Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Study Design
2.2. Dietary Assessment, Anthropometric Data and Blood Pressure
2.3. Blood Sampling and Laboratory Measurements
2.4. Endotoxin Measurement
2.5. Statistical Analysis
2.6. Clinical Trial and Ethical Considerations
3. Results
3.1. Clinical Characteristics of the NAFLD Patient Cohort and Healthy Controls
3.2. Differences in Nutritional Intake in Patients with NAFLD and Healthy Controls
3.3. Levels of Bacterial Endotoxin and LBP
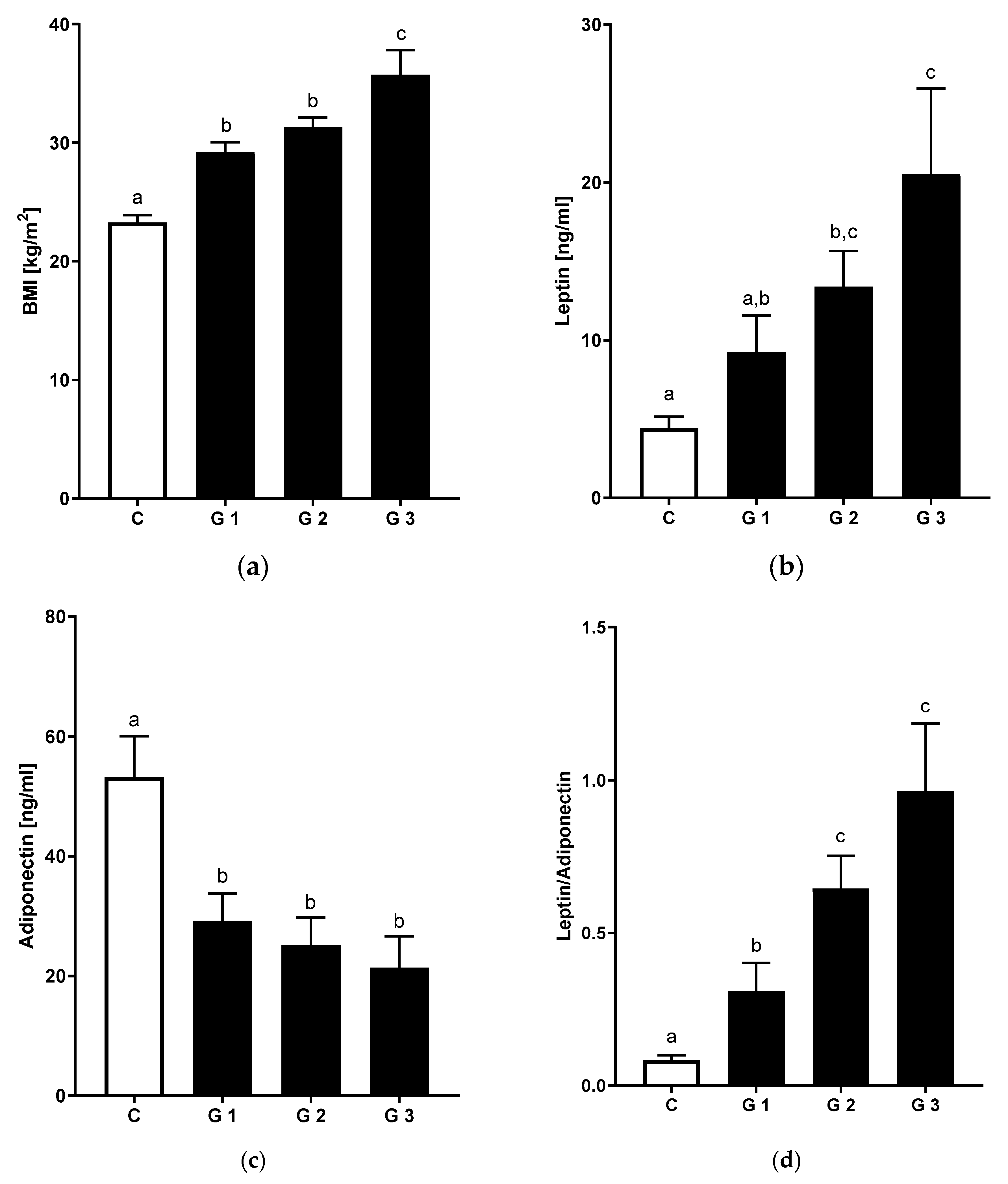
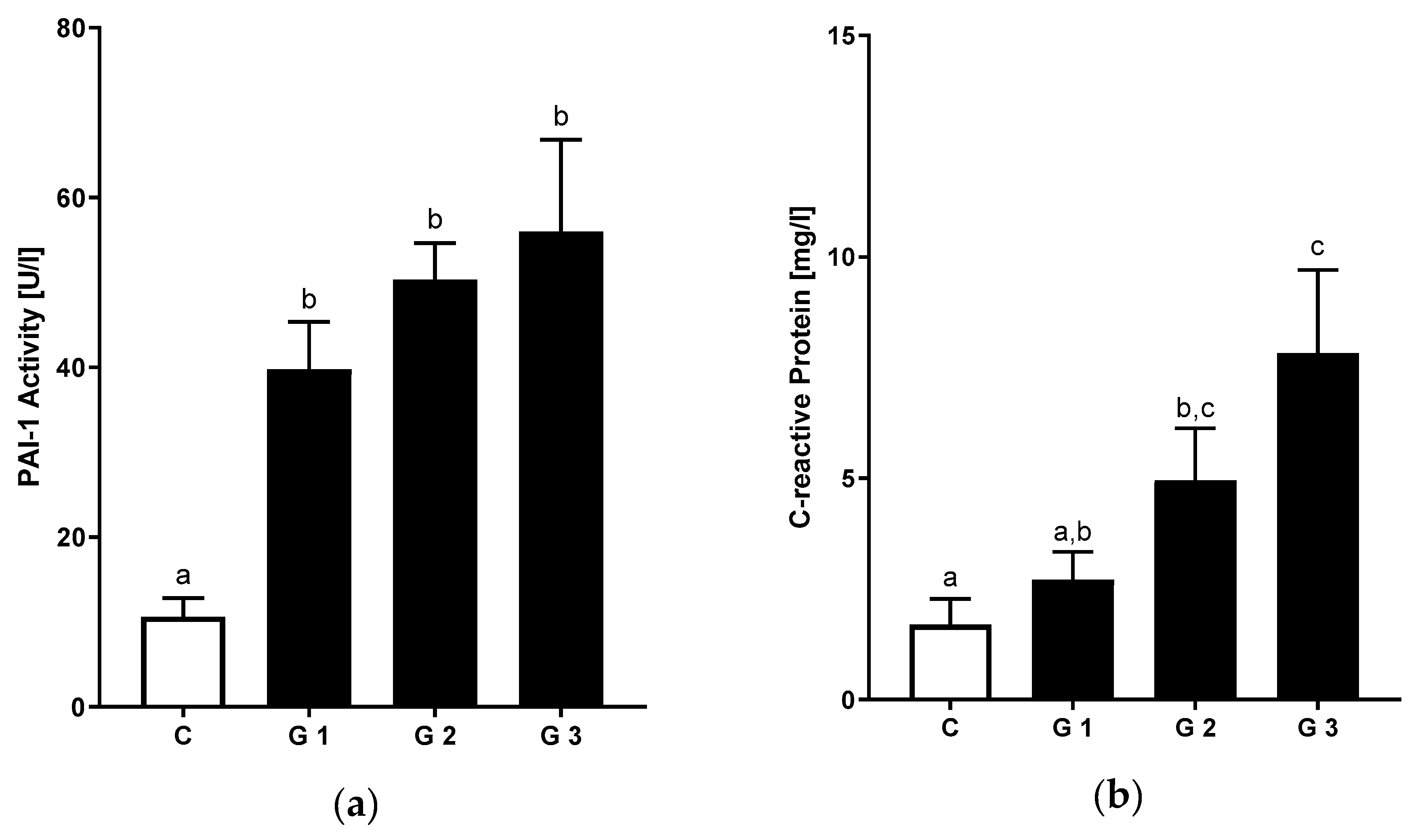
3.4. Relation of Hepatic Steatosis and Inflammatory Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Vipani, A.; Bresee, C.; Todo, T.; Kim, I.K.; Alkhouri, N.; Setiawan, V.W.; Tran, T.; Ayoub, W.S.; Lu, S.C.; et al. Nash leading cause of liver transplant in women: Updated analysis of indications for liver transplant and ethnic and gender variances. Am. J. Gastroenterol. 2018, 113, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Schuppan, D. Nonalcoholic steatohepatitis: The therapeutic challenge of a global epidemic. Curr. Opin. Lipidol. 2011, 22, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Schattenberg, J.M. Non-alcoholic steatohepatitis: Pathogenesis and novel therapeutic approaches. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 1), 68–76. [Google Scholar] [CrossRef]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.C.; Roudot-Thoraval, F. The burden of liver disease in europe: A review of available epidemiological data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef]
- Michel, M.; Schattenberg, J.M. Effectiveness of lifestyle interventions in nafld (nonalcoholic fatty liver disease) –how are clinical trials affected? Expert Opin. Investig. Drugs 2020, 1–5. [Google Scholar] [CrossRef]
- Cook, N.; Geier, A.; Schmid, A.; Hirschfield, G.; Kautz, A.; Schattenberg, J.M.; Balp, M.M. The patient perspectives on future therapeutic options in nash and patient needs. Front. Med. (Lausanne) 2019, 6, 61. [Google Scholar] [CrossRef]
- McManus, K.; Antinoro, L.; Sacks, F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int. J. Obes. 2001, 25, 1503–1511. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in nafld. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Le Marchand, L.; Rosen, H.R.; Setiawan, V.W. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: The multiethnic cohort. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canizales, J.; Dominguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Gonzalez-Cordova, A.F.; Vallejo-Cordoba, B.; Salazar-Lopez, N.J.; Gonzalez-Aguilar, G.A. Fiber and phenolic compounds contribution to the hepatoprotective effects of mango diets in rats fed high cholesterol/sodium cholate. Phytother. Res. 2019, 33, 2996–3007. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Faga, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Nier, A.; Brandt, A.; Rajcic, D.; Bruns, T.; Bergheim, I. Short-term isocaloric intake of a fructose- but not glucose-rich diet affects bacterial endotoxin concentrations and markers of metabolic health in normal weight healthy subjects. Mol. Nutr. Food Res. 2019, 63, e1800868. [Google Scholar] [CrossRef] [PubMed]
- Liebig, S.; Stoeckmann, N.; Geier, A.; Rau, M.; Schattenberg, J.M.; Bahr, M.J.; Manns, M.P.; Jaeckel, E.; Schulze-Osthoff, K.; Bantel, H. Multicenter validation study of a diagnostic algorithm to detect nash and fibrosis in nafld patients with low nafld fibrosis score or liver stiffness. Clin. Transl. Gastroenterol. 2019, 10, e00066. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Thuy, S.; Ladurner, R.; Volynets, V.; Wagner, S.; Strahl, S.; Konigsrainer, A.; Maier, K.P.; Bischoff, S.C.; Bergheim, I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J. Nutr. 2008, 138, 1452–1455. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Wong, V.W.; Chu, W.C.; Wong, G.L.; Li, L.S.; Leung, J.; Chim, A.M.; Yeung, D.K.; Sea, M.M.; Woo, J.; et al. Diet-quality scores and prevalence of nonalcoholic fatty liver disease: A population study using proton-magnetic resonance spectroscopy. PLoS ONE 2015, 10, e0139310. [Google Scholar] [CrossRef] [PubMed]
- Klementova, M.; Belinova, L.; Haluzik, M.; Pavlovicova, R.; Hill, M.; Pelikanova, T.; Kahleova, H. The effect of two isocaloric and energy-matched plant-based and processed-meat meals on glucose metabolism, gastrointestinal hormones, and satiety in subjects with t2d, obese subjects, and healthy controls—A randomized crossover study. Diabetes 2018, 67. [Google Scholar] [CrossRef]
- Giorgio, V.; Miele, L.; Principessa, L.; Ferretti, F.; Villa, M.P.; Negro, V.; Grieco, A.; Alisi, A.; Nobili, V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig. Liver Dis. 2014, 46, 556–560. [Google Scholar] [CrossRef]
- Krawczyk, M.; Maciejewska, D.; Ryterska, K.; Czerwinka-Rogowska, M.; Jamiol-Milc, D.; Skonieczna-Zydecka, K.; Milkiewicz, P.; Raszeja-Wyszomirska, J.; Stachowska, E. Gut permeability might be improved by dietary fiber in individuals with nonalcoholic fatty liver disease (nafld) undergoing weight reduction. Nutrients 2018, 10, 1793. [Google Scholar] [CrossRef]
- Volynets, V.; Kuper, M.A.; Strahl, S.; Maier, I.B.; Spruss, A.; Wagnerberger, S.; Konigsrainer, A.; Bischoff, S.C.; Bergheim, I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (nafld). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Nier, A.; Brandt, A.; Conzelmann, I.B.; Ozel, Y.; Bergheim, I. Non-alcoholic fatty liver disease in overweight children: Role of fructose intake and dietary pattern. Nutrients 2018, 10, 1329. [Google Scholar] [CrossRef]
- Ruiz, A.G.; Casafont, F.; Crespo, J.; Cayon, A.; Mayorga, M.; Estebanez, A.; Fernadez-Escalante, J.C.; Pons-Romero, F. Lipopolysaccharide-binding protein plasma levels and liver tnf-alpha gene expression in obese patients: Evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes. Surg. 2007, 17, 1374–1380. [Google Scholar] [CrossRef]
- Labenz, C.; Prochaska, J.H.; Huber, Y.; Nagel, M.; Straub, B.K.; Wild, P.; Galle, P.R.; Schattenberg, J.M. Cardiovascular risk categories in patients with nonalcoholic fatty liver disease and the role of low-density lipoprotein cholesterol. Hepatol. Commun. 2019, 3, 1472–1481. [Google Scholar] [CrossRef]
- Zeb, I.; Li, D.; Budoff, M.J.; Katz, R.; Lloyd-Jones, D.; Agatston, A.; Blumenthal, R.S.; Blaha, M.J.; Blankstein, R.; Carr, J.; et al. Nonalcoholic fatty liver disease and incident cardiac events: The multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2016, 67, 1965–1966. [Google Scholar] [CrossRef]
- Rosso, C.; Kazankov, K.; Younes, R.; Esmaili, S.; Marietti, M.; Sacco, M.; Carli, F.; Gaggini, M.; Salomone, F.; Moller, H.J.; et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jung, H.S.; Yun, K.E.; Cho, J.; Cho, Y.K.; Ryu, S. Cohort study of non-alcoholic fatty liver disease, nafld fibrosis score, and the risk of incident diabetes in a korean population. Am. J. Gastroenterol. 2013, 108, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Kang, D.; Chang, Y.; Ryu, S.; Cho, S.J.; Paik, S.W.; Song, Y.B.; Pastor-Barriuso, R.; Guallar, E.; Cho, J.; et al. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: A cohort study. J. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef]

| Parameter | Controls | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| n | 14 | 20 | 31 | 12 |
| Age | 47.4 ± 1.2 | 51.5 ± 2.4 | 47.9 ± 2.4 | 52.2 ± 4.2 |
| Sex [m/f] | 4/10 | 11/9 | 20/11 | 2/10 $ |
| BMI [kg/m2] | 23.3 ± 0.7 a | 29.2 ± 0.8 b | 31.3 ± 0.8 b | 35.7 ± 2.1 c |
| Waist circumference [cm] | 77.7 ± 2.3 a | 100.8 ± 2.8 b | 109.8 ± 2.2 b,c | 115.1 ± 4.3 c |
| ALT activity [U/L] | 18.4 ± 1.5 a | 58.7 ± 6.9 b | 82.6 ± 9.6 b | 59.4 ± 8.0 b |
| AST activity [U/L] | 18.4 ± 1.4 a | 38.2 ± 3.2 b | 47.4 ± 3.8 b | 54.3 ± 9.0 b |
| γ-GT activity [U/L] | 18.4 ± 2.3 a | 124.7 ± 22.4 b | 69.2 ± 8.5 b | 263.9 ± 100.5 b |
| Systolic Blood Pressure [mmHg] # | 124.4 ± 2.6 a | 136.9 ± 3.8 a,b | 141.1 ± 3.6 b | 142.4 ± 5.6 b |
| Diastolic Blood Pressure [mmHg] # | 82.3 ± 1.7 | 88.9 ± 2.4 | 88.1 ± 1.7 | 85.7 ± 2.6 |
| Triglycerides [mg/dL] | 87.0 ± 16.1 a | 147.5 ± 17.2 b | 174.2 ± 15.7 b | 193.1 ± 23.4 b |
| Total Cholesterol [mg/dL] | 195.6 ± 7.2 a | 232.1 ± 9.2 a,b | 208.5 ± 6.0 a,b | 240.6 ± 18.5 b |
| HDL-Cholesterol [mg/dL] | 72.9 ± 3.4 a | 55.6 ± 4.5 b | 43.7 ± 1.5 c | 51.1 ± 3.1 b,c |
| LDL-Cholesterol [mg/dL] | 107.2 ± 6.7 a | 147.0 ± 7.7 b | 129.4 ± 6.1 a,b | 150.8 ± 15.8 b |
| Fasting Blood Glucose [mg/dL] # | 79.2 ± 2.8 a | 97.2 ± 2.3 b | 114.9 ± 5.8 b | 137.3 ± 18.5 b |
| Fasting Insulin [mU/L] # | 4.4 ± 0.4 a | 11.2 ± 1.1 b | 14.9 ± 1.7 b | 27.6 ± 6.6 c |
| HOMA-IR # | 0.9 ± 0.1 a | 2.8 ± 0.3 b | 4.6 ± 2.8 b | 10.6 ± 3.8 b |
| Uric Acid [mg/dL] | 4.2 ± 0.3 a | 6.0 ± 0.4 b | 6.3 ± 0.2 b | 6.2 ± 0.4 b |
| Parameter | Controls | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| n | 12 # | 19 # | 31 | 12 |
| Total Energy [kcal/] | 2194 ± 185 | 2167 ± 115 | 2184 ± 110 | 1860 ± 118 |
| Protein [g/day] | 82 ± 8 | 85 ± 6 | 83 ± 5 | 79 ± 7 |
| Fat [g/day] | 93 ± 10 | 96 ± 6 | 98 ± 7 | 73 ± 5 |
| Carbohydrates [g/day] | 242 ± 18 | 230 ± 18 | 233 ± 13 | 206 ± 23 |
| Fiber [g/day] | 26 ± 2 a | 19 ± 1 a,b | 21 ± 1 a,b | 17 ± 2 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nier, A.; Huber, Y.; Labenz, C.; Michel, M.; Bergheim, I.; Schattenberg, J.M. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 699. https://doi.org/10.3390/nu12030699
Nier A, Huber Y, Labenz C, Michel M, Bergheim I, Schattenberg JM. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020; 12(3):699. https://doi.org/10.3390/nu12030699
Chicago/Turabian StyleNier, Anika, Yvonne Huber, Christian Labenz, Maurice Michel, Ina Bergheim, and Jörn M. Schattenberg. 2020. "Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD)" Nutrients 12, no. 3: 699. https://doi.org/10.3390/nu12030699
APA StyleNier, A., Huber, Y., Labenz, C., Michel, M., Bergheim, I., & Schattenberg, J. M. (2020). Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients, 12(3), 699. https://doi.org/10.3390/nu12030699






