, 37, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.; Kelley, S.; Thackray, V. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Al-Nakkash, L.; Herbst-Kralovetz, M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Fontané, L.; Benaiges, D.; Goday, A.; Llauradó, G.; Pedro-Botet, J. Influencia de la microbiota y de los probióticos en la obesidad. Clin. Investig. Arterioscler. 2018, 30, 271–279. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017, 8, 521–543. [Google Scholar] [CrossRef]
- Anahtar, M.; Gootenberg, D.; Mitchell, C.; Kwon, D. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef]
- Pramanick, R.; Mayadeo, N.; Warke, H.; Begum, S.; Aich, P.; Aranha, C. Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota? Microb. Pathog. 2019, 134, 103599. [Google Scholar] [CrossRef]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.; Dinleyici, E.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Bernal, J.; Mendiola, J.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011, 55, 758–774. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Sharma, M.; Dwivedi, P.; Singh Rawat, A.; Dwivedi, A. Nutrition nutraceuticals: A proactive approach for healthcare. Nutraceuticals 2016, 4, 79–116. [Google Scholar]
- Kothari, D.; Patel, S.; Kim, S. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Lara-Villoslada, F.; Sierra, S.; Díaz-Ropero, M.; Olivares, M.; Xaus, J. Safety Assessment of the Human Milk-Isolated Probiotic Lactobacillus salivarius CECT5713. J. Dairy Sci. 2007, 90, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel on Dietetic Products, Nutrition and Allergies. General scientific guidance for stakeholders on health claim applications. EFSA J. 2016, 14, 4367. [Google Scholar] [CrossRef]
- de Benito, A.; Ibáñez, C.; Moncho, W.; Martínez, D.; Vettorazzi, A.; de Cerain, A. Database on the taxonomical characterisation and potential toxigenic capacities of microorganisms used for the industrial production of food enzymes and feed additives, which do not have a recommendation for Qualified Presumption of Safety. EFSA Supporting Publ. 2017, 14. [Google Scholar] [CrossRef]
- Krauss-Silva, L.; Moreira, M.; Alves, M.; Braga, A.; Camacho, K.; Batista, M.; Almada-Horta, A.; Rebello, M.R.; Guerra, F. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: Preliminary results. Trials 2011, 12, 239. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Li, Y.; Zhang, T.; Wang, Q.; He, J.; Wang, L.; Li, L.; Yang, N.; Fang, Y. Probiotics for the treatment of women with bacterial vaginosis: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 2019, 864, 172660. [Google Scholar] [CrossRef]
- Falagas, M.; Betsi, G.; Athanasiou, S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 2007, 13, 657–664. [Google Scholar] [CrossRef]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J. Treatment of Infectious Mastitis during Lactation: Antibiotics versus Oral Administration of Lactobacilli Isolated from Breast Milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- de Andrés, J.; Jiménez, E.; Espinosa-Martos, I.; Rodríguez, J.; García-Conesa, M. An Exploratory Search for Potential Molecular Targets Responsive to the Probiotic Lactobacillus salivarius PS2 in Women With Mastitis: Gene Expression Profiling vs. Interindividual Variability. Front. Microbiol. 2018, 9, 2166. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.; Mehrabany, E. Effects of Probiotics on the Recurrence of Bacterial Vaginosis. J. Low. Genit. Tract Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, B.; Gismondo, M. The use of probiotics in medical practice. Int. J. Antimicrob. Agents 2000, 16, 531–536. [Google Scholar] [CrossRef]
- Gardiner, G.; Heinemann, C.; Baroja, M.; Bruce, A.; Beuerman, D.; Madrenas, J.; Reid, G. Oral administration of the probiotic combination Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 for human intestinal applications. Int. Dairy J. 2002, 12, 191–196. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Canese, K. An Updated PubMed Is on Its Way. NLM Tech. Bull. 2019, 427. Available online: https://www.nlm.nih.gov/pubs/techbull/ma19/ma19_pubmed_update.html (accessed on 13 March 2020).
- Higgins, J.P.; Altman, D.G.; Sterne, J. Chapter 8. In Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0.; Higgins, J.P., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Jiménez, E.; Fernández, L.; Maldonado, A.; Martin, R.; Olivares, M.; Xaus, J.; Rodriguez, J.M. Oral Administration of Lactobacillus Strains Isolated from Breast Milk as an Alternative for the Treatment of Infectious Mastitis during Lactation. Appl. Environ. Microbiol. 2008, 74, 4650–4655. [Google Scholar] [CrossRef]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Jiménez, E.; Martín, V.; Rodriguez, J.M. Prevention of Infectious Mastitis by Oral Administration of Lactobacillus salivarius PS2 During Late Pregnancy. Clin Infect. Dis. 2015, 62, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017–e00019. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.; Rulisa, S.; Gloor, G.; Macklaim, J.; Sumarah, M.; Reid, G. Pilot assessment of probiotics for pregnant women in Rwanda. PLoS ONE 2018, 13, e0195081. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.; Cardot, J. Vaginal Impact of the Oral Administration of Total Freeze-Dried Culture of LCR 35 in Healthy Women. Infect. Dis. Obstet. Gynecol. 2012, 2012, 503648. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.; Duru, M.; Eze, C.; Egharevba, J.; Aiyebelehin, A.; Bruce, A.; Reid, G. Oral use of probiotics as an adjunctive therapy to fluconazole in the treatment of yeast vaginitis: A study of Nigerian women in an outdoor clinic. Microb. Ecol. Health Dis. 2009, 21, 72–77. [Google Scholar]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Lechowicz, M.; Konopka, E.; Majewska, U.; Borszewska-Kornacka, M.; Mikula, M.; Cukrowska, B.; et al. Effect of Saccharomyces boulardii and Mode of Delivery on the Early Development of the Gut Microbial Community in Preterm Infants. PLoS ONE 2016, 11, e0150306. [Google Scholar] [CrossRef]
- Kiess, A.; Hirai, J.; Triplett, M.; Parker, H.; McDaniel, C. Impact of oral Lactobacillus acidophilus gavage on rooster seminal and cloacal Lactobacilli concentrations. Poult. Sci. 2016, 95, 1934–1938. [Google Scholar] [CrossRef]
- dos Santos, M.; Ramachandran, R.; Kiess, A.; Wamsley, K.; McDaniel, C. Impact of in vitro inoculation and dietary supplementation with Bacillus subtilis on sperm quality of aged White Leghorn roosters. J. Appl. Poult. Res. 2018, 27, 304–315. [Google Scholar] [CrossRef]
- Valcarce, D.; Riesco, M.; Martínez-Vázquez, J.; Robles, V. Diet Supplemented with Antioxidant and Anti-Inflammatory Probiotics Improves Sperm Quality after Only One Spermatogenic Cycle in Zebrafish Model. Nutrients 2019, 11, 843. [Google Scholar] [CrossRef]
- Verna, E.; Lucak, S. Use of probiotics in gastrointestinal disorders: What to recommend? Therap. Adv. Gastroenterol. 2010, 3, 307–319. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.; Unniappan, S.; Carnevali, O. Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 2017, 7, 5512. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Beuerman, D.; Heinemann, C.; Bruce, A. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol. 2001, 32, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Halis, G.; Arici, A. Endometriosis and Inflammation in Infertility. Ann. N. Y. Acad. Sci. 2004, 1034, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.; Jiménez-Almazán, J.; Alonso, R.; Alama, P.; Remohi, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.; Koenig, S.; McCulle, S.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2010, 108, 4680–4687. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.; Gajer, P.; Tarca, A.; Fadrosh, D.; Bieda, J.; Chaemsaithong, P.; Miranda, J.; Chaiworapongsa, T.; Ravel, J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014, 2, 18. [Google Scholar] [CrossRef]
- Romero, R.; Chaiworapongsa, T.; Kuivaniemi, H.; Tromp, G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: A role for genetic epidemiology in the prevention of preterm birth. Am. J. Obstet. Gynecol. 2004, 190, 1509–1519. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.; Arrieta, M.; Ramer-Tait, A.; Walter, J. A critical assessment of the sterile womb and in utero colonisation hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- de Goffau, M.; Lager, S.; Sovio, U.; Gaccioli, F.; Cook, E.; Peacock, S.; Parkhill, J.; Charnock-Jones, D.S.; Smith, G.C.S. Human placenta has no microbiome but can contain potential pathogens. Nature 2019, 572, 329–334. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Prince, A.; Ma, J.; Kannan, P.; Alvarez, M.; Gisslen, T.; Harris, R.; Sweeney, E.; Knox, C.; Lambers, D.; Jobe, A.; et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am. J. Obstet. Gynecol. 2016, 214, e1–e627. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Zehnder, J.; Druzin, M.; Brown, P. Gene expression patterns in human placenta. Proc. Natl. Acad. Sci. USA 2006, 103, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Gómez-López, N.; Kusanovic, J.P.; Pacora, P.; Panaitescu, B.; Erez, O.; Yoon, B.H. Clinical Chorioamnionitis at Term: New Insights into the Etiology, Microbiology, and the Fetal, Maternal and Amniotic Cavity Inflammatory Responses. Nogyogy. Es Szulesz. Tovabbk. Szle. 2018, 20, 103–112. [Google Scholar]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Boil. 2014, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- de Goffau, M.; Lager, S.; Salter, S.; Wagner, J.; Kronbichler, A.; Charnock-Jones, D.; Peacock, S.J.; Smith, G.C.S.; Parkhill, J. Recognizing the reagent microbiome. Nat. Microbiol. 2018, 3, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.; Bengmark, S.; Enck, P.; Haller, D.; Herz, U.; Kalliomaki, M.; Kudo, S.; Lenoir-Wijnkoop, I.; Mercenier, A.; Myllyluoma, E.; et al. Guidance for Substantiating the Evidence for Beneficial Effects of Probiotics: Current Status and Recommendations for Future Research. J. Nutr. 2010, 140, 671S–676S. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: A systematic review. BMJ Open 2014, 4, e005047. [Google Scholar] [CrossRef]
- Azaïs-Braesco, V.; Bresson, J.; Guarner, F.; Corthier, G. Not all lactic acid bacteria are probiotics, but some are. Br. J. Nutr. 2010, 103, 1079–1081. [Google Scholar] [CrossRef]
- Rijkers, G.; de Vos, W.; Brummer, R.; Morelli, L.; Corthier, G.; Marteau, P. Health benefits and health claims of probiotics: Bridging science and marketing. Br. J. Nutr. 2011, 106, 1291–1296. [Google Scholar] [CrossRef]
- Kumar, H.; Salminen, S.; Verhagen, H.; Rowland, I.; Heimbach, J.; Bañares, S.; Young, T.; Nomoto, K.; Lalonde, M. Novel probiotics and prebiotics: Road to the market. Curr. Opin. Biotechnol. 2015, 32, 99–103. [Google Scholar] [CrossRef]
- Pavlova, S.; Kilic, A.; Kilic, S.; So, J.; Nader-Macias, M.; Simoes, J.; Tao, L. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 2002, 92, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.; Hidalgo-Cantabrana, C.; Delgado, S.; Margolles, A.; Sánchez, B. Filling the gap between collection, transport and storage of the human gut microbiota. Sci. Rep. 2019, 9, 8327. [Google Scholar] [CrossRef] [PubMed]
- Cribby, S.; Taylor, M.; Reid, G. Vaginal Microbiota and the Use of Probiotics. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 256490. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, S.; Khodaverdi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef]
- Sashihara, T.; Sueki, N.; Ikegami, S. An analysis of the effectiveness of heat-killed lactic acid bacteria in alleviating allergic diseases. J. Dairy Sci. 2006, 89, 2846–2855. [Google Scholar] [CrossRef]
- Sashihara, T.; Sueki, N.; Furuichi, K.; Ikegami, S. Effect of growth conditions of Lactobacillus gasseri OLL2809 on the immunostimulatory activity for production of interleukin-12 (p70) by murine splenocytes. Int. J. Food Microbiol. 2007, 120, 274–281. [Google Scholar] [CrossRef]
- Qin, C.; Xu, L.; Yang, Y.; He, S.; Dai, Y.; Zhao, H.; Zhou, Z. Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reproduction 2013, 147, 53–64. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, W.; Liu, W.; Gatlin, D.; Zhang, Y.; Yao, B.; Ringø, E. Identification of highly-adhesive gut Lactobacillus strains in zebrafish (Danio rerio) by partial rpoB gene sequence analysis. Aquaculture 2012, 370, 150–157. [Google Scholar] [CrossRef]
- Treven, P.; Mrak, V.; Bogovič-Matijašić, B.; Horvat, S.; Rogelj, I. Administration of probiotics Lactobacillus rhamnosus GG and Lactobacillus gasseri K7 during pregnancy and lactation changes mouse mesenteric lymph nodes and mammary gland microbiota. J. Dairy Sci. 2015, 98, 2114–2128. [Google Scholar] [CrossRef]
- Schultz, M.; Göttl, C.; Young, R.J.; Iwen, P.; Vanderhoof, J.A. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonisation. J. Pediatric Gastroenterol. Nutr. 2004, 38, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, B.B.; Obermajer, T.; Zorič Peternel, M.; Trachatova, S.; Spanova, A.; Rogelj, I. Detection of the Lactobacillus gasseri K7 probiotic strain in feces and in human milk with molecular methods. Med. Razgl. 2009, 48, 137–138. [Google Scholar]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef] [PubMed]
- Jeżewska-Frąckowiak, J.; Seroczyńska, K.; Banaszczyk, J.; Jedrzejczak, G.; Żylicz-Stachula, A.; Skowron, P.M. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar] [CrossRef]
- Medina, M.; De Palma, G.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Bifidobacterium strains suppress in vitro the pro-inflammatory milieu triggered by the large intestinal microbiota of coeliac patients. J. Inflamm. Res. 2008, 5, 19. [Google Scholar] [CrossRef]
- Jang, S.; Jeong, J.; Choi, S.; Kim, H.; Han, M.; Kim, D. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 Attenuate Gardnerella vaginalis-Infected Bacterial Vaginosis in Mice. Nutrients 2017, 9, 531. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef]
- Di Pierro, F.; Polzonetti, V.; Patrone, V.; Morelli, L. Microbiological Assessment of the Quality of Some Commercial Products Marketed as Lactobacillus crispatus-Containing Probiotic Dietary Supplements. Microorganisms 2019, 7, 524. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Herman, L.; Koutsoumanis, K.; Lindavist, R.; Norrung, B.; et al. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2017, 15, e04664. [Google Scholar]
- Food and Agriculture Organization/World Health Organization. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations/World Health Organization: London, UK, 2002. [Google Scholar]

| Parameters | Inclusion Criteria |
|---|---|
| Population | Human |
| Intervention | Probiotic strains and doses |
| Comparison | Oral probiotics versus placebo |
| Outcome | Improvement on parameters of fertility |
| Setting | Clinical trials (CTs) |
| Reference | Population Characteristics Size (n) | Probiotic Strain | Doses and Administration Pattern | Period of Intervention (Weeks) | Disease | Clinical Parameters Variability |
|---|---|---|---|---|---|---|
| Zhang et al. [33] | 14 PCOS patients | Bifidobacterium lactis V9 | 1 × 106 CFU/day | 10 | PCOS | The study showed a potential mechanism by which the probiotic Bifidobacterium lactis V9 modulates sex hormone levels in patients with PCOS through the gut–brain axis. |
| McMillan et al. [34] | 38 Pregnant women <36 weeks | Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 | 1 × 109 CFU/day | 4 | Pregnancy associated disorders | Women in the placebo group were more likely to give birth preterm. However, the sample size that finished the study was not large enough to detect significant differences. |
| Krauss-Silva et al. [18] | 4204 Pregnant women <20 weeks | Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-15 | >2 × 106 CFU/day | 6–12 | Pregnancy associated disorders | The efficacy of probiotics tested to avoid spontaneous premature delivery cannot be statistically estimated because the study sample was insufficient. |
| de Andrés et al. [23] | 31 Women (23 with mastitis) | Lactobacillus salivarius PS2 | 3 × 109–3 × 1010 CFU/day | 3 | Mastitis | The results proved the involvement of modulation of inflammatory and cell-growth related pathways and genes in the somatic cells following the intake of L. salivarius PS2. |
| Jiménez et al. [31] | 20 Women with mastitis | Lactobacillus salivarius CECT5713 and Lactobacillus gasseri CECT5714 | 1 × 1010 CFU/day | 4 | Mastitis | Both probiotics appears to be an efficient alternative for the treatment of infectious mastitis during lactation. |
| Arroyo et al. [21] | 352 Women with mastitis | Lactobacillus fermentum CECT5716 Lactobacillus salivarius CECT5713 | 1 × 109 CFU/day | 3 | Mastitis | L. fermentum CECT5716 or L. salivarius CECT5713 seem to be an efficient alternative to antibiotics for the treatment of infectious mastitis during lactation. |
| Fernández et al. [32] | 108 Healthy pregnant women | Lactobacillus salivarius PS2 | 1 × 109 CFU/day | ~8 | Mastitis—Pregnancy | Administration of L. salivarius PS2 during late pregnancy appears to be an efficient method to prevent infectious mastitis in a susceptible population. |
| Zeber-Lubecka et al. [37] | 39 Preterm infants | Saccharomyces boulardii | 2 × 109 CFU/day | 6 | Microbiota dysbiosis | There were no statistical differences between babies supplemented with probiotic and without probiotic. |
| Anukam et al. [36] | 59 Women with vaginal dysbiosis | Fluconazol, Lactobacillus rhamnosus GR-1 y Lactobacillus reuteri RC-14 | 5 × 109 CFU/day | 24 | Vaginal dysbiosis | Probiotics did not affect the cure rate but did lead to fewer vulvovaginitis recurrences with its long-term use. |
| Bohbot and Cardot [35] | 20 Healthy women | Lactobacillus casei variety rhamnosus (LCR35) | Group 1: 1 × 108 CFU/day Group 2: 2 × 108 CFU/day | 4 | Vaginal dysbiosis | Probiotic decreased the Nugent score in both groups, but it was slightly more significant in group 2. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Moreno, A.; Aguilera, M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients 2020, 12, 757. https://doi.org/10.3390/nu12030757
López-Moreno A, Aguilera M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients. 2020; 12(3):757. https://doi.org/10.3390/nu12030757
Chicago/Turabian StyleLópez-Moreno, Ana, and Margarita Aguilera. 2020. "Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis" Nutrients 12, no. 3: 757. https://doi.org/10.3390/nu12030757
APA StyleLópez-Moreno, A., & Aguilera, M. (2020). Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients, 12(3), 757. https://doi.org/10.3390/nu12030757






