Advances in Medical Nutrition Therapy: Parenteral Nutrition
Abstract
1. Introduction
2. Advances in Pharmaceutical Preparation: “All-in-One” Admixtures
3. Consideration for Support of Parenteral Nutrition
3.1. Enteral Versus Parenteral Nutrition
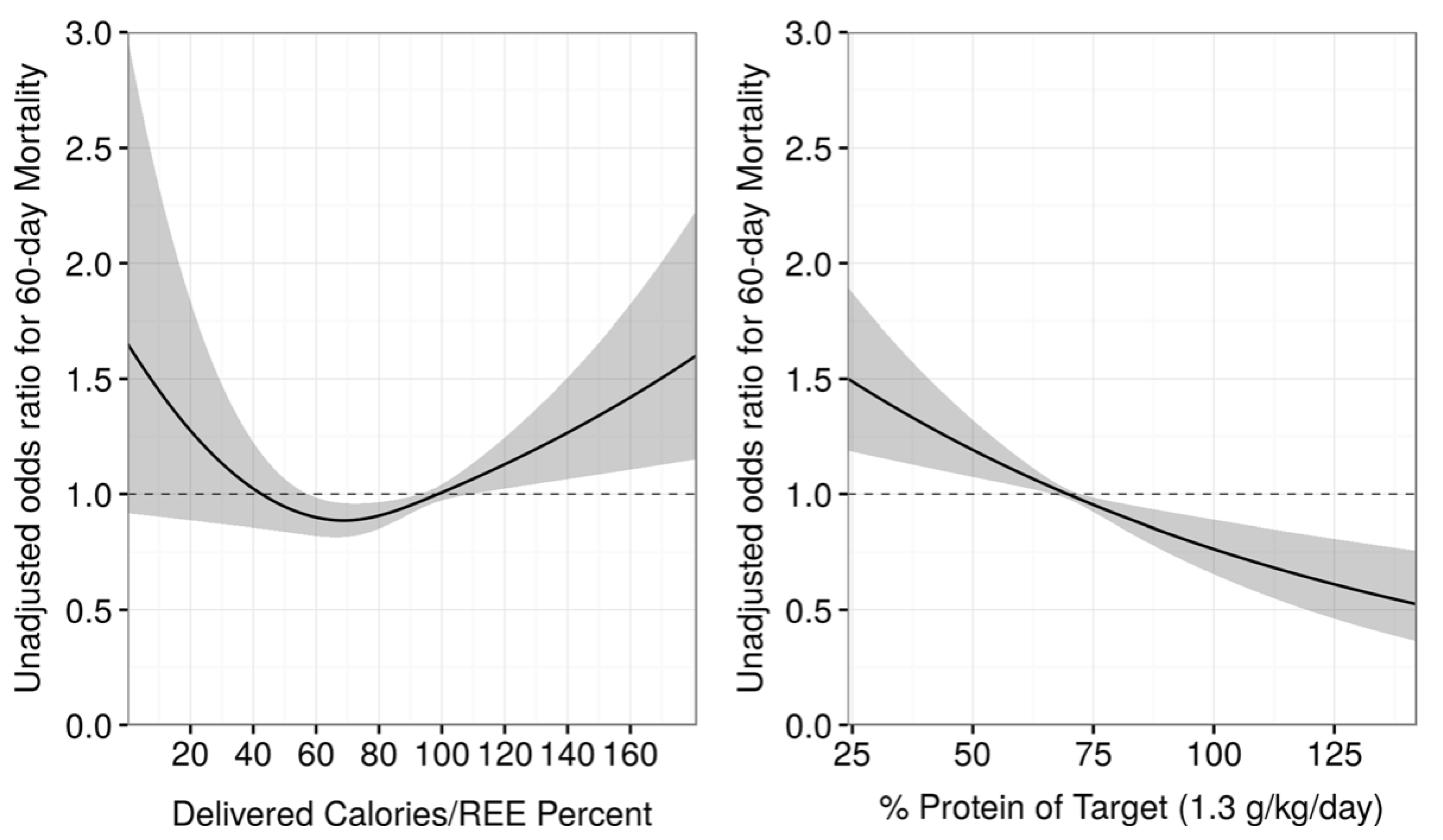
3.2. The use of Indirect Calorimetry
3.3. Venous Access Care and Infection Risks
3.4. Glucose Control
4. Development of Lipid Emulsion
5. Improved Protein Intake
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muhlebach, S.; Franken, C.; Stanga, Z.; Working group for developing the guidelines for parenteral nutrition of the German Association for nutritional medicine. Practical Handling of AIO admixture- guidelines on Parenteral Nutrition, Chapter 10. Ger. Med. Sci. 2009, 7, 1–8. [Google Scholar]
- Barnett, M.I.; Pertkiewicz, M.; Cosslett, A.G.; Muhlebach, S. Basics in clinical nutrition: Parenteral nutrition admixture, how to prepare parenteral nutrition (PN) admixtures. e-SPEN e-J. Clin. Nutr. Metab. 2009, 4, e114–e116. [Google Scholar] [CrossRef]
- Emilio Alfonso, J.; Berlana, D.; Boullata, J. Clinical, Ergonomic and Economic outcomes with multichamber bags compared with (Hospital) Pharmacy compounded bags and multibottle systems: A systemic Literature review. J. Parenter. Enter. Nutr. 2017, 41, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Sierro, C.; Griffiths, W. Convenience and Cost-efficiency by the Use of Multicompartment Bags for Total Parenteral Nutrition. In Proceedings of the XVIII ESPEN Congress on Clinical Nutrition and Metabolism, Geneva, Switzerland, 8–11 September 1996. [Google Scholar]
- Pichard, C.; Schwarz, G.; Frei, A.; Kyle, U.; Jolliet, P.; Morel, P.; Romand, J.A.; Sierro, C. Economic investigation of the use of three compartment total parenteral nutrition bag: Prospective randomized unblinded controlled study. Clin. Nutr. 2000, 19, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, J. Total Nutrient admixtures (3-in-1): Pros vs. Cons for adults. Nutr. Clin. Pract. 2015, 30, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Baras, Z.; Theilla, M.; Singer, P. From compound to “ready to use” parenteral nutrition bags use in a tertiary medical center: An observational study. Clin. Nutr. 2019, 38, S270–S271. [Google Scholar] [CrossRef]
- Elke, G.; van Zanten, A.R.H.; Heyland, D.K. Enteral versus parenteral nutrition in critically ill patients: An updated systemic review and meta-analysis of randomized controlled trails. Crit. Care 2016, 20, 117. [Google Scholar] [CrossRef]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral compared with parenteral nutrition: A meta-analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef]
- Ridley, E.J.; Daives, A.R.; McGuinness, S.; for the Supplemental Parenteral Nutrition Clinical Investigators. Supplemental parenteral nutrition versus usual care in critically ill adults: A pilot randomized control study. Crit. Care 2018, 22, 12. [Google Scholar] [CrossRef]
- McClave, S.A.; Heyland, D.K. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr. Clin. Pract. 2009, 24, 305–315. [Google Scholar] [CrossRef]
- Gungabissoon, U.; Hacquoil, K.; Bains, C. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. J. Parenter. Enter. Nutr. 2015, 39, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Debaveye, Y. Early versus late parenteral nutrition in critically ill adults (EPaNIC). N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Geilen, J.; Hiesmayr, M. Timing of parenteral nutrition: A controversy. Clin. Nutr. ESPEN 2019, 38, S57. [Google Scholar]
- Harvey, S.E.; Parrott, F.; Rowan, K.M.; for the CALORIES Trail Investigators. Trail of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Reignier, J.; Boisrame-Helms, J.; Le Gouge, A.; for the NUTRIREA-2 Trail Investigators and the Clinical Research in Intensive Care and Sepsis (CRICS) Group. Enteral versus Parenteral early nutrition in ventilated adults with shock: A randomized, controlled, multicenter, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Blaser, A.R.; Starkopf, J.; Oudemans-van Straaten, H.M.; the ESICM Working Group on Gastrointestinal Function. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Bischoff, S.C. ESPEN guidelines on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Doig, G.S.; Simpson, F.; Peake, S.; for the Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short term relative contraindication to early enteral nutrition. A randomized controlled trail. JAMA 2013, 309, 2130–2138. [Google Scholar] [CrossRef]
- Oshima, T.; Berger, M.M.; Pichard, C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2017, 36, 651–662. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Berger, M.M.; Pichard, C. Optimization of energy provision with supplemental parenteral nutrition in critically ill patients: A randomized controlled trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Braunschweig, C. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Schuetz, P.; Cederholm, T. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin. Nutr. 2018, 37, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Bozzetti, F. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.P.; Singer, P.; Mendoza Lopez, R.V. Resting energy expenditure and body composition in patients with head and neck cancer: An observational study leading to a new predictive equation. Nutrition (Burbank Los Angel. Cty. Calif.) 2018, 51–52, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Zanella, P.B.; Avila, C.C.; de Souza, C.G. Estimating resting energy expenditure by different methods as compared with indirect calorimetry for patients with pulmonary hypertension. Nutr. Clin. Pract. 2018, 33, 217–223. [Google Scholar] [CrossRef]
- Tatucu-Babet, O.A.; Ridley, E.J.; Tierney, A.C. Prevalence of underprescription or overprescription of energy needs in critically ill mechanically ventilated adults as determined by indirect calorimetry: A systematic literature review. J. Parenter. Enter. Nutr. 2016, 40, 212–225. [Google Scholar] [CrossRef]
- Zusman, O.; Kagan, I.; Singer, P. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin. Nutr. 2019, 38, 1206–1210. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef]
- Stapel, S.N.; de Grooth, H.J.; Oudemans-van Straaten, H.M. Ventilator-derived carbon dioxide production to assess energy expenditure in critically ill patients: Proof of concept. Crit. Care 2015, 19, 370. [Google Scholar] [CrossRef]
- Rousing, M.L.; Hahn-Pedersen, M.H.; Preiser, J.C. Energy expenditure in critically ill patients estimated by population-based equations, indirect calorimetry and CO2-based indirect calorimetry. Ann. Intensive Care 2016, 6, 16. [Google Scholar] [CrossRef]
- Oshima, T.; Graf, S.; Pichard, C. Can calculation of energy expenditure based on CO2 measurements replace indirect calorimetry? Crit. Care 2017, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Kagan, I.; Zusman, O.; Singer, P. Validation of carbon dioxide production (Vco2) as a tool to alculte resting energy expenditure (REE) in mechanically ventilated critically ill patients: A retrospective observational study. Crit. Care 2018, 22, 186. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Oshima, T.; Genton, L. Innovations in energy expenditure assessments. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, A.O.; Tran, L.B.; Kaye, A.D. Total parenteral and enteral nutrition in the ICU. Evolving concept. Anesthesiol. Clin. 2017, 35, 181–190. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Healthcare-Associated Infections: Prevention and Control in Primary and Community Care; Clinical Guideline; NICE: London, UK, 2012. [Google Scholar]
- Prevent Central line Infection—Getting Started Kit; Canadian Patient Safety Institute (CPSI). Available online: https://www.patientsafetyinstitute.ca/en/toolsResources/Documents/Interventions/Central%20Line-Associated%20Bloodstream%20Infection/CLI%20Getting%20Started%20Kit.pdf (accessed on 7 March 2020).
- Ling, M.L.; Apisarnthanarak, A.; LEE, C.M. APSIC guide for prevention of Central Line Associated Bloodstream Infections (CLABSI). Anti Microb. Resist. Infect. Control 2016, 5, 16. [Google Scholar] [CrossRef]
- Healthcare Infection Control Practices Advisory Committee (HICPAP) of the Centers of Disease Control and Prevention. In Guidelines for the Prevention of Intravascular Catheter Related Infections (2011), Update 2017. Available online: https://www.cdc.gov/infectioncontrol/guidelines/bsi/updates.html (accessed on 7 March 2020).
- Inchingolo, R.; Pasciuto, G.; Richeldi, L. Educational interventions alone and combined with port protector reduce the rate of central venous catheter infection and colonization in respiratory semi intensive care unit. BMC Infect. Dis. 2019, 19, 215. [Google Scholar] [CrossRef]
- Parienti, J.J.; Mongardon, N.; Cheyron, D.; for the 3SITES study group. Intravascular complication of central venous catheterization by insertion site. N. Engl. J. Med. 2015, 373, 1220–1229. [Google Scholar] [CrossRef]
- Arvaniti, K.; Lathyris, D.; Matamis, D.; for the Catheter Related Infections of ICU (CRI-ICU). Comparison of Oligon catheters and chlorhexidine-impregnated sponges with standard multilumen central venous catheters for prevention of associated colonization and infections in intensive care unit patients: A multicenter, randomized, controlled study. Crit. Care Med. 2012, 40, 420–429. [Google Scholar] [CrossRef]
- Timsit, J.F.; Mimoz, O.; Lucet, J.C. Randomized Controlled Trial of Chlorhexidine Dressing and Highly Adhesive Dressing for Preventing Catheter-related Infections in Critically Ill Adults. Am. J. Respir. Crit. Care Med. 2012, 186, 1272–1278. [Google Scholar] [CrossRef]
- Timsit, J.F.; Schwebel, C.; Lucet, J.C.; For the dressing study group. Chlorhexidine-Impregnated Sponges and Less Frequent Dressing Changes for Prevention of Catheter-Related Infections in Critically Ill Adults. A Randomized Controlled Trial. JAMA 2009, 301, 1231–1241. [Google Scholar] [CrossRef]
- Wouters, Y.; Theilla, M.; Wanten, G.J.A. Randomised clinical trial:2% Taurolidine versus 0.9% saline locking in patients on home parenteral nutrition. Aliment. Pharmacol. Ther. 2018, 48, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M. The 2013 Arvid Wretlind lecture: Evolving concept in parenteral nutrition. Clin. Nutr. 2014, 33, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.C.; Sicoutris, C.; Gracias, V.H. Poor glycemic control is associated with increase mortality in critically ill trauma patients. Am. Surg. 2007, 73, 434–460. [Google Scholar]
- Capes, S.E.; Hunt, D.; Gerstein, H.C. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with or without diabetes: A systemic review. Lancet 2000, 355, 773–778. [Google Scholar] [CrossRef]
- Capes, S.E.; Hunt, D.; Gerstein, H.C. Stress hyperglycemia and prognosis of stroke in non-diabetic and diabetic patients: A systemic review. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef]
- Van Den Berghe, G.; Wouters, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- The NICE STUDY Investigators. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Engel, C.; Reinhart, K. German competence network sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in sever sepsis. N. Engl. J. Med. 2008, 358, 125–139. [Google Scholar] [CrossRef]
- Devos, P.; Preiser, J.C.; Melot, C. Impact of tight glycemic control by intensive insulin therapy on ICU mortality and rate of hypoglycemia: Final results of the Glucontrol study. Intensive Care Med. 2007, 33, s189. [Google Scholar]
- Yatabe, T.; Inoue, S.; Egi, M. The optimal target for acute glycemic control in critically ill patients: A network meta-analysis. Intensive Care Med. 2017, 43, 16–28. [Google Scholar] [CrossRef]
- Egi, M.; Bellomo, R.; Hart, G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006, 105, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.; O’Brien, J.M., Jr.; Preiser, J.C. Glucose variability and mortality in patients with sepsis. Crit. Care Med. 2008, 36, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S. Glycemic variability: A strong independent predictor of mortality in critically ill patients. Crit. Care Med. 2008, 36, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.S.; Salama, A.; Singh, R. Association of time in blood glucose range with outcomes following cardiac surgery. BMC Anesthesiol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S.; Preiser, J.C. Time in blood glucose range 70 to 140 mg/dL >80% is strongly associated with increased survival in non-diabetic patients. Crit. Care Med. 2015, 19, 179. [Google Scholar]
- Lanspa, M.J.; Krinsley, J.S.; Hirshberg, E.L. Percentage of time in range 70 to 139 mg/dL is associated with reduced mortality among critically ill patients receiving insulin infusion. Chest 2019, 156, 878–886. [Google Scholar] [CrossRef]
- Rady, M.Y.; Johnson, D.J.; Helmers, R.A. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin. Proc. 2005, 80, 1558–1567. [Google Scholar] [CrossRef]
- Egi, M.; Bellomo, R.; Bailey, M. Blood Glucose concentration and outcome of critical illness: The impact of diabetes. Crit. Care Med. 2008, 36, 2249–2255. [Google Scholar] [CrossRef]
- Krinsley, J.S. Glycemic variability and mortality in critically ill patients: The impact of diabetes. J. Diabetes Sci. Technol. 2009, 3, 1292–1301. [Google Scholar] [CrossRef]
- Green, J.P.; Berger, T.; Panacek, E.A. Hyperlactatemia affect the association of hyperglycemia with mortality in non-diabetic adults with sepsis. Acad. Emerg. Med. 2012, 19, 1268–1275. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Bellomo, R. Stress hyperlactatemia modifies the relationship between stress hyperglycemia and outcome: A retrospective observational study. Crit. Care Med. 2014, 42, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.F.; Wieringa, N.; Nijsten, M.W. The association of early combined lactate and glucose levels with subsequent renal and liver dysfunction and hospital mortality in critically ill patients. Crit. Care Med. 2017, 21, 218. [Google Scholar]
- Calder, P.C.; Adolph, M.; Singer, P. Lipid in the intensive care unit: Recommendations from the ESPEN expert group. Clin. Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Battistella, F.D.; Widergren, J.T.; MacColl, K. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J. Trauma 1997, 43, 52–58. [Google Scholar] [CrossRef]
- Furukawa, K.; Yamamori, H.; Tashiro, T. Influences of soybean oil emulsion on stress response and cell mediated immune function in moderately or severely stressed patients. Nutrition 2002, 18, 235–240. [Google Scholar] [CrossRef]
- Heller, A.R.; Fischer, S.; Koch, T. Effects of parenteral nutrition with n-3 fatty acids on the result of therapy—A multicentre analysis with 661 patients. Aktuel Ernahr. 2005, 30, 15–22. [Google Scholar]
- Calder, P.C. Functional roll of fatty acids and their effect on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Tribole, E. What happened to do no harm? The issue of dietary omega-6 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 78–79. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Barbosa, V.M.; Calder, P.C. Olive oil in parenteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 165–174. [Google Scholar] [CrossRef]
- Dai, Y.J.; Sun, L.L.; Wang, W. Comparison of formulas based on lipid emulsions of olive oil, soybean oil, or several oils for parenteral nutrition: A systematic review and meta-analysis. Adv. Nutr. 2016, 15, 279–286. [Google Scholar] [CrossRef]
- De Miranda Torrinhas, R.S.; Santana, R.; Waitzberg, D.L. Parenteral fish oil as a pharmacological agent to modulate postoperative immune response: A randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin. Nutr. 2013, 32, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Lai, S.L.; LaI, H.S. Effects of Fish Oil on Inflammatory Modulation in Surgical Intensive Care Unit Patients. Nutr. Clin. Pract. 2012, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.; Mayer, K.; Muscaritoli, M. Ω-3 Fatty acid enriched parenteral nutrition in hospitalized patients: Systematic review with meta-analysis and trial sequential analysis. J. Parenter. Enter. Nutr. 2020, 44, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J. Parenteral nutrition: Amino acids. Nutrients 2017, 9, 257. [Google Scholar] [CrossRef]
- Nicolo, M.; Heyland, D.K.; Compher, C. Clinical outcome related to protein delivery in the critically ill population: A multicenter, multinational observation study. J. Parenter. Enter. Nutr. 2016, 40, 45–51. [Google Scholar] [CrossRef]
- Compher, C.; Chittams, J.; Heyland, D.K. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: A multicenter, multinational observational study. Crit. Care Med. 2017, 45, 156–163. [Google Scholar] [CrossRef]
- Allingstrup, M.J.; Kondrup, J.; Wils, J. Early goal directed nutrition versus standard of care in adult intensive patients: The single center, randomized, outcome assessor blinded EAT-ICU trail. Intensive Care Med. 2017, 43, 1637–1647. [Google Scholar] [CrossRef]
- Heyland, D.K.; Stapleton, R.; Compher, C. Should we prescribe more protein to critically ill patients? Nutrients 2018, 10, 462. [Google Scholar] [CrossRef]
- Arends, J.; Bodoky, G.; Znader, A. ESPEN guidelines on enteral nutrition: Non-surgical oncology. Clin. Nutr. 2006, 25, 245–259. [Google Scholar] [CrossRef]
- Herndon, D.N.; Tompkins, R.G. Support of the metabolic response to burn injury. Lancet 2004, 363, 1895–1902. [Google Scholar] [CrossRef]
- Bendavid, I.; Zusman, O.; Singer, P. Early administration of protein in critically ill patients: A retrospective cohort study. Nutrients 2019, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, W.A.C.; Van Setten, C.H.; Van Zanten, A.R.H. Timing of PROTein Intake and clinical outcome of adult critically ill patients on prolonged mechanical VANTilation: The PROTEINVENT retrospective study. Clin. Nutr. 2019, 38, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Bistrian, B.R. Nutrition in critical illness: A current conundrum. F1000Res 2016, 5, 2531. [Google Scholar] [CrossRef] [PubMed]
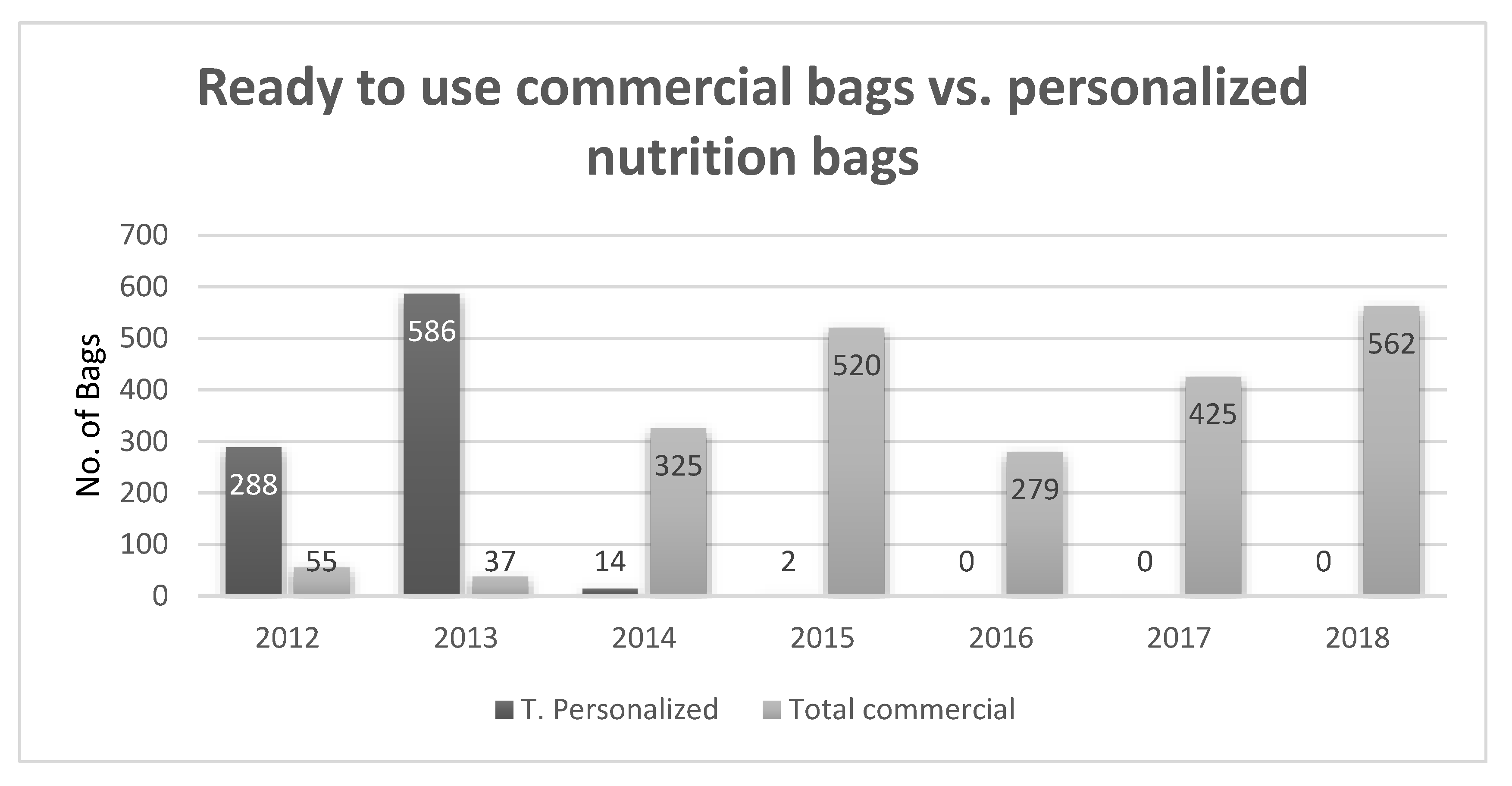
| Study | Type of Study | Results |
|---|---|---|
| Turpin et al. 2011 | Retrospective | Risk of BSI: 11.3% in commertial bags vs. 16.1% in personalized compounded bags, OR 1.56 (CI 1.37–1.79) |
| Turpin et al. 2012 | Retrospective | Risk of BSI: 19.6% in commertial bags vs. 25.9% in personalized compounded bags, OR 1.54 (CI 1.39–1.69) |
| Pontes-Arruda et al. 2012 | Prospective randomized | Incidence BSI:16.8% in commertial bags vs. 22.5% in personalized compounded bags. No significante difference in sepsis/septic shock incidence |
| Pontes-Arruda et al. 2012 | Retrospective | Risk of BSI: 24.9% in commertial bags vs. 29.6% in personalized compounded bags, OR 1.29 (CI 1.06–1.59) |
| Turpin et al. 2014 | Retrospective | Risk of BSI: HR 1.39 (CI 0.82–2.35) personalized compounded bags vs. commertial bags |
| HR 1.85 (CI 1.17–2.94) commertial bags with ward addition vs. commertial bags alone | ||
| HR 2.53 (CI 1.66–3.86) multibottle system vs.commertial bags | ||
| Liu et al. 2014 | Retrospective | Rate of BSI: 19.6% in commertial bags vs. 25.9% in personalized compounded bags |
| Rate of infection: 52.5% in commertial bags vs. 54.7% in personalized compounded bags | ||
| Magee et al. 2014 | Retrospective | No significant difference between groups in infection rate |
| Manufacturer | Product | Amino Acids (g/L) | Energy (kcal/L) |
|---|---|---|---|
| B Braun | Nutriflex | 70 | 1054 |
| Baxter | Clinimix | 50 | 340 |
| Baxter | Triomel 4 | 25 | 700 |
| Baxter | Triomel 7 | 44 | 1140 |
| Baxter | Triomel 9 | 56 | 1070 |
| Fresenius | Smofkabiven | 51 | 1116 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellerman Itzhaki, M.; Singer, P. Advances in Medical Nutrition Therapy: Parenteral Nutrition. Nutrients 2020, 12, 717. https://doi.org/10.3390/nu12030717
Hellerman Itzhaki M, Singer P. Advances in Medical Nutrition Therapy: Parenteral Nutrition. Nutrients. 2020; 12(3):717. https://doi.org/10.3390/nu12030717
Chicago/Turabian StyleHellerman Itzhaki, Moran, and Pierre Singer. 2020. "Advances in Medical Nutrition Therapy: Parenteral Nutrition" Nutrients 12, no. 3: 717. https://doi.org/10.3390/nu12030717
APA StyleHellerman Itzhaki, M., & Singer, P. (2020). Advances in Medical Nutrition Therapy: Parenteral Nutrition. Nutrients, 12(3), 717. https://doi.org/10.3390/nu12030717




