Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture
Abstract
1. Introduction
2. Materials and Methods
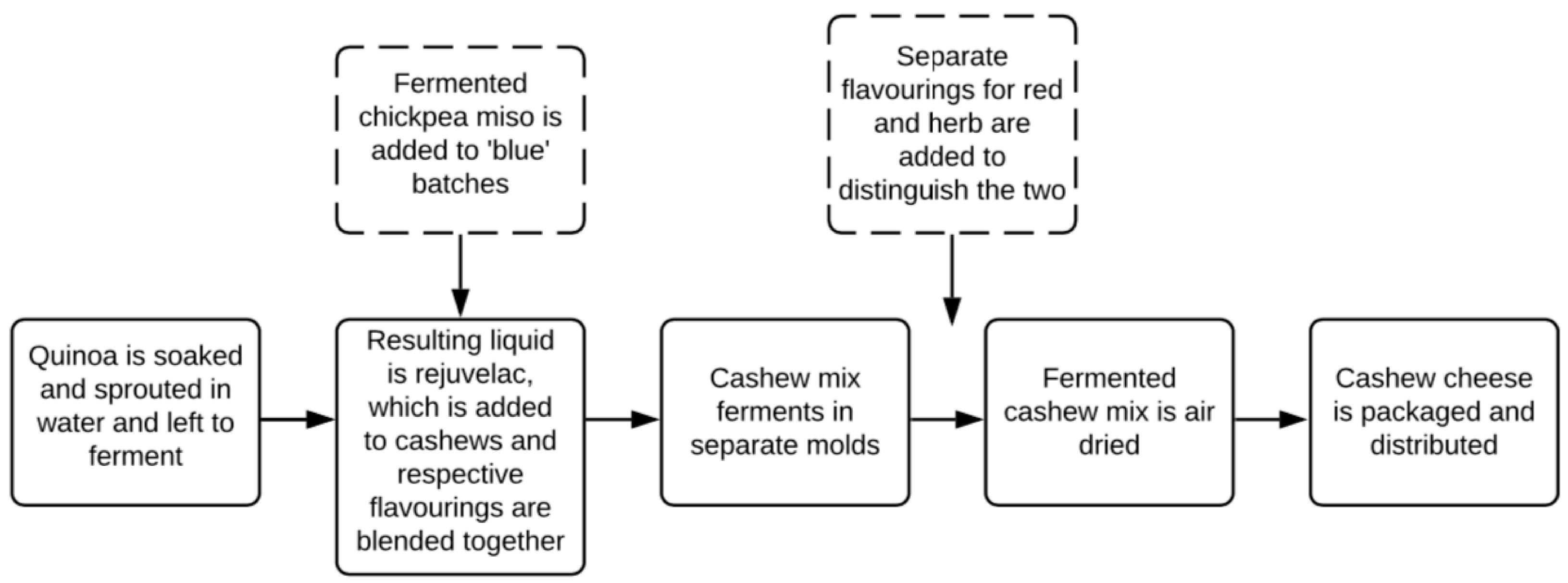
2.1. Fermented Cashew Cheese-Like Product Production Process
2.2. Product Sampling
2.3. Nutritional Analysis
2.4. Culturing of Microorganisms
2.5. PCR and 16S rRNA Gene Analysis of Bacterial Isolates and 18S rRNA Gene Analysis of Yeast Isolates
2.6. Microbiota Extraction, Sequencing, and Analysis
2.7. Cashew Allergenicity Testing of Products at Different Stages of Production
3. Results
3.1. Nutritional Analysis
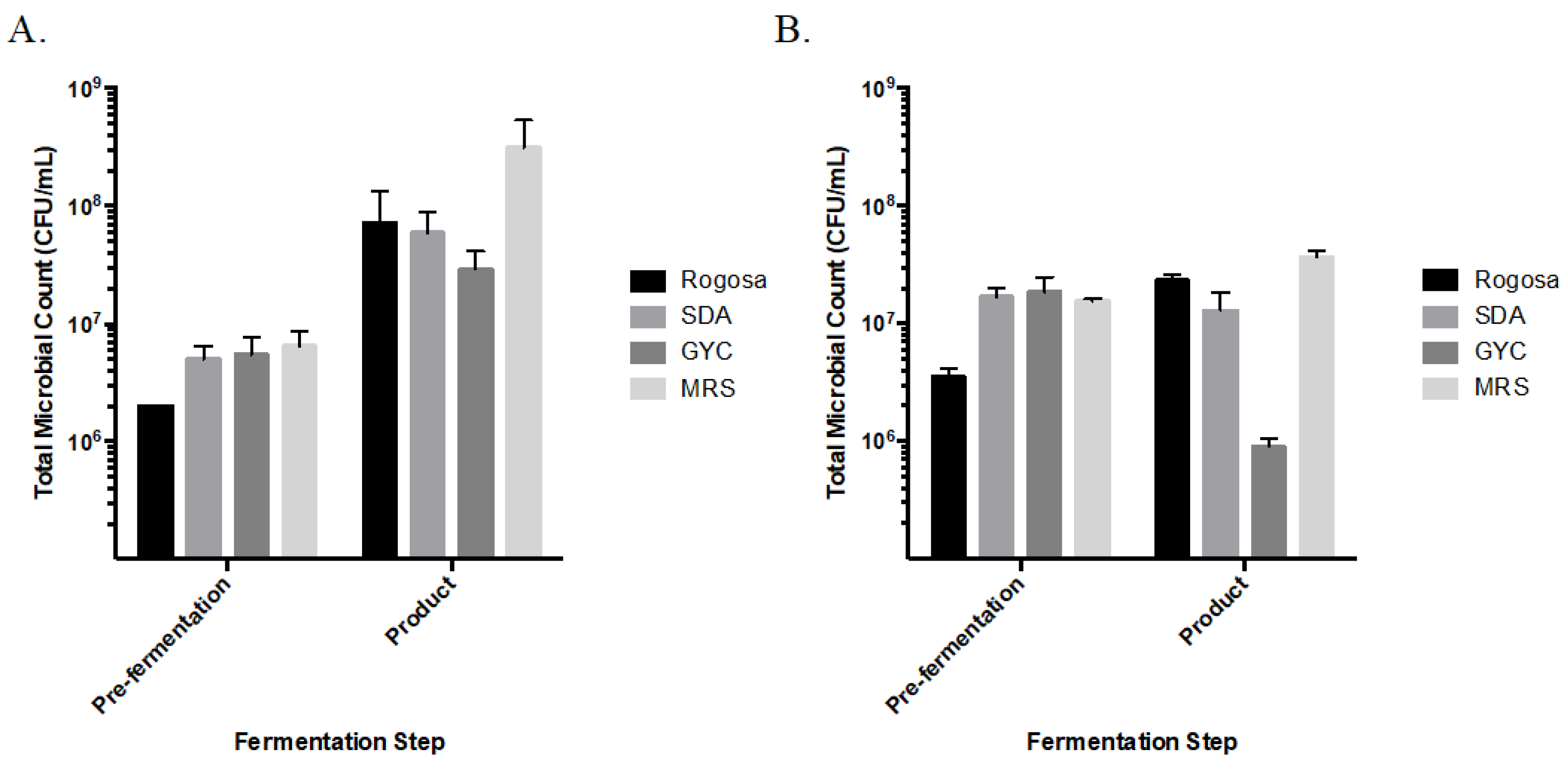
3.2. Viable Microbial Counts
3.3. Identification of Bacterial Isolates
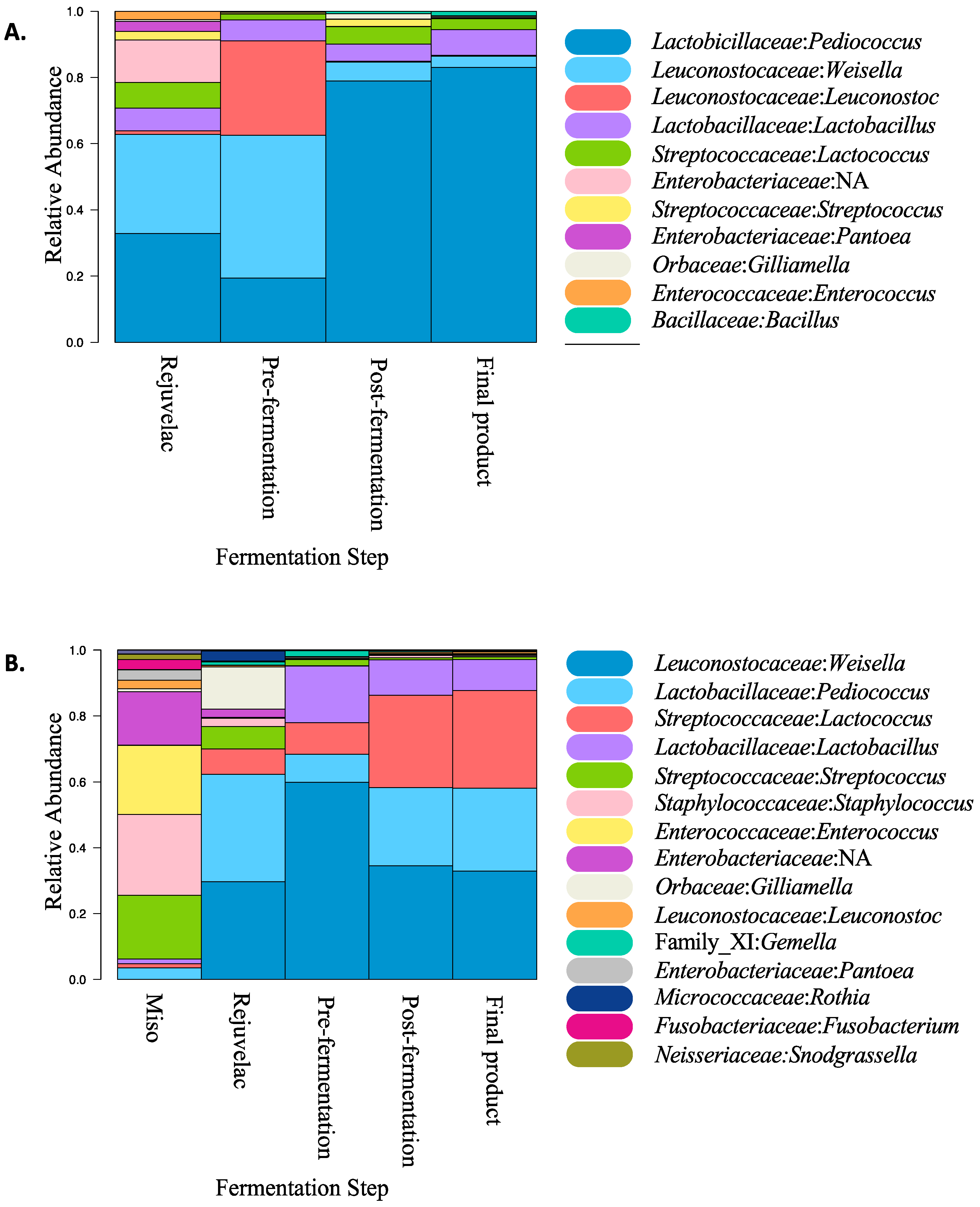
3.4. Microbiota Characterisation of Fermentation Stages and Products
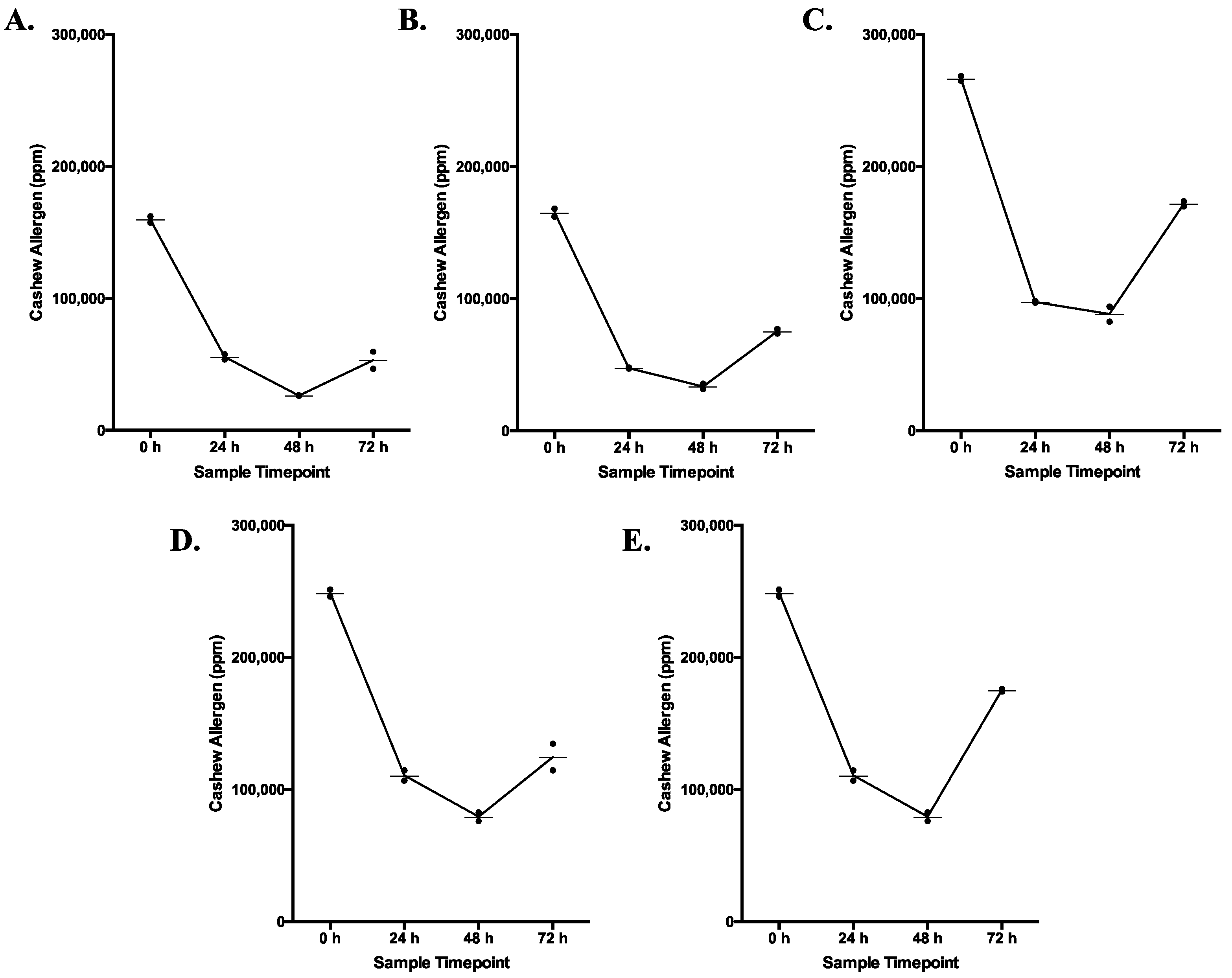
3.5. Cashew Allergenicity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nuraida, L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci. Hum. Wellness 2015, 4, 47–55. [Google Scholar] [CrossRef]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A review of fermented foods with beneficial effects on brain and cognitive function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef]
- Navarrete-Bolaños, J.L. Improving traditional fermented beverages: How to evolve from spontaneous to directed fermentation. Eng. Life Sci. 2012, 12, 410–418. [Google Scholar] [CrossRef]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Macori, G.; Cotter, P.D. Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 2018, 49, 172–178. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Johnson, M.E. A 100-year review: Cheese production and quality. J. Dairy Sci. 2017, 100, 9952–9965. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Kolars, J.C.; Levitt, M.D.; Aouji, M.; Savaiano, D.A. Yogurt—An autodigesting source of lactose. N. Engl. J. Med. 1984, 310, 1–3. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zhang, Y.; Chen, F. Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. J. Sci. Food Agric. 2010, 90, 2015–2020. [Google Scholar] [CrossRef]
- Song, Y.-S.; Frias, J.; Martinez-Villaluenga, C.; Vidal-Valdeverde, C.; de Mejia, E.G. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 2008, 108, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, B.; Berg, A.V.; Bollrath, C.; Berdel, D.; Schauer, U.; Rieger, C.; Haschke-Becher, E.; Wahn, U. Safety and efficacy of a new extensively hydrolyzed formula for infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2008, 19, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bahna, S.L. Hypoallergenic formulas: Optimal choices for treatment versus prevention. Ann. Allergy Asthma Immunol. 2008, 101, 453–459. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Mattison, C.P.; Grimm, C.C.; Reed, S. Simple methods to reduce major allergens Ara h 1 and Ana o 1/2 in peanut and cashew extracts. Food Sci. Nutr. 2017, 5, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Pedrosa, M.M.; Rodríguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract. Int. Arch. Allergy Immunol. 2012, 157, 41–50. [Google Scholar] [CrossRef]
- Kasera, R.; Singh, A.B.; Lavasa, S.; Prasad, K.N.; Arora, N. Enzymatic hydrolysis: A method in alleviating legume allergenicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 76, 54–60. [Google Scholar] [CrossRef]
- van der Valk, J.P.M.; Dubois, A.E.J.; van Wijk, R.G.; Wichers, H.J.; de Jong, N.W. Systematic review on cashew nut allergy. Allergy 2014, 69, 692–698. [Google Scholar] [CrossRef]
- Clark, A.T.; Anagnostou, K.; Ewan, P.W. Cashew nut causes more severe reactions than peanut: Case-matched comparison in 141 children. Allergy 2007, 62, 913–916. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef]
- Sharafi, S.; Rasooli, I.; Beheshti-Maal, K. Isolation, characterization and optimization of indigenous acetic acid bacteria and evaluation of their preservation methods. Iran. J. Microbiol. 2010, 2, 38–45. [Google Scholar]
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, S.L.; Montes de Oca, C.E.; Laiño, J.E.; Suarez, N.E.; Vignolo, G.; LeBlanc, J.G.; Rollán, G. Ancestral Andean grain quinoa as source of lactic acid bacteria capable to degrade phytate and produce B-group vitamins. Food Res. Int. 2016, 89, 488–494. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Komagata, K. Lactic acid bacteria in fermented foods in Thailand. World J. Microbiol. Biotechnol. 1995, 11, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Quero, G.M.; Cho, G.-S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Olstorpe, M.; Schnürer, J.; Passoth, V. Growth inhibition of various Enterobacteriaceae species by the yeast Hansenula anomala during storage of moist cereal grain. Appl. Environ. Microbiol. 2012, 78, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Amorim, A.; Nascimento, J.D.S. A highlight for non-Escherichia coli and non-Salmonella sp. Enterobacteriaceae in dairy foods contamination. Front. Microbiol. 2017. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, K.B. Antibacterial activity of the noni fruit extract against Listeria monocytogenes and its applicability as a natural sanitizer for the washing of fresh-cut produce. Food Microbiol. 2019. [Google Scholar] [CrossRef]
- Kim, J.Y.; Young, J.A.; Gunther, N.W.; Lee, J.-L. Inhibition of Salmonella by bacteriocin-producing lactic acid bacteria derived from U.S. kimchi and broiler chicken. J. Food Saf. 2015, 35, 1–12. [Google Scholar] [CrossRef]
- Camargo, A.C.; Todorov, S.D.; Chihib, N.E.; Drider, D.; Nero, L.A. Lactic acid bacteria (LAB) and their bacteriocins as alternative biotechnological tools to control Listeria monocytogenes biofilms in food processing facilities. Mol. Biotechnol. 2018, 60, 712–726. [Google Scholar] [CrossRef]
- Bearson, S.; Bearson, B.; Foster, J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997, 147, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 77. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Pasini, F.; Riciputi, Y.; Vannini, L.; Gozzi, G.; Balestra, F.; Caboni, M.F.; Gardini, F.; Montanari, C. Fermented nut-based vegan food: Characterization of a home made product and scale-up to an industrial pilot-scale production. J. Food Sci. 2018, 83, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.V.; Meinardi, C.A.; Zalazar, C.A. Production of flavour compounds from fat during cheese ripening by action of lipases and esterases. Protein Pept. Lett. 2009, 16, 1235–1243. [Google Scholar] [CrossRef]
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional composition of raw fresh cashew (Anacardium occidentale L.) kernels from different origin. Food Sci. Nutr. 2016, 4, 329–338. [Google Scholar] [CrossRef]
- Government of Canada Dietary Reference Intakes. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/tables.html#rvv (accessed on 13 June 2019).
- Yu, J. An overview of peanut allergenicity and enzymatic treatment for reducing it. Med. Res. Arch. 2018. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Cheng, H.; Maleki, S. Enzymatic treatment of peanut kernels to reduce allergen levels. Food Chem. 2011, 127, 1014–1022. [Google Scholar] [CrossRef]
- Teuber, S.S.; Sathe, S.K.; Peterson, W.R.; Roux, K.H. Characterization of the soluble allergenic proteins of cashew nut (Anacardium occidentale L.). J. Agric. Food Chem. 2002, 50, 6543–6549. [Google Scholar] [CrossRef]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Tawde, P.; Sathe, S.K.; Roux, K.H. Ana o 1, a cashew (Anacardium occidental) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002, 110, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Ana o 2, a major cashew (Anacardium occidentale L.) nut allergen of the legumin family. Int. Arch. Allergy Immunol. 2003, 132, 27–39. [Google Scholar] [CrossRef]
- Robotham, J.M.; Wang, F.; Seamon, V.; Teuber, S.S.; Sathe, S.K.; Sampson, H.A.; Beyer, K.; Seavy, M.; Roux, K.H. Ana o 3, an important cashew nut (Anacardium occidentale L.) allergen of the 2S albumin family. J. Allergy Clin. Immunol. 2005, 115, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Davoren, M.; Peake, J. Cashew nut allergy is associated with a high risk of anaphylaxis. Arch. Dis. Child. 2005, 90, 1084–1085. [Google Scholar] [CrossRef]
- Liu, M.; Bayjanov, J.R.; Renckens, B.; Nauta, A.; Siezen, R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genom. 2010, 36. [Google Scholar] [CrossRef]
- Taylor, S.L.; Baumert, J.L.; Kruizinga, A.G.; Remington, B.C.; Crevel, R.W.R.; Brooke-Taylor, S.; Allen, K.J.; Houben, G. Establishment of Reference Doses for residues of allergenic foods: Report of the VITAL Expert Panel. Food Chem. Toxicol. 2014, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]

| Compound | Pre-Fermentation | Post-Fermentation | Final Product | Unit |
|---|---|---|---|---|
| saturated fat | 18.6 | 19.7 | 18.2 | % |
| monounsaturated fat | 11.3 | 12.4 | 10.9 | % |
| polyunsaturated fat | 3 | 3.3 | 2.9 | % |
| trans fat | <0.1 | <0.1 | <0.1 | % |
| total fat | 35.1 | 37.6 | 33.9 | % |
| omega 3 fat | <0.1 | <0.1 | <0.1 | % |
| omega 9 fat | 11.2 | 12.4 | 10.8 | % |
| omega 6 fatty acids | 3 | 3.3 | 2.9 | % |
| calories from fat | 316 | 338 | 305 | cal/100 g |
| crude protein content | 8.3 | 8.1 | 8.2 | % |
| crude ash content | 2.5 | 1.5 | 1.4 | % |
| carbohydrates | 10.2 | 8.9 | 13.1 | % |
| energy | 389 | 406 | 390 | cal/100 g |
| energy | 1629 | 1700 | 1632 | kJ/100 g |
| cholesterol | <2 | <2 | <2 | mg/100 g |
| fructose | <0.2 | <0.2 | <0.2 | g/100 g |
| glucose | <0.2 | <0.2 | <0.2 | g/100 g |
| sucrose | <0.2 | <0.2 | <0.2 | g/100 g |
| maltose | <0.2 | <0.2 | <0.2 | g/100 g |
| lactose | <0.2 | <0.2 | <0.2 | g/100 g |
| total sugar | <0.2 | <0.2 | <0.2 | g/100 g |
| total fibre | <0.4 | <0.4 | <0.4 | % |
| sodium | 610 | 591 | 626 | mg/100 g |
| calcium | 30 | 31 | 41 | mg/100 g |
| iron | 1.6 | 2.6 | 1.7 | mg/100 g |
| potassium | 171 | 167 | 176 | mg/100 g |
| vit C | <2 | <2 | <2 | mg/100 g |
| vit B1, HCl | 0.00118 | 0.00107 | 0.00123 | % |
| vit B2 | 0.00097 | 0.00044 | 0.00078 | % |
| vit B6 | 0.00098 | 0.00108 | 0.00112 | % |
| vit B12 | <0.1 | <0.1 | <0.1 | ppm |
| moisture | 44 | 43.9 | 43.4 | % |
| copper | - | - | 8.6 | ppm |
| magnesium | - | - | 732.9 | ppm |
| zinc | - | - | 17 | ppm |
| selenium | - | - | <0.1 | ppm |
| manganese | - | - | 6.7 | ppm |
| nickel | - | - | 0.8 | ppm |
| phosphorous | - | - | 2059 | ppm |
| pH of ‘brie’ | 3.0 | 2.9 | 2.8 | - |
| pH of ‘blue’ | 3.0 | 2.9 | 2.9 | - |
| Sample | Species and Media | Genera |
|---|---|---|
| Rejuvelac | Weisella cibaria (MRS) | Weisella |
| Weisella cibaria (GYC) | Weisella | |
| ‘Brie’ | Leuconostoc citreum (SDA) | Leuconostoc |
| Leuconostoc citreum (GYC) | Leuconostoc | |
| ‘Blue’ | Lactococcus lactis (MRS) | Lactococcus |
| Weisella cibaria (MRS) | Weisella |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.M.; Al, K.F.; Craven, L.J.; Seney, S.; Coons, M.; McCormick, H.; Reid, G.; O’Connor, C.; Burton, J.P. Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture. Nutrients 2020, 12, 648. https://doi.org/10.3390/nu12030648
Chen JM, Al KF, Craven LJ, Seney S, Coons M, McCormick H, Reid G, O’Connor C, Burton JP. Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture. Nutrients. 2020; 12(3):648. https://doi.org/10.3390/nu12030648
Chicago/Turabian StyleChen, Jennifer M., Kait F. Al, Laura J. Craven, Shannon Seney, Margaret Coons, Heather McCormick, Gregor Reid, Colleen O’Connor, and Jeremy P. Burton. 2020. "Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture" Nutrients 12, no. 3: 648. https://doi.org/10.3390/nu12030648
APA StyleChen, J. M., Al, K. F., Craven, L. J., Seney, S., Coons, M., McCormick, H., Reid, G., O’Connor, C., & Burton, J. P. (2020). Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture. Nutrients, 12(3), 648. https://doi.org/10.3390/nu12030648





