Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fruits and Mice Supplementation
2.3. Measurements of Serum Parameters
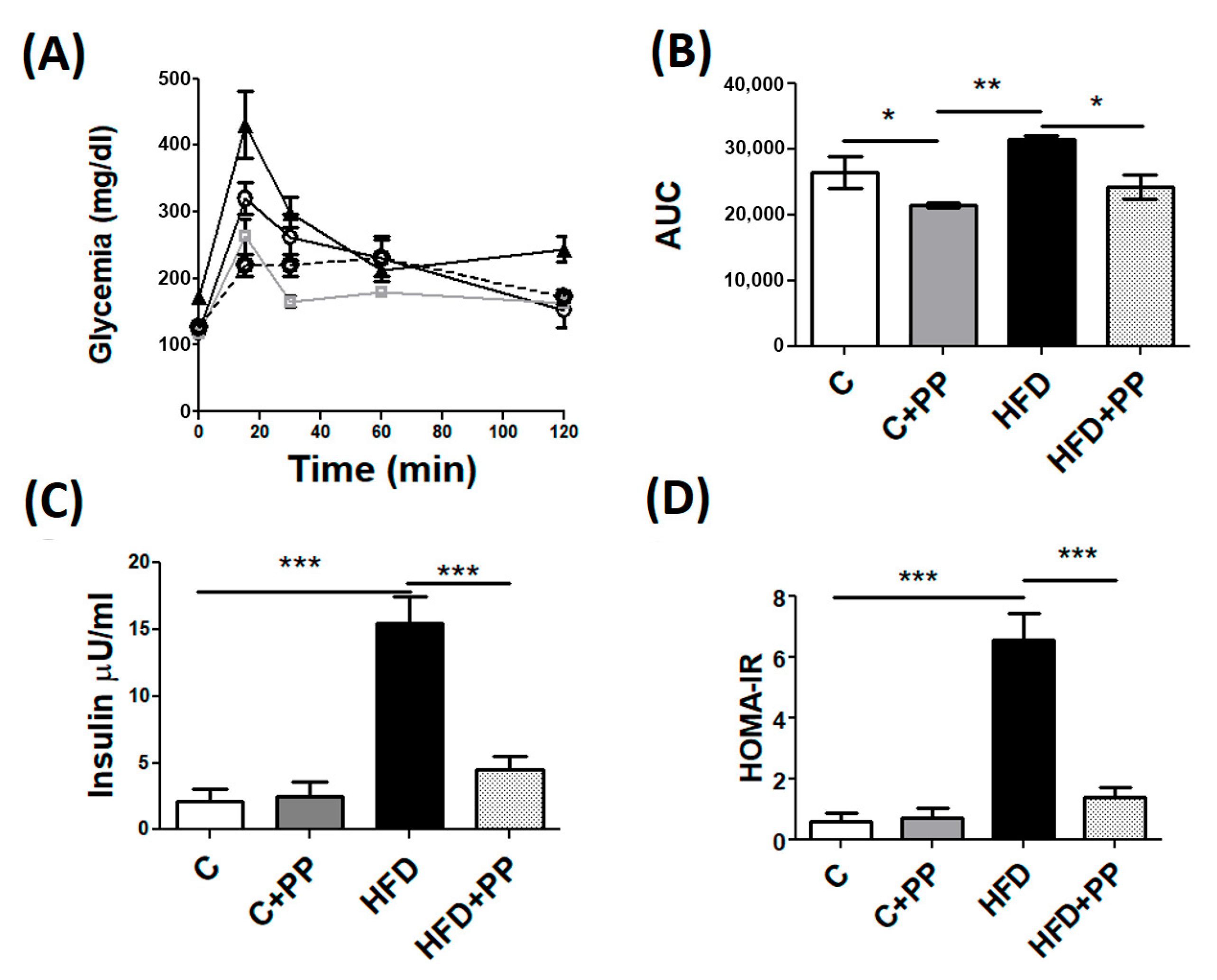
2.4. Intraperitoneal Glucose Tolerance Test (IpGTT)
2.5. Skeletal Fiber Culture
2.6. Glucose Uptake Assay
2.7. Quantitative PCR
2.8. Lipoperoxidation Labeling
2.9. Statistics
3. Results
3.1. Weights and Metabolic Parameters in HFD-Fed Mice Supplemented with PP
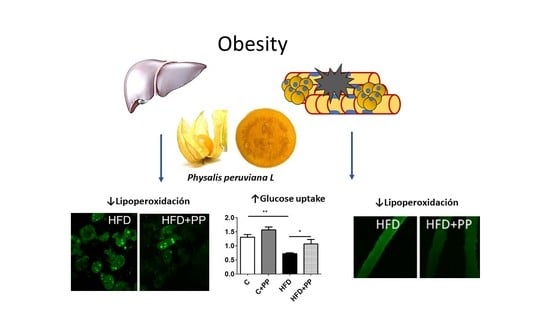
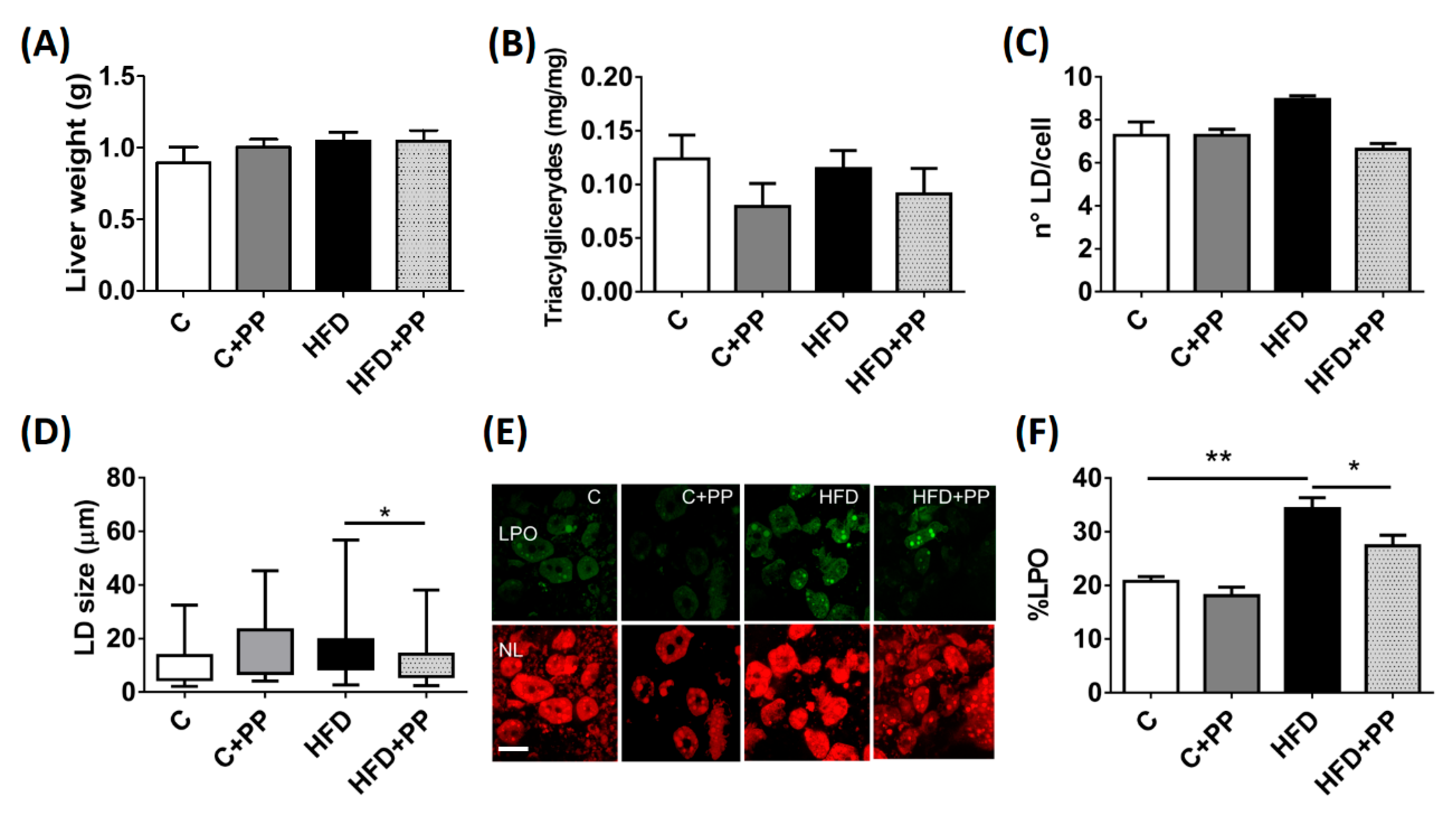
3.2. Hepatic Obesity-Induced Lipoperoxidation is Prevented by PP Supplementation
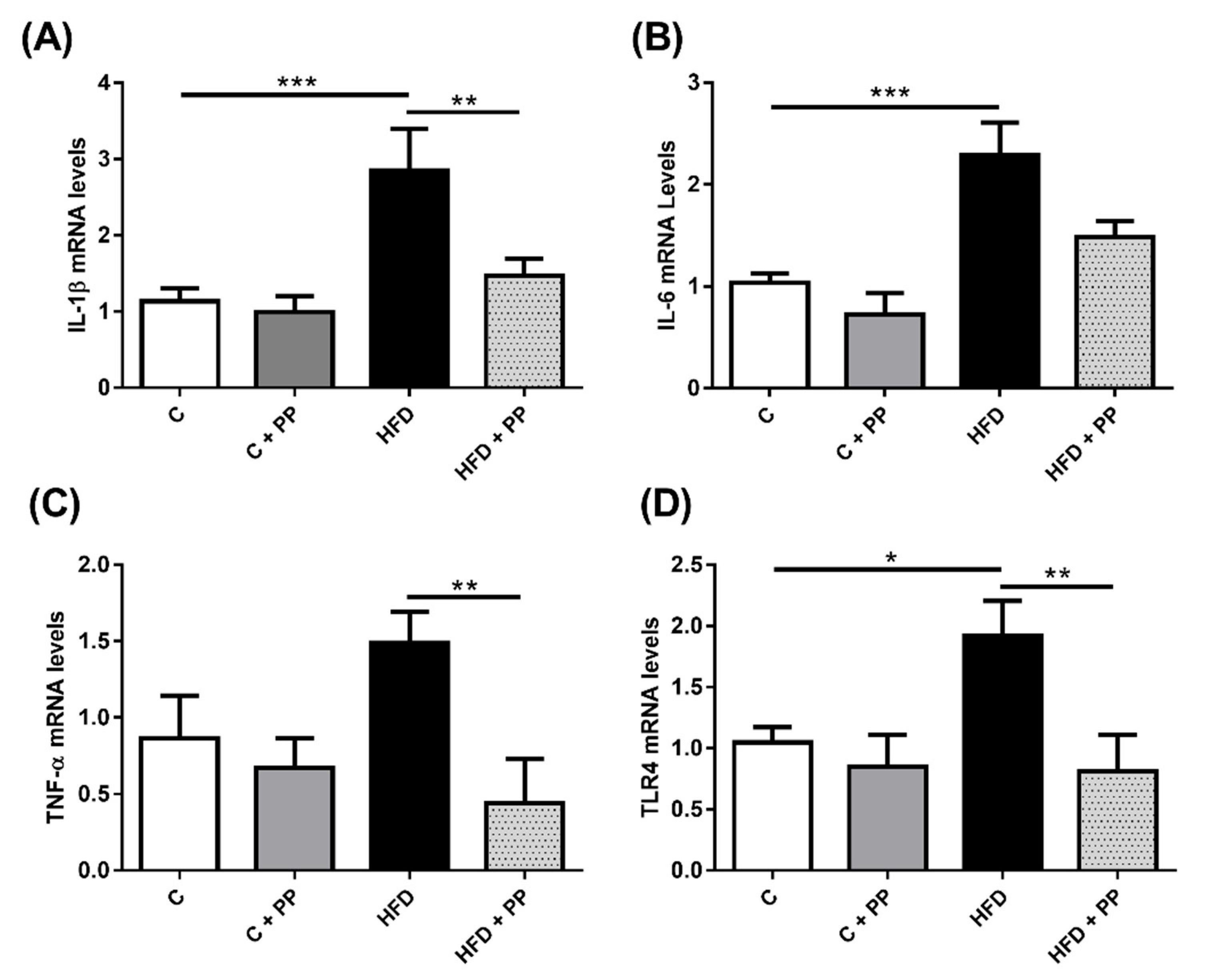
3.3. Pro-Inflammatory Markers in the Liver of HFD-Induced Obese Mice are Prevented with PP Supplementation
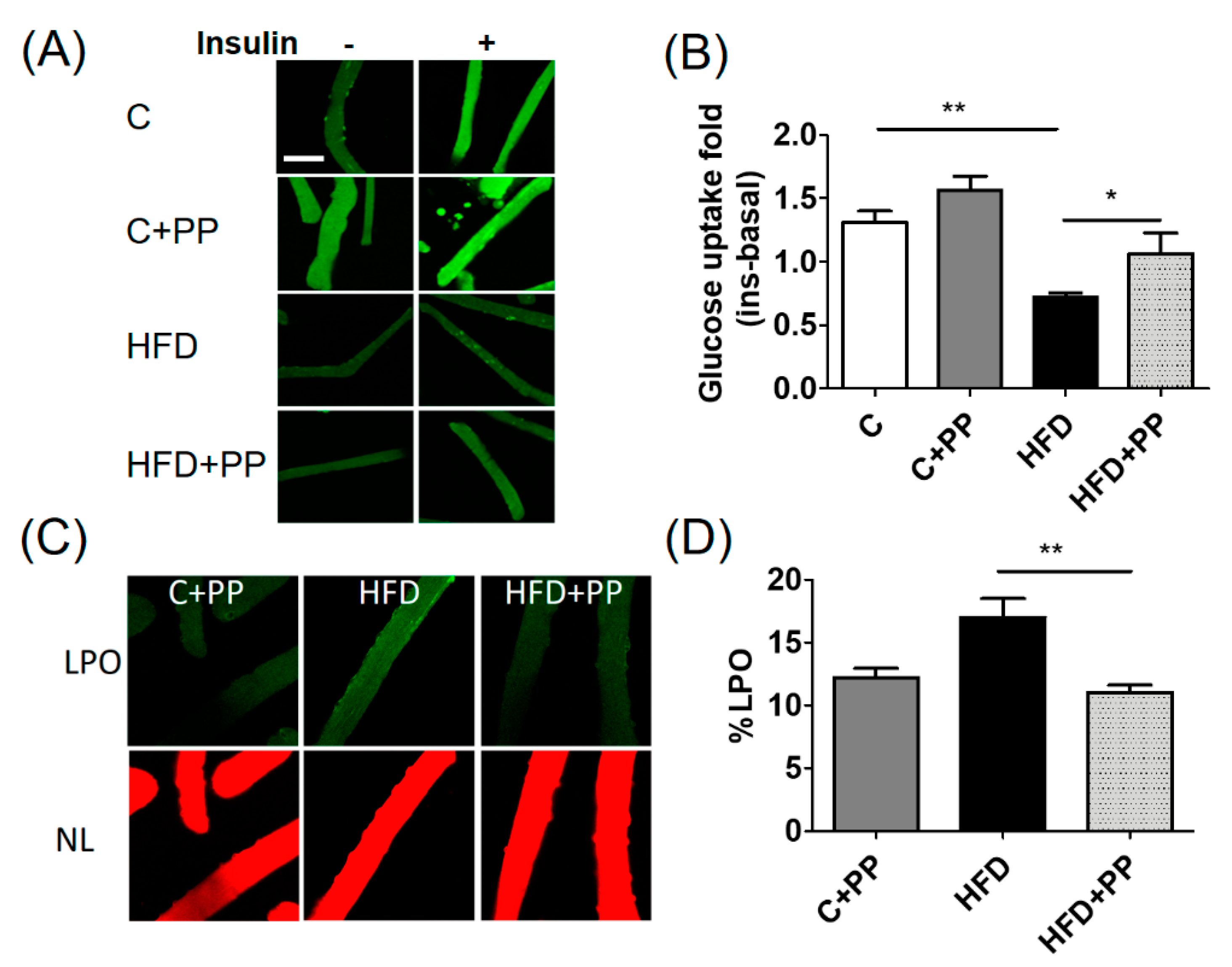
3.4. PP Improves Insulin-Dependent Glucose Uptake and Reduces Lipoperoxidation Level in Skeletal Muscle Fibers from HFD-Fed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grundy, S.M. Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 2016, 64, 1082–1086. [Google Scholar] [CrossRef]
- Veum, V.L.; Laupsa-Borge, J.; Eng, Ø.; Rostrup, E.; Larsen, T.H.; Nordrehaug, J.E.; Nygård, O.K.; Sagen, J.V.; Gudbrandsen, O.A.; Dankel, S.N.; et al. Visceral adiposity and metabolic syndrome after very high-fat and low-fat isocaloric diets: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Maruyama, T.; Yoshikawa, N.; Matsumiya, R.; Ma, Y.; Ito, N.; Tasaka, Y.; Kuribara-Souta, A.; Miyata, K.; Oike, Y.; et al. A muscle-liver-fat signalling axis is essential for central control of adaptive adipose remodelling. Nat. Commun. 2015, 6, 6693. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving concepts in the pathogenesis of NASH: Beyond steatosis and inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef] [PubMed]
- Tateya, S.; Kim, F.; Tamori, Y. Recent Advances in Obesity-Induced Inflammation and Insulin Resistance. Front. Endocrinol. 2013, 4, 93. [Google Scholar] [CrossRef]
- Rosso, C.; Kazankov, K.; Younes, R.; Esmaili, S.; Marietti, M.; Sacco, M.; Carli, F.; Gaggini, M.; Salomone, F.; Møller, H.J.; et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 1012–1021. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, D.; Yu, N.; An, T.; Miao, J.; Mo, F.; Gu, Y.; Zhang, D.; Gao, S.; Jiang, G. Curcumin improves glycolipid metabolism through regulating peroxisome proliferator activated receptor γ signalling pathway in high-fat diet-induced obese mice and 3T3-L1 adipocytes. R. Soc. Open Sci. 2017, 4, 170917. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.-S.; Kim, L.; Park, H.J.; Lee, D.; Kim, H. Anti-Obesity Effects of the Flower of Prunus persica in High-Fat Diet-Induced Obese Mice. Nutrients 2019, 11, 2176. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Zura-Bravo, L.; Lemus-Mondaca, R.; Martinez-Monzó, J.; Quispe-Fuentes, I.; Puente, L.; Di Scala, K. Influence of drying temperature on dietary fibre, rehydration properties, texture and microstructure of Cape gooseberry (Physalis peruviana L.). J. Food Sci. Technol. 2015, 52, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Tejera, E.; Granda-Albuja, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits Consumed in the Western Coastal Region of Ecuador as a Source of Health-Promoting Compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.-M.; Wang, J.; Xie, X.-J.; Xu, L.-J.; Tang, S.-Q. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. Int. J. Mol. Med. 2019, 44, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, Y.; Yi, R.; Zhao, X. Preventive Effect of Blueberry Extract on Liver Injury Induced by Carbon Tetrachloride in Mice. Foods 2019, 8, 48. [Google Scholar] [CrossRef]
- Aranaz, P.; Romo-Hualde, A.; Zabala, M.; Navarro-Herrera, D.; Ruiz de Galarreta, M.; Gil, A.G.; Martinez, J.A.; Milagro, F.I.; González-Navarro, C.J. Freeze-dried strawberry and blueberry attenuates diet-induced obesity and insulin resistance in rats by inhibiting adipogenesis and lipogenesis. Food Funct. 2017, 8, 3999–4013. [Google Scholar] [CrossRef]
- Bazalar Pereda, M.S.; Nazareno, M.A.; Viturro, C.I. Nutritional and Antioxidant Properties of Physalis peruviana L. Fruits from the Argentinean Northern Andean Region. Plant Foods Hum. Nutr. 2019, 74, 68–75. [Google Scholar] [CrossRef]
- Areiza-Mazo, N.; Robles, J.; Zamudio-Rodriguez, J.A.; Giraldez, L.; Echeverria, V.; Barrera-Bailon, B.; Aliev, G.; Sahebkar, A.; Ashraf, G.M.; Barreto, G.E. Extracts of Physalis peruviana Protect Astrocytic Cells Under Oxidative Stress With Rotenone. Front. Chem. 2018, 6, 276. [Google Scholar] [CrossRef]
- Park, H.A.; Lee, J.-W.; Kwon, O.-K.; Lee, G.; Lim, Y.; Kim, J.H.; Paik, J.-H.; Choi, S.; Paryanto, I.; Yuniato, P.; et al. Physalis peruviana L. inhibits airway inflammation induced by cigarette smoke and lipopolysaccharide through inhibition of extracellular signal-regulated kinase and induction of heme oxygenase-1. Int. J. Mol. Med. 2017, 40, 1557–1565. [Google Scholar] [CrossRef][Green Version]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Aref, A.M.; Othman, M.S.; Kassab, R.B.; Abdel Moneim, A.E. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid. Med. Cell. Longev. 2014, 2014, 381413. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Al-Quraishy, S.; Diab, M.M.S.; Othman, M.S.; Aref, A.M.; Abdel Moneim, A.E. The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem. Toxicol. 2014, 74, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Campos, C.; Díaz-Vegas, A.; Galgani, J.E.; Juretic, N.; Osorio-Fuentealba, C.; Bucarey, J.L.; Tapia, G.; Valenzuela, R.; Contreras-Ferrat, A.; et al. Insulin-dependent H2O2 production is higher in muscle fibers of mice fed with a high-fat diet. Int. J. Mol. Sci. 2013, 14, 15740–15754. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Jornayvaz, F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef]
- Negrin, K.A.; Roth Flach, R.J.; DiStefano, M.T.; Matevossian, A.; Friedline, R.H.; Jung, D.; Kim, J.K.; Czech, M.P. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS ONE 2014, 9, e107265. [Google Scholar] [CrossRef]
- Jia, L.; Vianna, C.R.; Fukuda, M.; Berglund, E.D.; Liu, C.; Tao, C.; Sun, K.; Liu, T.; Harper, M.J.; Lee, C.E.; et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 2014, 5, 3878. [Google Scholar] [CrossRef]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.C.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 signaling in obese NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G270–G278. [Google Scholar] [CrossRef]
- Franco, L.A.; Ocampo, Y.C.; Gómez, H.A.; De la Puerta, R.; Espartero, J.L.; Ospina, L.F. Sucrose esters from Physalis peruviana calyces with anti-inflammatory activity. Planta Med. 2014, 80, 1605–1614. [Google Scholar] [CrossRef]
- Tapia, G.; Valenzuela, R.; Espinosa, A.; Romanque, P.; Dossi, C.; Gonzalez-Mañán, D.; Videla, L.A.; D’Espessailles, A. N-3 long-chain PUFA supplementation prevents high fat diet induced mouse liver steatosis and inflammation in relation to PPAR-α upregulation and NF-κB DNA binding abrogation. Mol. Nutr. Food Res. 2014, 58, 1333–1341. [Google Scholar] [CrossRef]
- Pino-de la Fuente, F.; Quezada, L.; Sepúlveda, C.; Monsalves-Alvarez, M.; Rodríguez, J.M.; Sacristán, C.; Chiong, M.; Llanos, M.; Espinosa, A.; Troncoso, R. Exercise regulates lipid droplet dynamics in normal and fatty liver. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158519. [Google Scholar] [CrossRef]
- Puente, L.; Nocetti, D.; Espinosa, A. Physalis peruviana Linnaeus, an Update on its Functional Properties and Beneficial Effects in Human Health. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Cham, Switzerland, 2019; pp. 447–463. [Google Scholar]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanuš, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef] [PubMed]
| C (a) | C + PP (b) | HFD (c) | HFD + PP (d) | |
|---|---|---|---|---|
| Total Weight (g) | 22.7 ± 1.1 ** (d) *** (c) | 26.8 ± 0.8 ** (c, d) | 38.6 ± 1.6 ** (b) *** (a) | 35.8 ± 3.4 ** (a, b) |
| Visceral Fat Weight (mg) | 147.0 ± 0.5 ** (d) | 156.0 ± 1.6 *** (b) | 407.0 ± 7.0 ** (a), *** (b) | 214.0 ± 3.1 ** (a, c) |
| Fasting glycemia (mg/dL) | 140.6 ± 22.4 ** (c, d) | 127.3 ± 3.9 *** (c), ** (d) | 190.5 ± 7.2 ** (a), *** (b) | 168.1 ± 10.7 ** (a, c), *** (b) |
| Triacylglycerides (mg/dL) | 66.7 ± 8.9 | 46.0 ± 2.4 | 52.2 ± 4.1 | 55.3 ± 2.9 |
| Total cholesterol (mg/dL) | 94.5 ± 3.2 | 89.2 ± 3.8 | 135.2 ± 5.7 ** (a, b) | 130.7 ± 9.6 ** (a, b) |
| ALT (UI/l) | 53.0 ± 5.3 | 62.0 ± 12.3 | 52.0 ± 6.5 | 67.0 ± 7.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino-de la Fuente, F.; Nocetti, D.; Sacristán, C.; Ruiz, P.; Guerrero, J.; Jorquera, G.; Uribe, E.; Bucarey, J.L.; Espinosa, A.; Puente, L. Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice. Nutrients 2020, 12, 700. https://doi.org/10.3390/nu12030700
Pino-de la Fuente F, Nocetti D, Sacristán C, Ruiz P, Guerrero J, Jorquera G, Uribe E, Bucarey JL, Espinosa A, Puente L. Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice. Nutrients. 2020; 12(3):700. https://doi.org/10.3390/nu12030700
Chicago/Turabian StylePino-de la Fuente, Francisco, Diego Nocetti, Camila Sacristán, Paulina Ruiz, Julia Guerrero, Gonzalo Jorquera, Ernesto Uribe, José Luis Bucarey, Alejandra Espinosa, and Luis Puente. 2020. "Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice" Nutrients 12, no. 3: 700. https://doi.org/10.3390/nu12030700
APA StylePino-de la Fuente, F., Nocetti, D., Sacristán, C., Ruiz, P., Guerrero, J., Jorquera, G., Uribe, E., Bucarey, J. L., Espinosa, A., & Puente, L. (2020). Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice. Nutrients, 12(3), 700. https://doi.org/10.3390/nu12030700